Symptom Overview
Many chemotherapy agents have the potential to impair fertility.1,2 Infertility resulting from cancer treatments can affect the quality of life for young cancer survivors.3 In 2017, an estimated 852,630 female patients were diagnosed with cancer in the United States, of whom 10.5% were aged <45 years.4
Overall, female cancer survivors are 40% less likely to become pregnant compared with the general population.5 In addition, female patients are less likely than male patients to receive information about fertility preservation before initiating cancer treatment, despite higher subsequent pregnancy rates for female partners of male cancer survivors.3,5
Barriers to oncofertility support for female patients diagnosed with cancer include the cost of and inability to afford treatment, the inability to delay definitive cancer treatment, and the tolerability of fertility-preserving procedures.3
Infertility is defined as the inability to conceive after 1 year of frequent intercourse without contraception.1,2 Infertility resulting from cancer treatment can be temporary or permanent. For some female patients this manifests as amenorrhea, and for others, premature menopause may follow.1,2 In addition, fertility may be compromised despite the maintenance of or resumption of cyclic menses.1
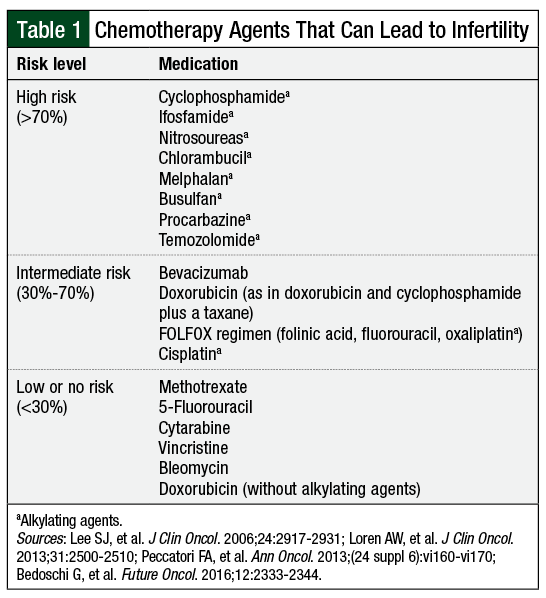
Of the chemotherapy agents known to affect fertility (Table 1),1,2,5,6 alkylating agents carry the greatest risk (Table 1), having demonstrated direct primordial follicle toxicity and apoptosis.6 Other chemotherapeutic agents, such as the antimetabolites and vinca alkaloids, are associated with a low risk for direct primordial follicle toxicity.6 Much of these data, however, are extrapolated from animal models and in-vitro studies with ovarian xenografts.
Little is known about the effects of newer targeted therapies and immunotherapy on fertility. Of note, bevacizumab has been associated with ovarian failure.2 In 2010, the US Food and Drug Administration (FDA) issued a warning about the potential for ovarian failure with bevacizumab use, recommending that providers inform patients of this risk.2 For other agents, caution is a reasonable approach.
Etiology
Female fertility after anticancer therapy is dependent on multiple factors, including the underlying character of the disease, the patient’s age, pretreatment fertility status, and adjunctive regional radiation.1,2,5 The effects on fertility can result from various functional changes to the reproductive system, which can occur by alterations in hormonal balance, anatomical and vascular changes to the reproductive system, decline in the number of primordial follicles, inhibition of oogenesis, and ovarian failure.1 The complex nature of compromised fertility in patients with cancer necessitates individualized fertility preservation strategies.
Decisions about fertility preservation options are complicated by the risk to the patient from delaying cancer treatment, whether the patient can tolerate the necessary procedures, and if the patient has a hormone-sensitive cancer.1,2 Nevertheless, practice guidelines from the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology, as well as an ethics report from the American Society for Reproductive Medicine, recommend that all patients with cancer, including parents of pediatric patients who receive chemotherapy, should be referred to a fertility expert to discuss fertility considerations.2,5,7
Fertility Preservation Treatment Options
Fertility preservation treatment options for female patients include ovarian stimulation and egg retrieval, followed by oocyte cryopreservation or in-vitro fertilization and embryo cryopreservation.1,2,5,7 These processes are considered standard of care for postpubertal females who can delay definitive chemotherapy. These processes can take anywhere from 2 to 6 weeks, and oocyte harvest is done before chemotherapy is initiated.1
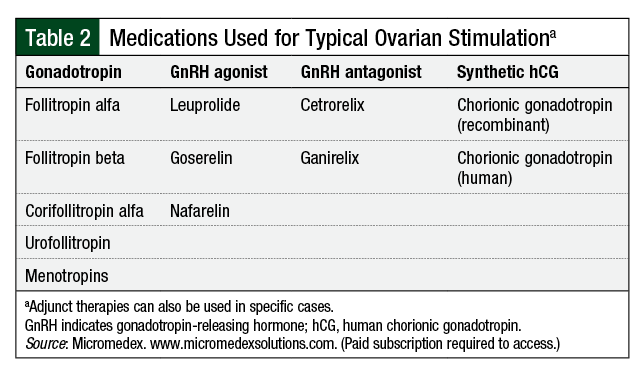
Egg retrieval uses a complex schedule of assorted medications designed to mimic and take control of the natural ovulation cycle (Table 2).8
For female patients with hormone-sensitive tumors, ovarian stimulation regimens incorporating letrozole or tamoxifen have been described.2,9 Protocols using letrozole may be preferred to tamoxifen, because they result in lower peak estradiol levels than tamoxifen.9 Studies support similar outcomes for follicle stimulation and oocyte harvest compared with traditional ovarian stimulation regimens.2,9 Short-term follow-up does not indicate a negative impact on cancer-free survival.2
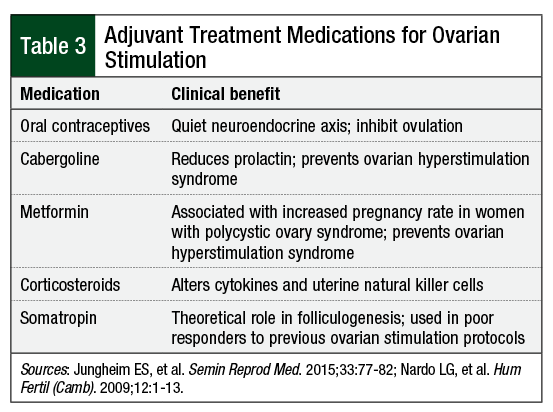
Other adjuvant treatment medications for ovarian stimulation, such as metformin, corticosteroids, somatropin, and dehydroepiandrosterone, may be considered in certain patients (Table 3).10,11 The use of these medications is based primarily on bench research (ie, invitro research that is clinical in nature and includes test tubes and cell cultures, not patient samples) or theoretical efficacy.
Other fertility preservation options described in the literature include ovarian tissue cryopreservation and medical ovarian suppression.1,2,7 Ovarian tissue cryopreservation with autologous transplant is a promising option for patients who are not eligible for ovarian stimulation.1,7 The procedure is currently considered investigational for this purpose and may be offered through clinical trials only.7,12 In ovarian tissue cryopreservation, ovarian tissue is removed laparoscopically, which can be performed as an outpatient procedure, and is then preserved and later transplanted after definitive cancer treatment.1
Ovarian tissue cryopreservation is beneficial, because it does not necessitate the delay in cancer treatment as required for ovarian stimulation or for sexual maturity.2 However, there has been theoretical concern that this option could reintroduce cancer cells.2,13 Therefore, this procedure is not considered in patients with ovarian cancer involvement. Furthermore, animal models have demonstrated that lymphoma and leukemia cell lines can be transmitted from ovarian and testis grafts, respectively.13 Despite this concern, cases have been reported of live births after ovarian tissue cryopreservation autologous transplant in patients whose cancer was in remission, including patients with hematologic or with solid tumor cancers.13,14
Ovarian suppression using gonadotropin-releasing hormone (GnRH) agonists for the duration of treatment with chemotherapy has been explored as a means of preserving fertility.2,5 The results of studies evaluating GnRH agonists for fertility preservation are conflicting, and have been criticized for using resumption of menses as the primary outcome measure, but it is in fact a surrogate measure that does not always translate to preserved fertility.2,5 Therefore, it is difficult to draw conclusions about the efficacy of this strategy. Ovarian suppression for the purpose of fertility preservation is not recommended by current practice guidelines for most patients with cancer.2,5,7,12
A recent update of the ASCO guideline recognized that fertility preservation methods are not always feasible for patients with cancer.12 The guideline has broadened its recommendations to include the use of GnRH agonists, although this is limited to the setting of young women with breast cancer who are ineligible for other fertility preservation methods. The use of GnRH agonists is not currently recommended for other patients with cancer.12
Fertility preservation for patients receiving pelvic irradiation may include ovarian transposition, and for those with a gynecologic malignancy, anatomy-conserving surgery is recommended where appropriate.1,2
Ovarian Stimulation: Course of Therapy
Medical ovarian stimulation mimics the natural menstrual cycle to stimulate follicular development and then takes control of ovulation to promote development of multiple mature oocytes (Figure).10,15
Traditionally, stimulatory medications are initiated 3 days after the onset of menses, which could require 2 to 6 weeks to allow for the appropriate timing of medications.10,15 Daily intramuscular injections of follicle-stimulating hormone (FSH) are administered with or without luteinizing hormone. Most stimulation protocols start with 150 IU to 300 IU of gonadotropin, given daily; 225 IU of gonadotropin is the standard starting dose for most patients.10 Higher doses may be used when the goal is to stimulate a larger cohort of ovarian follicles. Stimulation with gonadotropin typically takes 2 weeks for adequate folliculogenesis.15
Follicle development is monitored by serial transvaginal ultrasounds and blood tests.15 At the appropriate time, a “trigger injection” is administered to start the ovulatory cascade.10,15 This is typically given when 2 leading follicles are bigger than 16 mm to 18 mm, as visualized by ultrasound.10,15
Administration timing varies by provider. The trigger injection mimics the luteinizing hormone surge that starts ovulation. This can be done with human chorionic gonadotropin or a GnRH agonist. Approximately 36 hours after the trigger administration, the oocytes are subsequently collected by ultrasound-guided transvaginal needle aspiration under intravenous sedation.15
Guidelines describing the optimal ovarian stimulation regimen are lacking.10,15 The medications used and the schedule sequence for ovarian stimulation are largely determined by the provider’s preference, experience, and the patient’s response throughout the course of treatment.10 The medication regimens are individualized to the patient’s needs and characteristics.
There are currently 3 general protocols in use. These protocols include (1) long GnRH agonist, (2) flare, and (3) GnRH antagonist. The long GnRH regimens include GnRH agonists, gonadotropin, and human chorionic gonadotropin analogs.10,15 These regimens are initiated in the midluteal phase of the cycle preceding FSH stimulation.15 GnRH agonists are used to downregulate endogenous gonadotropins or to quiet the neuroendocrine axis. This allows for folliculogenesis to be controlled with exogenous gonadotropin.15 Long GnRH regimens can result in improved response, although they require careful planning and longer time commitments, making them impractical for patients with cancer.
As with the long GnRH regimens, flare regimens use GnRH agonists, but the GnRH agonist is initiated 2 to 3 days before menses. Flare regimens are used for patients who are poor responders to conventional ovarian stimulation regimens, and have no role for emergent oocyte harvest for patients with cancer.10 In contrast, the GnRH antagonist regimens are preferred for patients in need of urgent oocyte harvest, such as newly diagnosed patients with cancer who need to start treatment with chemotherapy, because the regimen can be initiated quickly and requires a shorter cycle of ovarian stimulation.10
GnRH antagonist regimens allow for random or midcycle initiation, because GnRH antagonists immediately block the production of endogenous gonadotropins.16 Ultimately, the ovarian stimulation protocol used, as well as the combination of medications, are dependent on the phase of the menstrual cycle in which treatment is initiated, and a GnRH antagonist may not always be necessary.16 In patients with cancer, treatment with chemotherapy is delayed until ovarian stimulation is complete.
When GnRH antagonists are used, endogenous luteinizing hormone levels are extremely suppressed.10 Gonadotropins with luteinizing hormone activity should be included in these regimens. Otherwise, the choice of gonadotropin depends on drug availability, convenience, and cost, taking into consideration that most health insurance companies do not offer coverage for assisted reproductive techniques.
The alternatives to fertility preservation are limited when the risk for delaying chemotherapy is too high. Ovarian tissue cryopreservation may be an option for some individuals where institutional review board–approved protocols are available.1,2 One case report describes successful emergent in vitro fertilization performed between chemotherapy cycles, although the long-term effects on the oocyte and subsequent offspring are unknown.17
Ovarian Suppression: Role of GnRH Agonists
As previously mentioned, the data describing the use of GnRH agonists are conflicting and extensively scrutinized. As summarized in the recent ASCO guideline update, 7 international guidelines offer mixed recommendations.12 The majority of the data describing the role of GnRH agonists in fertility preservation consists of low-evidence studies, such as animal models, in vitro studies, small retrospective studies, and uncontrolled observational trials.18-20 More recently, non–placebo-controlled trials have been conducted, and numerous literature reviews and meta-analyses have attempted to clarify the role of GnRH agonists.12,18-22 Despite this growing body of research, the results and the interpretations of these data remain controversial.
The majority of the data evaluating the role of GnRH agonists in fertility preservation has been conducted in patients with breast cancer, although some studies and meta-analyses have also included patients with ovarian cancer, lymphoma, and those receiving hematopoietic stem-cell transplants.12,18-22 Primordial follicle loss caused by chemotherapy is triggered through DNA damage and is not cell-cycle dependent.18 With this in mind, it is unlikely that the efficacy or outcomes with GnRH agonists would be different based on cancer type alone. Some researchers suggest that the beneficial effect of GnRH agonists is age-limited,19 which is also controversial.21
Fourteen clinical trials have evaluated the role in the setting of breast cancer, with more that are ongoing.21,23-36 The results of these studies should be interpreted with caution, because of their small sample sizes and short follow-up periods. The definitions of premature ovarian failure or insufficiency are inconsistent, with most studies relying on inappropriate surrogates, such as amenorrhea.19-22 Patients in these studies had a median age near 40 years, bringing into question baseline fertility.21 The results were also confounded by including patients who were candidates for adjuvant endocrine therapy for indications other than ovarian suppression or fertility preservation.21
In addition, little is known about the combined fertility preservation modalities used by patients who eventually conceive. In all, 10 of the 14 studies concluded that the concurrent use of GnRH agonists during chemotherapy is associated with a significant reduction in the risk for premature ovarian failure.21 The evidence for a role in fertility preservation is more limited, with only 1 study collecting pregnancy data as a predetermined end point, and that study did not adjust for intent to conceive.18,21,34
Meta-analyses and reviews that combined data from these trials have not always included studies in patients with cancers other than breast cancer.21 The majority of these meta-analyses and reviews, including a recent Cochrane review, are consistent in concluding that the concurrent use of GnRH agonists with chemotherapy reduces the risk for premature ovarian failure.21,22 These results were not significantly observed in subgroup analyses of patients with hematologic malignancies, although the included patient populations were small.21
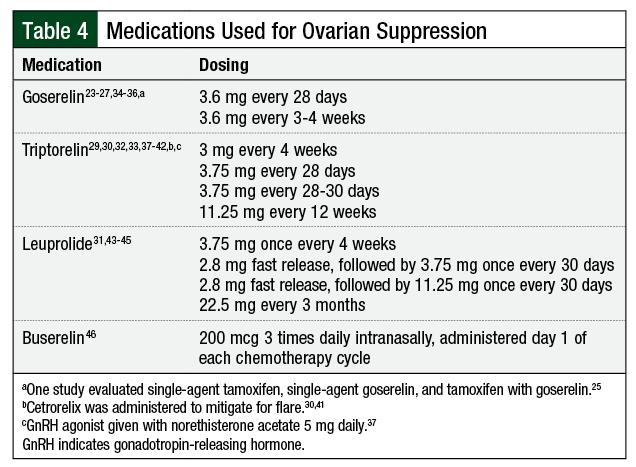
The majority of studies utilized triptorelin or goserelin, although leuprolide and buserelin were used in a few of the studies (Table 4).23-27,29-46 It remains unclear by which mechanism GnRH agonists provide ovarian protection. For some of these patients with cancer, GnRH agonists may carry other indications, such as adjuvant endocrine treatment or the prevention of thrombocytopenia-associated menorrhagia.20,47
Anecdotally at our institutions, insurance coverage has not been a barrier to care. It is unclear if this is because these medications may be used for other indications, although currently GnRH agonists are not approved by the FDA for ovarian suppression. Consideration should be given to the timing of the initiation of GnRH agonist treatment because of the ovulation flare, although this may not always be realistic.20 Only 2 studies described the use of a GnRH antagonist to mitigate the flare effect.30,41 The treatment side effects include hot flashes, headaches, mood swings, and osteoporosis.8
The efficacy of GnRH agonists as a means of fertility preservation has yet to be clearly demonstrated.12,18-22 Evidence suggests a role for ovarian suppression with a GnRH agonist in reducing premature ovarian failure. It is important to distinguish the nuances between premature ovarian failure or insufficiency and menopause. Women are still at risk for early-onset menopause, an end point that is not explored in these studies, likely because of the long-term follow-up requirements these data would necessitate.21
Ovarian suppression with a GnRH agonist has sometimes been used for patients who have no other fertility preservation option, without having a full understanding of the potential risks and benefits.1 The extent of this practice is unknown. The ASCO practice guidelines encourage patients who are interested in ovarian suppression as a means of fertility preservation to participate in clinical trials.12 This method should not be used to replace methods with proved and reliable results for subsequent pregnancy.19
Conclusion
Infertility is common in patients with cancer who receive treatment with chemotherapy. Many patients do not receive appropriate information regarding fertility treatment options. Surveys suggest that female patients with cancer are less likely to receive information regarding fertility preservation, despite poorer fertility outcomes after cancer treatment. Multiple barriers may limit female patients with cancer from pursuing fertility-preserving methods. These barriers likely weigh heavier on female patients, given the physiologic differences and the ease and timeliness of fertility-preserving options compared with male patients with cancer.
Current practice guidelines recommend that all patients with cancer be referred to a fertility specialist as part of the initial workup and design of their treatment plans. Oncology providers and fertility specialists should take the patient’s fertility goals into account, working together to develop care plans that use the best fertility-preserving methods.
Fertility preservation options are best personalized to the individual patient’s situation and needs. For female patients, ovarian stimulation and cryopreservation are the preferred standard of care for postpubertal patients. The method selected is dependent on patient characteristics, timing of menstrual cycle, and urgency of oocyte harvest. In addition, stimulation protocols have been established for patients with hormone-sensitive cancers. For those who are unable to tolerate ovarian stimulation or delays in definitive cancer treatment, and for prepubertal patients, fertility preservation options are currently limited to clinical trials. Ovarian tissue cryopreservation and transplantation may be a promising option for these patients. Patients interested in these options should be encouraged to enroll in clinical trials.
Author Disclosure Statement
Dr McCollam, Dr Shipman, Dr Bubalo, and Dr Krieg have
no conflicts of interest to report.
References
- Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
- Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500-2510.
- Logan S, Perz J, Ussher J, et al. Clinician provision of oncofertility support in cancer patients of a reproductive age: a systematic review. Psychooncology. 2018;27:748-756.
- American Cancer Society. Cancer facts & figures 2017. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer--facts-and-figures/2017/estimated-new-cases-for-the-four-major-cancers-by-sex-and-age-group-2017.pdf. Accessed September 29, 2017.
- Peccatori FA, Azim HA Jr, Orecchia R, et al; for the ESMO Guidelines Working Group. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;(24 suppl 6):vi160-vi170.
- Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol. 2016;12:2333-2344.
- Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;-83:1622-1628.
- Micromedex. www.micromedexsolutions.com. Accessed June 18, 2018. (Paid subscription required to access.)
- Oktay K, Buyuk E, Libertella N, et al. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347-4353.
- Jungheim ES, Meyer M, Broughton DE. Best practices for controlled ovarian stimulation in in vitro fertilization. Semin Reprod Med. 2015;33:77-82.
- Nardo LG, Granne I, Stewart J; for the Policy Practice Committee of the British Fertility Society. Medical adjuncts in IVF: evidence for clinical practice. Hum Fertil (Camb). 2009;12:1-13.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;-36:1994-2001.
- Bockstaele L, Tsepelidis S, Dechene J, et al. Safety of ovarian tissue autotransplantation for cancer patients. Obstet Gynecol Int. 2012;2012:495142.
- Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59-67.
- Robertson DM, Gilchrist RB, Ledger WL, Baerwald A. Random start or emergency IVF/in vitro maturation: a new rapid approach to fertility preservation. Women’s Health (Lond). 2016;12:339-349.
- Cai H, Shen H. Random-start controlled ovarian stimulation for emergency fertility preservation in a patient with myelodysplastic syndrome: a case report. Braz J Med Biol Res. 2016;49:e5227.
- Hussein G, Lood M. Random-start IVF treatment: an emergent fertility preservation technique between cytotoxic treatment courses and stem-cell transplantation in acute myelocytic leukemia. Middle East Fertil Soc J. 2015;20:297-300.
- Oktay K, Bedoschi G. Appraising the biological evidence for and against the utility of GnRHa for preservation of fertility in patients with cancer. J Clin Oncol. 2016;34:2563-2565.
- Blumenfeld Z, von Wolff M. GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update. 2008;14:543-552.
- Cima LN, Colita A, Fica S. Perspectives on the co-treatment with GnRHa in female patients undergoing hematopoietic stem cell transplantation. Endocr Connect. 2017;6:R162-R170.
- Lambertini M, Richard F, Nguyen B, et al. Ovarian function and fertility preservation in breast cancer: should gonadotropin-releasing hormone agonist be administered to all premenopausal patients receiving chemotherapy? Clin Med Insights Reprod Health. March 2019;13.
- Chen H, Xiao L, Li J, et al. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev. 2019;3:CD008018.
- Li M, Huang H, Liang Y, et al. Effect of Zoladex administered before chemotherapy on menstruation of patients with breast cancer. Chin J Clin Oncol. 2008;35:905-907.
- Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91:694-697.
- Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant gose-relin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat. 2009;117:-561-567.
- Gerber B, von Minckwitz G, Stehle H, et al. Effect of luteinizing hormone–releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011;-29:2334-2341.
- Sun JB, Ren YH, Li WQ. Effect of Zoladex administered before chemotherapy on menstruation of patients with breast cancer. Chin Disabil Med. 2011;19:-15-16.
- Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306:269-276.
- Munster PN, Moore AP, Ismail-Khan R, et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:533-538. Erratum in: J Clin Oncol. 2012;30:4048.
- Elgindy EA, El-Haieg DO, Khorshid OM, et al. Gonadatrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstet Gynecol. 2013;121:78-86.
- Song G, Gao H, Yuan Z. Effect of leuprolide acetate on ovarian function after cyclophosphamide–doxorubicin-based chemotherapy in premenopausal patients with breast cancer: results from a phase II randomized trial. Med Oncol. 2013;30:667. doi.org/10.1007/s12032-013-0667-8. Accessed October 15, 2019.
- Jiang FY, Zhang QQ, Zeng J. Protective effect of GnRHa on chemotherapy induced ovarian damage in breast cancer patients. Shandong Med J. 2013;53:16-18.
- Karimi-Zarchi M, Forat-Yazdi M, Vafaeenasab MR, et al. Evaluation of the effect of GnRH agonist on menstrual reverse in breast cancer cases treated with cyclophosphamide. Eur J Gynaecol Oncol. 2014;35:59-61.
- Moore HCF, Unger JM, Phillips KA, et al; for the POEMS/S0230 investigators. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy.N Engl J Med. 2015;372:923-932.
- Leonard RCF, Adamson DJA, Bertelli G, et al; for the Anglo Celtic Collaborative Oncology Group and National Cancer Research Institute Trialists. GnRH agonist for protection against ovarian toxicity during chemotherapy early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol. 2017;28:1811-1816.
- Zhang Y, Ji Y, Li J, et al. Sequential versus simultaneous use of chemotherapy and gonadotropin-releasing hormone agonist (GnRHa) among estrogen receptor (ER)-positive premenopausal breast cancer patients: effects on ovarian function, disease-free survival, and overall survival. Breast Cancer Res Treat. 2018;168:679-686.
- Demeestere I, Brice P, Peccatori FA, et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J Clin Oncol. 2016;34:2568-2574.
- Blumenfeld Z, Avivi I, Eckman A, et al. Gonadotropin-releasing hormone agonist decreases chemotherapy-induced gonadotoxicity and premature ovarian failure in young female patients with Hodgkin lymphoma. Fertil Steril. 2008;89:166-173.
- Castelo-Branco C, Nomdedeu B, Camus A, et al. Use of gonadotropin-releasing hormone agonists in patients with Hodgkin’s disease for preservation of ovarian function and reduction of gonadotoxicity related to chemotherapy. Fertil Steril. 2007;87:703-706.
- Dann EJ, Epelbaum R, Avivi I, et al. Fertility and ovarian function are preserved in women treated with an intensified regimen of cyclophosphamide, adriamycin, vincristine and prednisone (Mega-CHOP) for non-Hodgkin lymphoma. Hum Reprod. 2005;20:2247-2249.
- Huser M, Crha I, Ventruba P, et al. Prevention of ovarian function damage by a GnRH analogue during chemotherapy in Hodgkin lymphoma patients. Hum Reprod. 2008;23:863-868.
- Blumenfeld Z, Patel B, Leiba R, Zuckerman T. Gonadotropin-releasing hormone agonist may minimize premature ovarian failure in young women undergoing autologous stem cell transplantation. Fertil Steril. 2012;98:1266-1270.e1.
- Pereyra Pacheco B, Méndez Ribas JM, Milone G, et al. Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: a preliminary report. Gynecol Oncol. 2001;81:-391-397.
- Cheng YC, Takagi M, Milbourne A, et al. Phase II study of gonadotropin-releasing hormone analog for ovarian function preservation in hematopoietic stem cell transplantation patients. Oncologist. 2012;17:233-238.
- Phelan R, Mann E, Napurski C, et al. Ovarian function after hematopoietic cell transplantation: a descriptive study following the use of GnRH agonists for myeloablative conditioning and observation only for reduced-intensity conditioning. Bone Marrow Transplant. 2016;51:1369-1375.
- Waxman JH, Ahmed R, Smith D, et al. Failure to preserve fertility in patients with Hodgkin’s disease. Cancer Chemother Pharmacol. 1987;19:159-162.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast Cancer. Version 3.2019. September 6, 2019. www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed October 15, 2019.