With commentary by

Maria Shellock, BSN, RN
Nurse Manager
Massachusetts General Hospital Cancer Center/Protocol Office
Boston, MA
Introduction
Each year, approximately 250,000 cases of breast cancer are diagnosed in the United States.1 Triple-negative breast cancer (TNBC) accounts for 10% to 15% of these diagnoses, amounting to 25,000 to 37,500 new cases, most often among women who are black, aged <40 years, and/or BRCA1-positive.1,2 TNBC is generally more aggressive and challenging to treat than other types of breast cancer, resulting in an 8% to 16% lower relative survival rate 5 years after diagnosis.2,3 The scarcity of effective treatment options is largely explained by pathological negativity for 3 common drug targets: human epidermal growth factor receptor 2 and hormone receptors for both progesterone and estrogen.2,4 Because patients with TNBC lack these drug targets on their cancer cells, chemotherapy has historically been the mainstay treatment for TNBC, despite associations with early relapse and, after metastasis, a median overall survival (OS) of just 10 to 13 months.4
Fortunately, this paradigm is shifting, as emerging targeted therapies are expanding treatment options for patients with TNBC.4 Recently, one of these agents, sacituzumab govitecan-hziy (SG), gained FDA approval for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received ≥2 prior systemic therapies, ≥1 of them for metastatic disease.5 SG, a first-in-class agent, was also upgraded to a preferred therapy in this setting by the National Comprehensive Cancer Network (NCCN), based on results of the phase 3 ASCENT trial.6,7
This article offers an overview of SG clinical data, optimal administration of SG, and management of adverse events (AEs). After each evidence-based section, Maria Shellock, BSN, RN, Research Nurse Manager at Massachusetts General Hospital Cancer Center/Protocol Office, offers relevant commentary from a real-world perspective, helping bridge the gap between clinical trials and clinical practice.
Commentary by Maria Shellock, BSN, RN: TNBC often comes on fast and furious, so sometimes it feels like a train that’s already left the station. It also affects a younger demographic, and the tool chest of treatments is more limited, which can make a diagnosis feel like a bit of a panicked situation. Fortunately, a lot of promising treatment options are coming that are very exciting for this population. Antibody–drug conjugates such as SG are attacking the cancer in a much more targeted, smarter way.
I was fortunate to be involved in the very early trials evaluating SG. It’s been exciting to see metastatic patients doing so well, having their quality of life improved, and being able to stay on treatment longer. It’s really been a breakthrough. With SG, we’re finally seeing a significant jump in survival for patients with mTNBC, and in a way that they can still live their lives and feel well.
Overview of sacituzumab govitecan
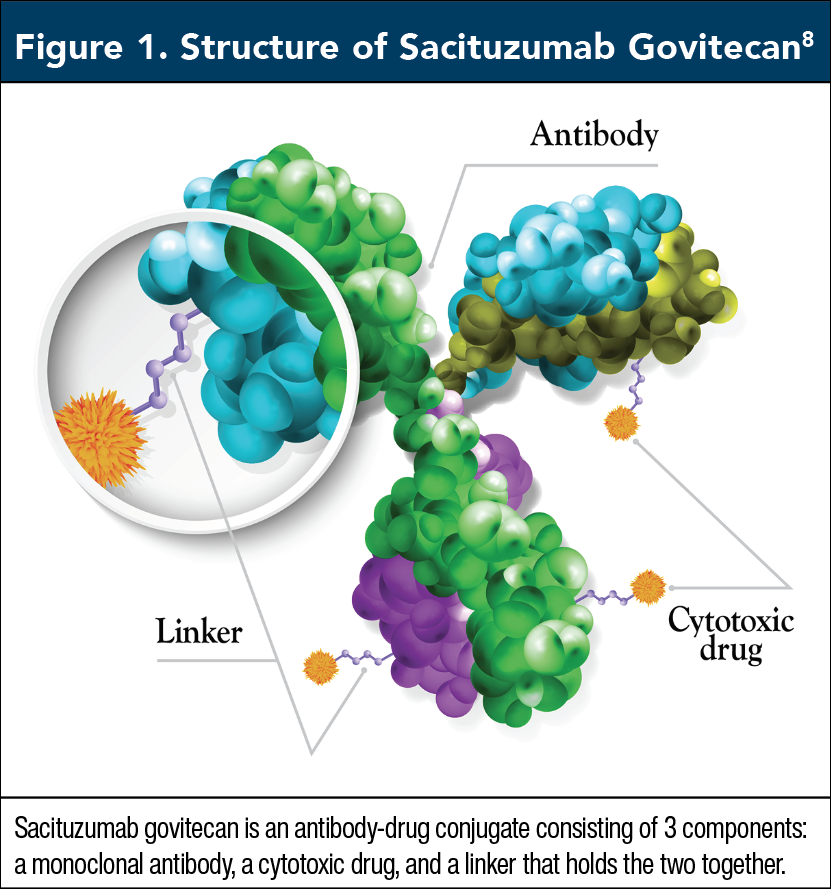
SG is an antibody–drug conjugate consisting of 3 components: a monoclonal antibody, a cytotoxic drug (SN-38), and a linker that holds the two together (Figure 1).8 During treatment, the antibody targets trophoblast cell-surface antigen-2 on TNBC cells, leading to internalization of the agent.9 Once inside a cancer cell, the linker is broken down, releasing SN-38, a topoisomerase I inhibitor that prevents repair of single-strand breaks in DNA, ultimately leading to cancer cell death and apoptosis.9 In simpler terms, SG targets cancer cells and delivers a cytotoxic drug directly into them. It is administered intravenously in a 21-day treatment cycle on days 1 and 8, at a dose of 10 mg/kg, continued until unacceptable toxicity or disease progression.9
Commentary by Maria Shellock, BSN, RN: SG is a smarter agent, there is a chemotherapy aspect to it, but it’s encapsulated in something and it’s not just floating through the system, able to touch everything in its path. It’s in a protected bubble, so to speak, and once it hooks up to cancer cells, it’s able to release directly inside of them. In contrast, traditional chemotherapy affects everything it touches, which is why it has more issues with tolerability and quality of life.
You need to gauge your patient when explaining what SG is. Although some people want the nitty gritty, scientific details, including the exact mechanism of action, most patients just want a basic explanation. I typically use an analogy. I ask the patient to imagine being locked out of their house. To get in, they can use a sledgehammer or a key. Traditional chemotherapy is more like a sledgehammer, whereas SG is more like a key. Both can get you through the front door, but one is going to do a lot more damage than the other.
Clinical data: Phase 1/2 trial
In 2017, Ocean and colleagues conducted a phase 1/2 trial that evaluated SG in 178 patients with a variety of advanced epithelial cancers.10 The study included a cohort of 53 patients with mTNBC, all of whom had an Eastern Cooperative Oncology Group performance status of 0 or 1, denoting full activity level or mild physical restrictions, and a median age of 53 years (range, 33-81 years).10,11 The mTNBC group was heavily pretreated; most patients (85%) had received ≥3 prior therapies, with a median of 5.10
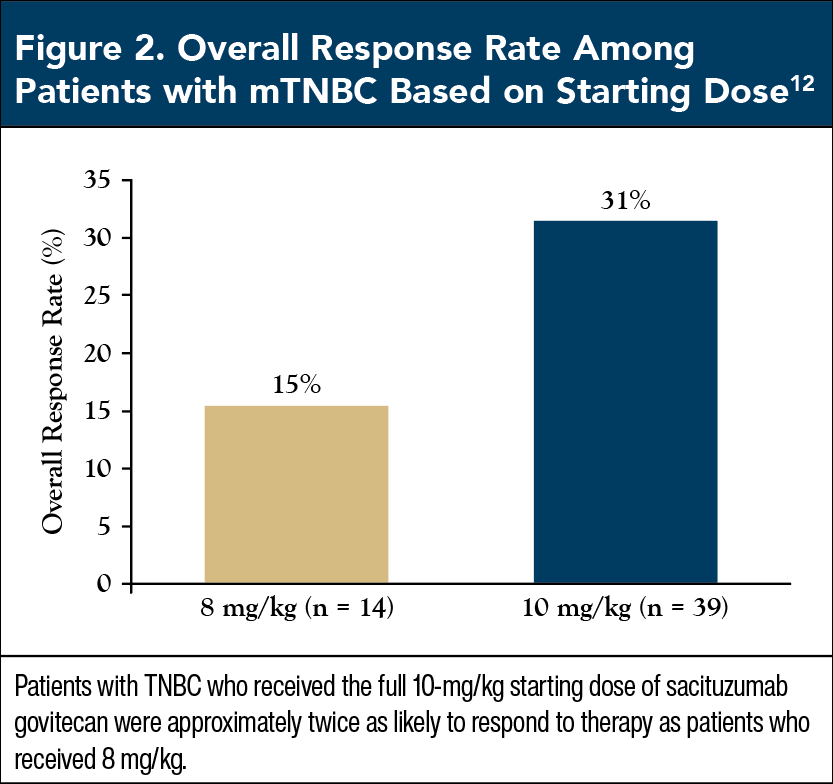
Based on early safety and tolerability findings, patients were given either 8 mg/kg or 10 mg/kg of SG as a starting dose, which revealed that AE rates were similar between doses.10 Although the 10-mg/kg dose was associated with slightly more grade 3 or 4 AEs, particularly diarrhea and neutropenia, the investigators concluded that the relative clinical benefits of the full dose were so great that they outweighed minor differences in safety.10
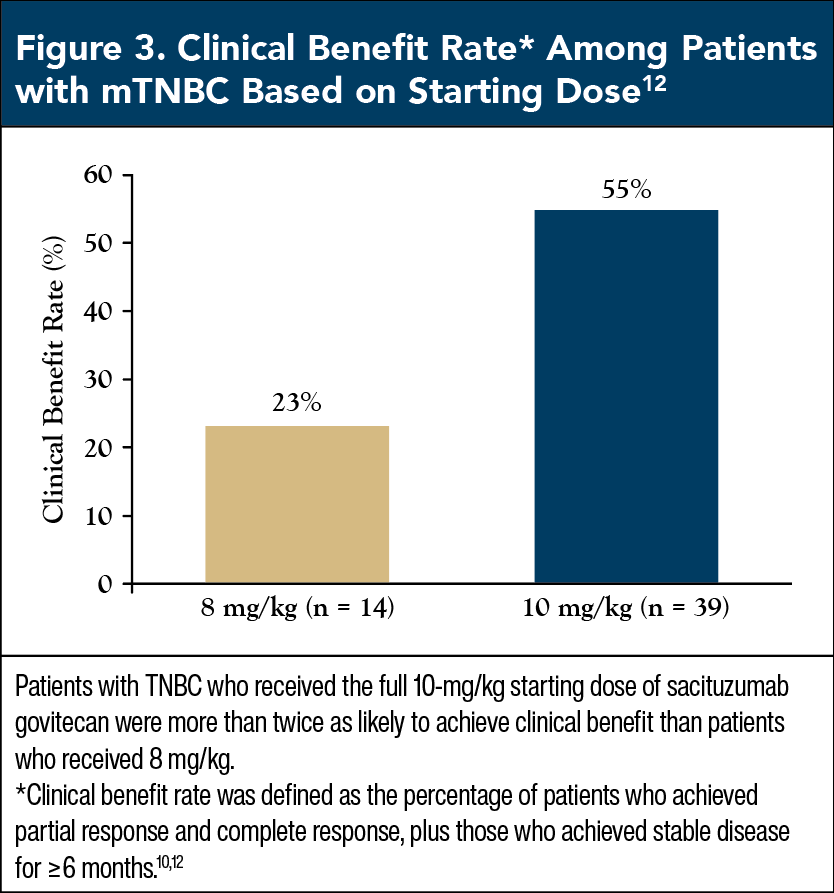
Patients with mTNBC who received the full dose of 10 mg/kg had an overall response rate of 31.4%, twice as high as the 15.4% rate among patients who received the reduced dose of 8 mg/kg (Figure 2).12 Similarly, 54.8% of mTNBC patients who received 10 mg/kg had clinical benefit, more than double the 23.1% rate among those who received 8 mg/kg (Figure 3).12 These findings provide strong support that patients with mTNBC should start SG at the recommended dose of 10 mg/kg, as this dose maximizes clinical benefit without significantly altering tolerability.9
Commentary by Maria Shellock, BSN, RN: Beginning therapy at any treatment’s recommended starting dose helps derive the maximum benefit from it, and we clearly see this effect with SG in the trial by Ocean and colleagues. In addition to these data, when we’re starting patients on a new treatment, it’s much easier to go down in dose than it is to go up. So, when patients first start SG, we really want to give them the full dose, because that gives them the best chance at a response, and maximum benefit.
Clinical data: Phase 3 ASCENT trial
Design
With topline results published in 2021, the phase 3 ASCENT trial offered a more comprehensive look at the efficacy and safety of SG for mTNBC. The trial involved 468 patients with relapsed or refractory mTNBC who did not have brain metastases and had previously received ≥2 standard chemotherapy regimens, including ≥1 taxane-based chemotherapies.7 Median patient age was 54 years, ranging from 27 to 82 years.7 Patients were randomized in a 1:1 ratio to receive either SG (n = 235) or physician’s choice chemotherapy (n = 233), most often eribulin (n = 126), followed by vinorelbine (n = 47), capecitabine (n = 31), and gemcitabine (n = 29).7 The primary end point was progression-free survival (PFS) determined by blinded independent central review.7 Secondary end points included objective response, investigator-assessed PFS, OS, and safety.7
Efficacy
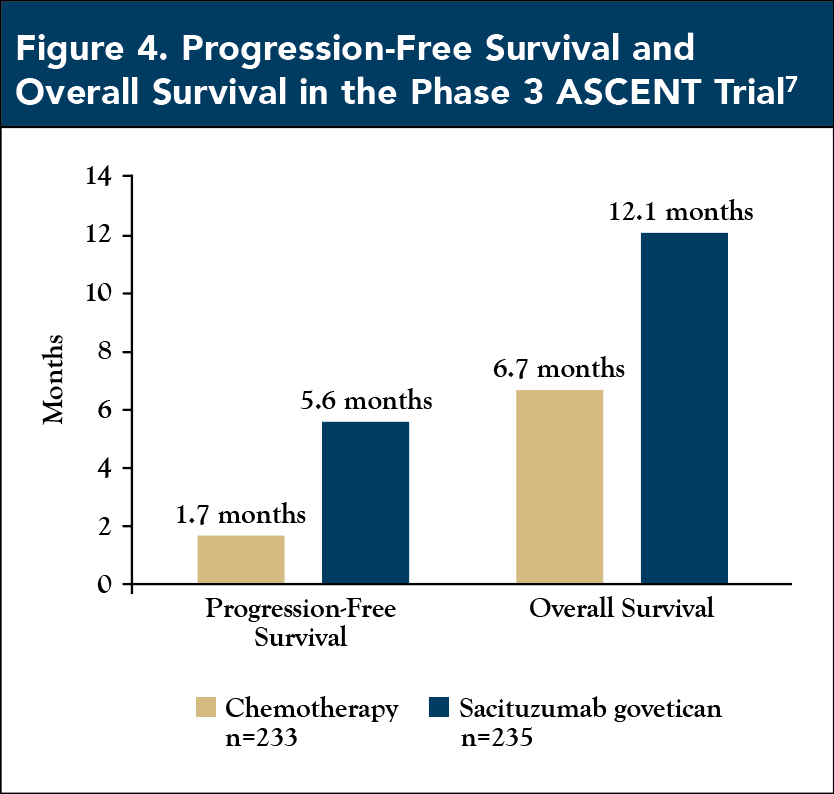
After a median follow-up of 17.7 months (range, 5.8-28.1 months), the objective response rate in the SG group was 35%, compared with 5% in the chemotherapy group. Similarly, median PFS was higher with SG (5.6 months), compared with chemotherapy (1.7 months), which translated to a 59% reduced risk of disease progression or death (hazard ratio [HR], 0.41; 95% confidence interval [CI], 0.32-0.52; P <.001) (Figure 4).7 SG also significantly prolonged life, based on a median OS of 12.1 months in the SG group, compared with 6.7 months in the chemotherapy group (HR, 0.48; 95% CI, 0.38-0.59; P <.001) (Figure 4).7 The PFS, OS, and clinical benefits of SG were observed across subgroups, including patients aged ≥65 years, those who had received ≥4 prior therapies, and those previously treated with a checkpoint inhibitor.7
Health-related quality of life
A separate analysis of the ASCENT trial explored the effects of SG versus chemotherapy on health-related quality of life (HRQOL). The data set included 419 mTNBC patients with or without brain metastases, among whom 236 received SG and 183 received chemotherapy.13 HRQOL was assessed with the European Organisation for Research and Treatment of Cancer Quality of Life Core 30 Questionnaire, which was administered at baseline, on the first day of each cycle, and 1 month after completing therapy.13
Domains of primary importance were global health status/QOL, role functioning, physical functioning, fatigue, and pain.13 Superior HRQOL benefit required a significant improvement from baseline that exceeded minimal-important-difference thresholds between treatment groups.13 Compared with chemotherapy, SG demonstrated superior HRQOL benefit in 4 of 5 primary HRQOL domains: global health status/QOL, physical functioning, fatigue, and pain.13 SG also demonstrated superiority in 3 secondary domains: emotional functioning, dyspnea, and insomnia.13 Although SG was more often associated with diarrhea and nausea/vomiting, these outcomes did not lead to a significant negative impact on global health status/QOL or functioning domains.
For each of the 5 primary HRQOL domains, the investigators also evaluated median time until first clinically meaningful deterioration.13 This revealed that SG was superior to chemotherapy in 4 of 5 domains: physical functioning (22.1 vs 12.1 weeks), role functioning (11.4 vs 7.1 weeks), fatigue (7.7 vs 6.0 weeks), and pain (21.6 vs 9.9 weeks).13 SG also had a minor, non-statistically significant association with sooner deterioration of global health status/QOL (14.1 vs 15.1 months; 95% CI, 0.70-1.07).13
Collectively, the data demonstrate that SG delivers greater HRQOL benefits than chemotherapy, while simultaneously extending both PFS and OS.
Safety
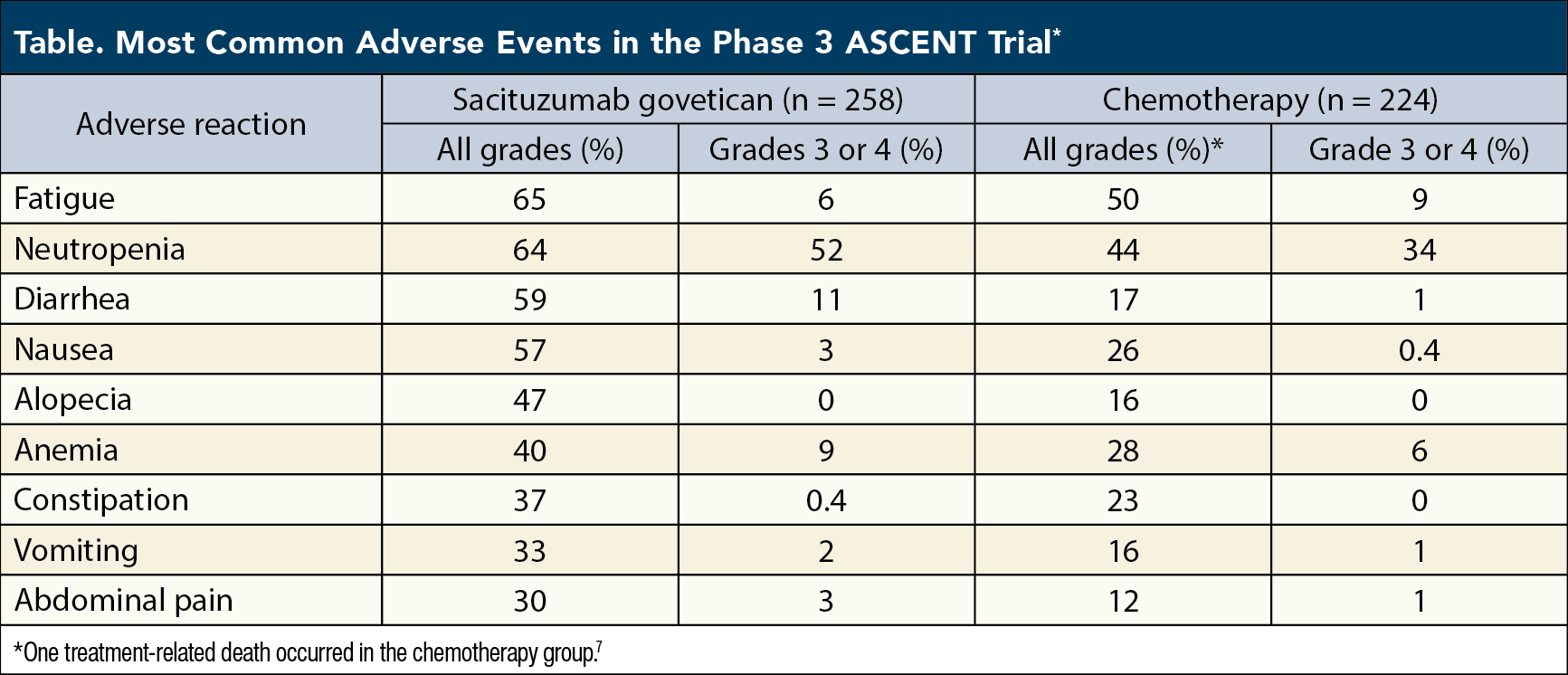
The ASCENT trial also offered a comprehensive overview of the safety profile for SG. Among 258 patients eligible for analysis, the most common AEs of any grade were fatigue (65%), neutropenia (64%), diarrhea (59%), nausea (57%), alopecia (47%), anemia (40%), constipation (37%), vomiting (33%), and abdominal pain (30%) (Table).9 Among grade 3 or 4 events, neutropenia occurred most often, in 52% of patients, followed by 11% of patients who developed grade 3 or 4 diarrhea or leukopenia (Table).9
Although grade 3 or 4 AEs were more common in the SG group than the chemotherapy group (64% vs 47%), rates of treatment discontinuation as a result of AEs were the same in both groups (5%) and dose reductions because of AEs were more common in the chemotherapy group (26% vs 22%).7 Furthermore, no deaths in the SG group were deemed treatment-related, whereas 1 death in the chemotherapy group resulted from treatment-related neutropenic sepsis.7
These findings highlight some of the AEs associated with SG that may require management, particularly neutropenia and diarrhea, which are included in a boxed warning.9 As will be described in a later section, a variety of steps can be taken both before and during treatment to manage these AEs, including prophylactic use of granulocyte colony-stimulating factor (G-CSF) to reduce the risk of neutropenia, and dose modifications to limit non-neutropenic toxicities, such as diarrhea.9
Special safety consideration: UGT1A1. When administering SG, adverse reactions may be slightly more common among a subset of patients homozygous for the uridine diphosphate-glucuronosyltransferase 1A1 (UGT1A1)*28 allele (ie, they have inherited 2 copies of the gene).14 Such patients have reduced activity of UGT1A1, an enzyme involved in metabolizing SN-38, the cytotoxic component of SG.15 These patients may therefore have higher levels of the drug in their system, leading to a modestly increased risk for hematologic toxicities, such as anemia and neutropenia, and possibly other AEs.14
In the ASCENT trial, 250 patients in the SG arm (97%) had UGT1A1 genotype data at baseline, with 59% of patients with 2 copies of the UGT1A1*28 allele developing grade ≥3 neutropenia, compared with 53% of patients with normal UGT1A1 genes. Grade ≥3 diarrhea was also more common among patients with 2 copies of UGT1A1*28, at 15%, compared with 10% for patients with normal UGT1A1 genes.15
As these risk elevations are relatively mild, patients with 2 copies of UGT1A1*28 should still receive SG at the recommended starting dose (10 mg/kg); however, they need to be monitored closely, with a plan to modify dose and add supportive medications as needed.15 In contrast, drugs that affect UGT1A1 enzyme function, including inhibitors and inducers, are contraindicated, and should be avoided when administering SG.14
Commentary by Maria Shellock, BSN, RN: One of the biggest takeaways from the ASCENT trial is that we are finally seeing the needle move in the right direction for patients with mTNBC. It’s a huge improvement for the objective response rate to go from only 5% with traditional chemotherapy to 35% with SG.
Patients always want to know, “How much time is this drug going to give me? How long am I going to have on this treatment?” Obviously, everyone’s different, so we don’t know how they’re going to respond. But when you’re able to provide head-to-head data comparing SG against traditional chemotherapies, and we’re seeing nearly double the median overall survival, that’s a huge improvement for these patients, and an option that they just haven’t had in the past. These data give patients the hope that they really need.
In addition to the positive response and survival findings, the quality-of-life data in the ASCENT trial were really encouraging. SG provides a better quality of life than traditional chemotherapy, and that makes sense, because your quality of life is going to be better when your treatment is smarter. When you’re able to protect as much of the normal body’s function by delivering this targeted cytotoxic drug, you’re able to improve a patient’s quality of life in terms of what they’re able to do every day, how long their side effects last, how fatigued they are, and how they perceive their own level of health.
Administering sacituzumab govitecan
SG was recently designated a preferred regimen by the NCCN, and is approved by the FDA for patients with unresectable locally advanced or mTNBC who have received ≥2 prior systemic therapies, ≥1 of them for metastatic disease.5,6 Patients should start on a full dose of 10 mg/kg SG infused intravenously on days 1 and 8 of a 21-day treatment cycle until unacceptable toxicity or disease progression.14
Although the FDA-approved drug label for SG states that 180 mg of the agent is present in each vial, this is a minimum fill amount; the target fill is 200 mg, so reconstitution with 20 mL of 0.9% Sodium Chloride Injection, USP will create a concentration of 10 mg/mL.14 This reconstituted solution should be diluted immediately to make an infusion solution.9 If not administered right away, the infusion solution can be refrigerated for up to 4 hours at 2°C to 8°C (36°F-46°F).9 After refrigeration, the solution should be administered within 4 hours, including duration of infusion.9
Prior to each infusion of SG, patients should be premedicated to reduce the risk of chemotherapy-induced nausea and vomiting.14 A 2- or 3-drug premedication regimen is recommended, such as an antipyretic with H1 and H2 blockers, or dexamethasone with an NK1 receptor antagonist.14 Corticosteroids are recommended for patients who have had prior infusion reactions.14
The first infusion of SG should be delivered over 3 hours, whereas subsequent infusions may be given more rapidly—over 1 to 2 hours, thereby increasing clinical efficiency for providers and infusion centers, and reducing treatment burden for patients.14 Each infusion should be followed by a 30-minute observation period for symptoms or signs of infusion-related reactions.14
Commentary by Maria Shellock, BSN, RN: The main thing we try to do before the first infusion of SG is to set expectations, to let patients know that there are supportive medications that can help mitigate side effects with good success. It might take us a cycle or two to get the right cocktail to make it manageable, but ultimately most patients really do tolerate SG quite well.
For premedication, we typically use an antipyretic such as acetaminophen and an H1 or H2 blocker, such as famotidine. For antiemetics, we usually give aprepitant and palonosetron. The exact protocol we choose—including use of steroids—depends on patient history, goals, and tolerance of each infusion.
For example, if the patient is someone who struggled with chemotherapy and had a long history of complicated nausea, then we will use steroids with the first infusion. Otherwise, we see how patients do, and add steroids if necessary. SG can be pretty emetogenic, so probably 80% of our patients end up using steroids. Adding that it can really be beneficial.
Prevention and management of adverse events
The most immediate AEs that may be encountered with administration of SG are hypersensitivity and infusion-related reactions, which may cause a variety of signs and symptoms, such as wheezing, angioedema, hypotension, and others.16 Data suggest that 37% of patients may have hypersensitivity reactions to SG within 24 hours; however, these reactions are typically mild, with only 2% of patients having grade 3 or 4 reactions, 0.3% of patients having anaphylactic reactions, and 0.3% of patients having reactions that require permanent treatment discontinuation.16 To reduce the risk of hypersensitivity and infusion-related reactions, premedication is recommended, as well as post-administration monitoring for ≥30 minutes.16 Emergency equipment and necessary interventional medications should be on hand during administration.16 Patients with grade 4 reactions—which are considered life-threatening, such as anaphylaxis—should permanently discontinue treatment.9,16
The most common AEs seen in the ASCENT study requiring SG dose reductions were neutropenia and diarrhea, at rates of 11% and 5%, respectively; although some patients develop both, others experience just 1 of the 2.9 The SG package insert offers a stepwise approach to handling these events.9 For the first occurrence of severe neutropenia (a significant drop in white blood cells, increasing risk of infection), a 25% dose reduction is recommended plus administration of G-CSF.9 G-CSF, also known as filgrastim, was given to 44% of patients in the ASCENT trial; it combats neutropenia by causing the bone marrow to create more white blood cells.9,17 A second occurrence of severe neutropenia requires a 50% dose reduction of SG, and a third occurrence necessitates treatment discontinuation.9 SG should also be discontinued if grade 3 or 4 neutropenia requires a dose delay of ≥3 weeks to recover to grade ≤1 neutropenia.9 For severe non-neutropenic toxicity, a similar approach is followed, with a 25% dose reduction at first occurrence, a 50% dose reduction at second occurrence, and discontinuation if the toxicity persists, or if recovery to grade ≤1 toxicity takes ≥3 weeks.9
Patients with diarrhea should first be evaluated for infectious causes, and if none are present, loperamide should be started at 4 mg, with additional doses of 2 mg for further episodes, up to 16 mg daily.9 Supportive therapies, such as fluid and electrolyte substitution, should also be given as needed.9 In the case of an excessive cholinergic response, which may present as diarrhea plus salivation and abdominal cramping, an appropriate anticholinergic premedication, such as atropine, should be given at the next infusion.9
A list of the most common AEs seen in the phase 3 ASCENT study is provided in the Table. See the SG package insert for a full list of AEs and frequencies, as well as instructions for dose modification and AE intervention.
Commentary by Maria Shellock, BSN, RN: Reacting to a patient’s side effects and intervening with supportive medications really makes or breaks how they do. We’ve been very successful managing side effects with SG, and that has made a big difference in how patients feel. In contrast, with traditional chemotherapy we use everything we can but, ultimately, it’s still not great, and they’re still really struggling.
In the ASCENT trial, the main adverse event we encountered was neutropenia. With more experience, we’ve gotten very comfortable managing neutropenia using G-CSF. We give most patients long-acting G-CSF on day 9 of the treatment cycle, and if necessary, short-acting G-CSF on days 4, 5, and possibly 6, depending on their levels. If a patient has a borderline neutrophil level before their first infusion, then we will probably start G-CSF right away, otherwise we pay close attention and respond as needed. Ideally, we teach patients how to self-inject G-CSF at home, which alleviates some of the treatment burden.
Diarrhea is the other common side effect highlighted by the ASCENT data; however, in our experience, this has been easily manageable with over-the-counter loperamide. Some patients are also using ondansetron, which may help reduce rates of diarrhea. Most often, patients note loose stools for a couple of days after their infusions, but then it resolves.
I also think patients should know that they will lose their hair when they take SG. The ASCENT data showed an alopecia rate of 47%, but that’s artificially low—many of the patients had already lost their hair from chemotherapy before they started SG therapy. Mouth sores and fatigue are also somewhat common with SG, but these issues tend to be relatively mild and manageable compared with some conventional chemotherapies.
Access to care
A variety of support services are available for patients who need help accessing SG. For patients with commercial or private insurance, the Trodelvy™ Savings Program can help offset the cost of medication up to $25,000 per year.18 Underinsured or uninsured patients may qualify for the Gilead Patient Assistance Program, which may allow them to receive SG at no cost.18 For more information, visit trodelvy.com/patient/mTNBC/access-support.
Beyond these manufacturer-supported services, patients may benefit from third-party assistance organizations, which provide a variety of services, including counseling, case management, social and emotional support, education, and financial assistance. Organizations include CancerCare (cancercare.org), the Triple Negative Breast Cancer Foundation (tnbcfoundation.org), the Tigerlily Foundation (tigerlilyfoundation.org), the Ellie Fund (elliefund.org), and others.
Commentary by Maria Shellock, BSN, RN: Certain patients’ insurance plans make it challenging to treat them, but more often we find that patients need help overcoming other barriers to care. Many patients with TNBC are underserved, or they’re younger, or their lives are more complicated—they may have young children, and they’re also trying to work. These patients may need help getting to and from their treatments, or they may need financial assistance with bills or food because they miss work while they’re being treated. To meet these needs, the main support system we access is our social workers. They really have been hugely supportive for this population. Beyond social workers, the Ellie Fund has been hugely helpful to us at Mass General but, also, to breast cancer patients everywhere. The Ellie Fund offers support for patients with things like gas cards, food cards, and vouchers for other goods and services. We’re not always able to offset the actual treatment costs, but we try to help support the patients and offset other costs when we can.
Conclusion
Although conventional chemotherapy was once the mainstay treatment for patients with mTNBC, emerging therapies such as antibody–drug conjugates are offering more effective ways of managing this disease. One of these new medications, SG, is a first-in-class targeted agent that delivers cytotoxic therapy directly into cancer cells. Compared with conventional chemotherapy, this approach offers 3-fold longer PFS, almost twice the OS, and significantly better HRQOL outcomes, including improved global health status and extended time until QOL deterioration. As a second-line agent preferred by the NCCN, SG has solidified its place as a key part of the mTNBC treatment flow, and with optimal administration, is sure to deliver the same promising outcomes in clinical practice that have been reported in phase 3 clinical trials.
References
- Moss JL, Tatalovich Z, Zhu L, et al. Triple-negative breast cancer incidence in the United States: ecological correlations with area-level sociodemographics, healthcare, and health behaviors. Breast Cancer. 2021;28:82-91.
- American Cancer Society. Triple-negative breast cancer. Updated January 27, 2021. www.cancer.org/cancer/breast-cancer/about/types-of-breast-cancer/triple-negative.html. Accessed October 12, 2021.
- American Cancer Society. Survival rates for breast cancer. Updated January 27, 2021. www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html. Accessed October 12, 2021.
- Malhotra MK, Emens LA. The evolving management of metastatic triple negative breast cancer. Semin Oncol. 2020;47:229-237.
- US Food and Drug Administration. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. Updated April 7, 2021. www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-sacituzumab-govitecan-triple-negative-breast-cancer. Accessed October 8, 2021.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer. Version 8.2021. Updated September 13, 2021. www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed October 8, 2021.
- Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529-1541.
- Gilead Sciences, Inc. Trodelvy: how it works. Updated 2021. https://trodelvy.com/patient/mTNBC/How-it-works. Accessed October 12, 2021.
- Trodelvy (sacituzumab govitecan-hziy) [package insert]. Foster City, CA; Gilead Sciences, Inc; April 2021.
- Ocean AJ, Starodub AN, Bardia A, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: safety and pharmacokinetics. Cancer. 2017;123:3843-3854.
- Eastern Cooperative Oncology Group. ECOG performance status. https://ecog-acrin.org/resources/ecog-performance-status. Accessed October 25, 2021.
- Ocean AJ, Starodub AN, Bardia A, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: safety and pharmacokinetics [online supporting materials]. Cancer. 2017;123.
- Loibl S, Loirat D, Tolaney S, et al. Health-related quality of life (HRQoL) in the ASCENT study of sacituzumab govitecan (SG) in metastatic triple-negative breast cancer (mTNBC). Presented at: 2021 European Society for Medical Oncology Virtual Congress; September 16-21, 2021.
- Gilead Sciences, Inc. Trodelvy: dosing, reconstitution, and administration guide. Updated 2021. https://trodelvyhcp.com/pdf/Dosing-Reconstitution-Admin-Booklet.pdf. Accessed October 12, 2021.
- Rugo H, Tolaney SM, Loirat D, et al. Impact of UGT1A1 status on the safety profile of sacituzumab govitecan in the phase 3 ASCENT study in patients with metastatic triple‑negative breast cancer. Presented at: San Antonio Breast Cancer Symposium; December 8-11, 2020; San Antonio, TX.
- Gilead Sciences, Inc. Trodelvy clinical trial brochure. Updated 2021. https://trodelvyhcp.com/pdf/Clinical_Trial_Brochure_FINAL.pdf. Accessed October 12, 2021.
- National Cancer Institute. G-CSF: NCI dictionary of cancer terms. www.cancer.gov/publications/dictionaries/cancer-terms/def/g-csf. Accessed October 22, 2021.
- Gilead Sciences, Inc. Trodelvy access support. www.trodelvy.com/patient/mTNBC/access-support#trdAssist. Accessed October 12, 2021.