Introduction
For this supplement, expert faculty provided detailed perspectives based on their extensive experience in the management of non–small-cell lung cancer (NSCLC), focusing on the incorporation and considerations for biomarker testing in precision medicine.
The evolving landscape of actionable biomarkers in NSCLC1,2
Which biomarkers do you typically test for in your practice?
Dr. Socinski – We have a comprehensive 51-gene panel that covers all of the currently actionable molecular biomarkers: EGFR, ALK, ROS1, BRAF, NTRK, METex14, RET, KRAS G12C, and HER2. We also test for PD-L1 expression using immunohistochemistry.3,4,5
Ms. Welch – We try to get full next-generation sequencing (NGS) of hundreds of genes on all of our patients with metastatic lung cancer.3,4
Dr. Arcila – We have a comprehensive NGS panel that tests 505 genes. We also have rapid screening assays for common mutations in EGFR and KRAS and for fusions.3,4,5
Consider a broad-based biomarker testing approach in order to identify actionable as well as emerging driver mutations, which may open the path for more treatment plans6
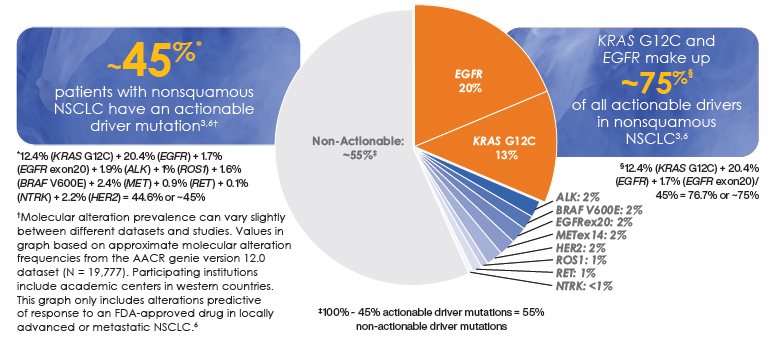
Prevalence of oncogenic drivers in NSCLC7
Dr. Arcila – We perform testing by normal">NGS at diagnosis and upon progression/relapse. Some normal">NGS assays may also be performed for monitoring of disease during the course of treatment.6
Ms. Welch – In my cancer center, comprehensive normal">NGS testing is done at diagnosis, sometimes with both plasma and tissue to expedite results. normal">NGS testing is repeated at the time of progression.8
Dr. Socinski – We typically do biomarker testing at lung cancer diagnosis but retesting at progression can also be therapeutically informative. By retesting, you may find a different actionable mutation that may help inform the patient’s treatment plan.8
It is advisable to test for actionable and emerging biomarkers in eligible patients with advanced NSCLC at diagnosis and during the course of the disease5,6,9
Data show that <50% of eligible patients with NSCLC receive biomarker testing
A retrospective, observational study conducted in the community setting revealed that among the 2257 eligible patients with metastatic NSCLC, 1724 (76.4%) had results available for at least one biomarker available at the time (ALK, EGFR, BRAF, ROS1, or PD-L1). Approximately half of 1724 had results available for at least 1 biomarker prior to first-line treatment.10
Guidelines recommend broad molecular testing for eligible patients with advanced NSCLC3
Adherence to testing for NCCN guideline-recommended biomarkers, regardless of therapy, decreased mortality risk by 11%11*
What has been the impact of integrating biomarker testing in routine clinical practice?
Ms. Welch – By routinely incorporating biomarker testing in appropriate patients, we get a better understanding of what may be driving these patients’ cancers.8
Dr. Arcila – Personalized treatment plans have contributed to an increased survival of patients with lung cancer in the past decade.11,12
NCCN, National Comprehensive Cancer Network.
*Study examined adult patients diagnosed with de novo mNSCLC between January 1, 2017 and September 30, 2019, with follow-up through December 31, 2019 using The US Oncology Network structured electronic health records data.10
Considerations for optimizing the biomarker testing journey in NSCLC
A. Broad molecular testing identifies actionable biomarkers in either a single assay or a combination of a few assays, and optimally also identifies emerging biomarkers3
What is the impact of broad-based multigene biomarker testing on clinical workflow compared with single-gene testing?
Dr. Socinski – Many of the biopsies in my lung cancer practice are technically difficult to obtain and you may not get bountiful tissue in the biopsy. Our patients are better served if we get all the information we need upfront with broad molecular testing and do not have to go back and subject the patient to a second biopsy using single-gene testing.6
Dr. Arcila – Next-generation sequencing technology enables comprehensive simultaneous screening for all required markers, decreasing overall cumulative costs, and patient sample requirements, compared to single-gene testing.6 The more comprehensive assays tend to be highly complex resulting in a longer turnaround time.6 An equally critical issue is that more comprehensive assays require a team of specialists to handle the breadth and depth of the biomarker information.6
Broad multigene testing can reduce the number of assays ordered and conserve tissue needed to assess all actionable biomarkers6
B. In reflex biomarker testing, the pathologist orders a group of preapproved biomarkers at the time of initial diagnosis14
- Considerations for implementing reflex testing protocols14
- Reduces the time from lung cancer diagnosis to delivery of all clinically actionable results
- Decreases turnaround time of molecular testing results
- Improves detection rate of targeted gene alterations
What impact does reflex testing have on patient care?
Dr. Socinski – At our center, the reflex biomarker testing occurs, for example, with the initial biopsy at the time of diagnosis. A benefit of reflex testing is that it allows the clock to start earlier and select the most appropriate treatment option in a timely manner.15
Ms. Welch – In my experience, one consideration from the medical oncology side with reflex testing is that by the time we meet the patient, we often already have the results, so we are able to make a treatment plan and thus avoid a delay in care.15
C. Rapid on-site evaluation (ROSE) is performed to increase tissue sample adequacy rate and diagnostic yield to help reduce rebiopsy rates. ROSE quickly guides appropriate sampling for molecular testing and provides a preliminary diagnosis to help direct immediate patient care16
The accuracy of ROSE is high and can be useful for obtaining instant diagnosis, contributing to a high success rate of molecular analysis17
What impact does ROSE have on clinical workflow efficiencies?
Dr. Arcila – Changes to our comprehensive quality assurance program in the way that we obtain, handle, and process the biopsies and the way that they are evaluated upfront increased our success rate of obtaining an adequate amount of tissue.17-20
Dr. Socinski – ROSE may improve workflow efficiency because the on-site pathologist can take a quick look and make sure that the clinician taking the biopsy (eg, the thoracic surgeon or interventional pulmonologist) has hit the target.16 This may increase efficiency, but it may also increase cost because not only do you have the person doing the biopsy, but additionally the pathologist and the related infrastructure necessary to perform ROSE.16
Ms. Welch – ROSE does add an additional layer of multidisciplinary coordination: it is necessary to have a cytopathologist or a similar clinician to evaluate the tissue.16 We have to coordinate these various roles at the same time that the interventional radiologists and surgeons are performing the biopsies.15
ROSE may help attain adequate tumor sample for molecular biomarker testing15
D. Liquid biopsy uses DNA shed from tumors into the circulation as a substrate for molecular biomarker testing.8,15 Although tumor tissue remains the gold standard for molecular analyses, liquid biopsy may be considered a key element in comprehensive testing when tissue-based testing is inadequate15
- Considerations for liquid biopsy
Pros - Liquid biopsy can be used when the tissue specimen is insufficient or of low quality for biomarker testing15
- Liquid biopsy is less invasive and shortens turnaround time18
- Liquid biopsy testing may be serially performed to follow treatment response and identify development of acquired resistance before observance of radiographic or clinical progression6
- Compared with tissue-based testing, liquid biopsy may better reflect the systemic tumor burden and intratumoral heterogeneity8
- Results based on liquid biopsy can complement tissue studies8
- Not all tumors shed sufficient DNA for detection15
- Negative test by liquid biopsy requires confirmation using tissue biopsy15,21
When might it be appropriate to consider liquid biopsy for biomarker testing, as well as its advantages and disadvantages?
Dr. Socinski – I consider obtaining a plasma based biopsy in addition to tissue biopsy almost every time I run biomarker tests, and I base that on guideline recommendations as well as evidence from a couple of studies.5 A study that looked into adding plasma based ctDNA NGS in addition to tissue NGS for detection of targetable mutations, has shown that concurrent testing led to an increase in detection rate of an actionable mutation (EGFR, ALL, MET, BRCA1, ROS1, RET, ERBB2, KRAS, and BRAF) from 20.5%-35.8% (n=229).22*
Ms. Welch – In my opinion, it is appropriate to consider liquid biopsy for biomarker testing; it has a much quicker turnaround time than tissue biopsy.8 Another advantage to liquid biopsy is that it may provide a broader reflection of the mutations that may be driving cancer growth.8 On the other hand, it is harder for liquid biopsies to pick up fusion mutations.8
Dr. Arcila – The liquid biopsy is minimally invasive and enables detection of genetic biomarkers when a biopsy is not possible. The main drawbacks of liquid biopsy testing are: It may be less sensitive compared to tissue particularly for tumors that have a low-shedding of circulating tumor DNA.8 This means a negative result should be considered a false-negative until proven otherwise, and it should be followed up with a tissue biopsy.22 And both malignant and premalignant conditions from hematopoietic cells may be detected in the liquid biopsy and may complicate interpretations.8
*In this single-center cohort study of 323 patients with non–small cell lung cancer, 229 had concurrent plasma and tissue next-generation sequencing or were unable to complete tissue testing. Plasma NGS was performed using a 73-gene commercial platform. Patients were enrolled at the Hospital of the University of Pennsylvania from April 1, 2016, through January 2, 2018.21
The IASLC consensus statement recommends concurrent use of liquid and tissue biopsy as this can increase detection of actionable and emerging biomarkers 8,21
E. Multidisciplinary team (MDT) collaboration may support patient care through early initiation of a treatment plan15
How has the collaboration between members of the MDT enhanced patient care?
Dr. Socinski – In 1995 I was hired at the University of North Carolina to start their MDT, which brought a team of a medical oncologist, the pulmonologist, thoracic surgeons, and radiation oncologists together with nurse navigation to start the multidisciplinary thoracic program. I can tell you I am such a better medical oncologist because of what I’ve learned from pulmonologists, surgeons, radiation oncologists, because they have a different perspective. I think at the end of the day, patients who go through a multidisciplinary approach have a more integrated and comprehensive management plan than when a multidisciplinary strategy is not implemented.15,23
Ms. Welch – In my practice, advanced practice providers are very involved in the biomarker testing process and in assisting with ordering the tests. Often, we take on the role of explaining to patients the importance of biomarker testing, the testing process, and the significance of the results.24
F. Standardized methods to document biomarker test results may facilitate future access as needed25,26
- Considerations for Consistent Reporting and Documenting
- Include all actionable mutations at the beginning of the report25
- Report all mutations at the variant level25
- Use uniform and unambiguous nomenclature to report variants (ie, KRAS G12C)25
- Retrieving Biomarker Results
- Store patients’ biomarker test reports in a reliable location in their electronic medical record (EMR), such as in your notes or their chart26
- Consider establishing the optimal location for test results with your multidisciplinary team for easy retrieval by providers, now and in the future26
What processes would you consider at your institution to document and integrate precision medicine information, for easy access of results at diagnosis and upon progression?
Ms. Welch – At our large community- based practice, we have a database platform that houses all of molecular testing results for our entire practice. This makes interrogating for specific mutations relatively easy to identify patients with specific genomic drivers.26 Next-generation sequencing reports are annotated in the electronic health record25 and molecular testing results are pulled forward into the patient’s notes. Many providers list NGS and PD-L1 results in the molecular profiling section of the Assessment/Plan. When a patient progresses, molecular testing is repeated and results are reviewed to help direct the next steps in patient care.8
Dr. Socinski – In the lung cancer program at Advent Health, we have many processes in place to help ensure no actionable biomarker is missed at disease progression.25 It starts with our navigators who keep a pretty close eye on our lung cancer patients. They send the pathology and molecular testing reports via e-mail to the oncologist. Secondly, we have a weekly thoracic tumor board and a biweekly molecular board where biomarker testing of our lung cancer patients is discussed.15,26 Lastly, operationally, the biomarker testing results are automatically uploaded into Epic, then into my inbox for review, and finally into the electronic medical record report.25 Details of the biomarker testing results are recorded in the pathology section of Epic for example: Molecular tests show KRAS G12C status, PD-L1 expression levels, etc.25 These multiple procedures work well at our cancer center to ensure that results are properly documented and easily accessible by all treating physicians on the case at different lines of therapy.26
Dr. Arcila – In our practice, our team developed and implemented a clinical variants results system. Following data analysis, variant results are stored in this system. A web user interface allows the users to access and interact with the content for review and generation of reports.26 The system also enables tracking of all biomarker test results from the time of initial diagnosis and across any other timepoint at which testing is done. Variants are annotated based on highly curated evidence, including the ranking for the level of evidence that a specific molecular alteration is predictive of drug response by FDA labeling and NCCN guidelines. Molecular testing reports are accessible through the EMR,25 along with all other pathology reports.
Consider reporting and documenting genomic test results in an easily retrievable format which may facilitate accessibility when necessary25,26
References
- US Food and Drug Administration. www.fda.gov. Accessed October 13, 2022.
- Cheng Y, Zhang T, Xu Q. MedComm. 2021;2:692-729.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Guideline Name V.2.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed June 13, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Owen DH, Singh N, Ismaila N, et al. J Clin Oncol. https://doi.org/10.1200/JCO.22.02124.
- Rolfo C, Mack PC, Scagliotti GV, et al. J Thorac Oncol. 2018;13:1248-1268.
- Pennell NA, Arcila ME, Gandara DR, West H. Am Soc Clin Oncol Educ Book. 2019;39:531-542.
- Data on file, Amgen [Analysis of AACR Genie v12.0].
- Rolfo C, Mack P, Scagliotti GV, et al. J Thorac Oncol. 2021;16:1647-1662.
- Lindeman NI, Cagle PT, Aisner DL, et al. J Mol Diagn. 2018;20:129-159.
- 10. Nadler ES, Vasudevan A, Davies K, Wang Y, Gupta R, Ogale S. J Clin Oncol. 2021;39:9079-9079.
- John A, Yang B, Shah R. Adv Ther. 2021;38:1552-1566.
- Chen R, Manochakian R, James L, et al. J Hemat Oncol. 2020;13:58.
- Matsuda H, Ogawa T, Sadatsuki T, et al. Respirat Investig. 2023;61:61-73.
- Anand K, Phung TL, Bernicker EH, et al. Clin Lung Cancer. 2020;21:437-442.
- Gregg JP, Li T, Yoneda KY. Transl Lung Cancer Res. 2019;8(3):286-301.
- Jain D, Allen TC, Aisner DL, et al. Arch Pathol Lab Med. 2018;142:253-262.
- Shikano K,Ishiwata T, Saegusa F, et al. J Thorac Dis. 2020;12:3057-3064.
- Arcila ME, Yang S-R, Momeni A, et al. JTO Clin Res Rep. 2020;1:1-13.
- Tian SK, Killian K, Rekhtman N, et al. Arch Pathol Lab Med. 2016;140:1200-1205.
- Turner SR, Buonocore D, Desmeules P, et al. Lung Cancer. 2018;119:85-90.
- Leighl NB, Page RD, Raymond VM, et al. Clin Cancer Res. 2019;25:4691-4700.
- Guibert N, Pradines A, Favre G, et al. Eur Respir Rev. 2020;29:190052.
- Kowalczyk A, Jassem J. Transl Lung Cancer Res. 2020;9:1690-1698.
- Martin JC. Clin J Oncol Nurs. 2020;24:648-656.
- Li MM, Datto M, Duncavage EJ, et al. J Mol Diagn. 2017;19:4-23.
- Kim ES, Roy UB, Ersek JL, et al. J Thorac Oncol. 2019;14:338-342.