Supported through funding from

Researchers and clinicians continue to make advances in the treatment of cancer. In 2021, despite the COVID-19 pandemic, there have been many exciting developments in biomarker testing and other diagnostics, treatment approaches and targets, and other aspects of cancer care.
Many annual congresses, such as the American Association for Cancer Research (AACR) Annual Meeting 2021 and the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, were conducted as virtual programs this year, enabling researchers to share information in hundreds of posters and presentations.
This supplement summarizes some of the most important data from these meetings that may affect the management of patients with early-stage and metastatic non–small-cell lung cancer (NSCLC). This material is divided into the following topic areas:
- Immunotherapy
- Targeted therapy
- Combination therapies
- Early data
- Novel agents
- Diagnostic testing
- Healthcare disparities
A total of 30 data presentations from AACR 2021 and ASCO 2021 are summarized in these categories. We hope that the information reviewed here can be applied to clinical practice and offers valuable insights into the important progress that is being made in the care of patients with NSCLC.
Immunotherapy
Two-Year Update from CheckMate-9LA Study of Nivolumab plus Ipilimumab and Chemotherapy in First-Line Advanced NSCLC
After 2 years, combination use of nivolumab and ipilimumab together with chemotherapy demonstrated durable survival and other efficacy benefits relative to chemotherapy alone in first-line patients with advanced NSCLC.
In the randomized phase 3 CheckMate-9LA trial, combination use of nivolumab (NIVO) and ipilimumab (IPI) plus 2 cycles of chemotherapy significantly improved overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) compared with chemotherapy alone (4 cycles) as first-line therapy for patients with advanced NSCLC. Clinical benefit was observed regardless of PD-L1 expression level and histology. At the 2021 ASCO Annual Meeting, researchers reported efficacy data with at least 2 years’ follow-up from the CheckMate-9LA study.1
Adult patients with stage IV recurrent NSCLC, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and no known sensitizing EGFR or ALK alterations were stratified by PD-L1 score (<1% vs ≥1%), sex, and histology (squamous vs nonsquamous). They were randomized 1:1 to NIVO 360 mg every 3 weeks plus IPI 1 mg/kg every 6 weeks plus chemotherapy (2 cycles; n = 361) or chemotherapy alone (4 cycles; n = 358).1 Patients with nonsquamous NSCLC in the chemotherapy-alone arm could receive pemetrexed maintenance. The primary end point was OS. Secondary end points included PFS and ORR by blinded independent central review (BICR), and efficacy by different PD-L1 levels. Safety was exploratory.1
After minimum follow-up of 24.4 months for OS (database lock, February 18, 2021), patients treated with NIVO + IPI + chemotherapy continued to derive OS benefit compared with chemotherapy.1 The median OS for each arm was 15.8 months versus 11.0 months, respectively (hazard ratio [HR], 0.72; 95% confidence interval [CI], 0.61-0.86).1 Two-year OS rates were 38% versus 26%, respectively.1 (See Figure 1.)
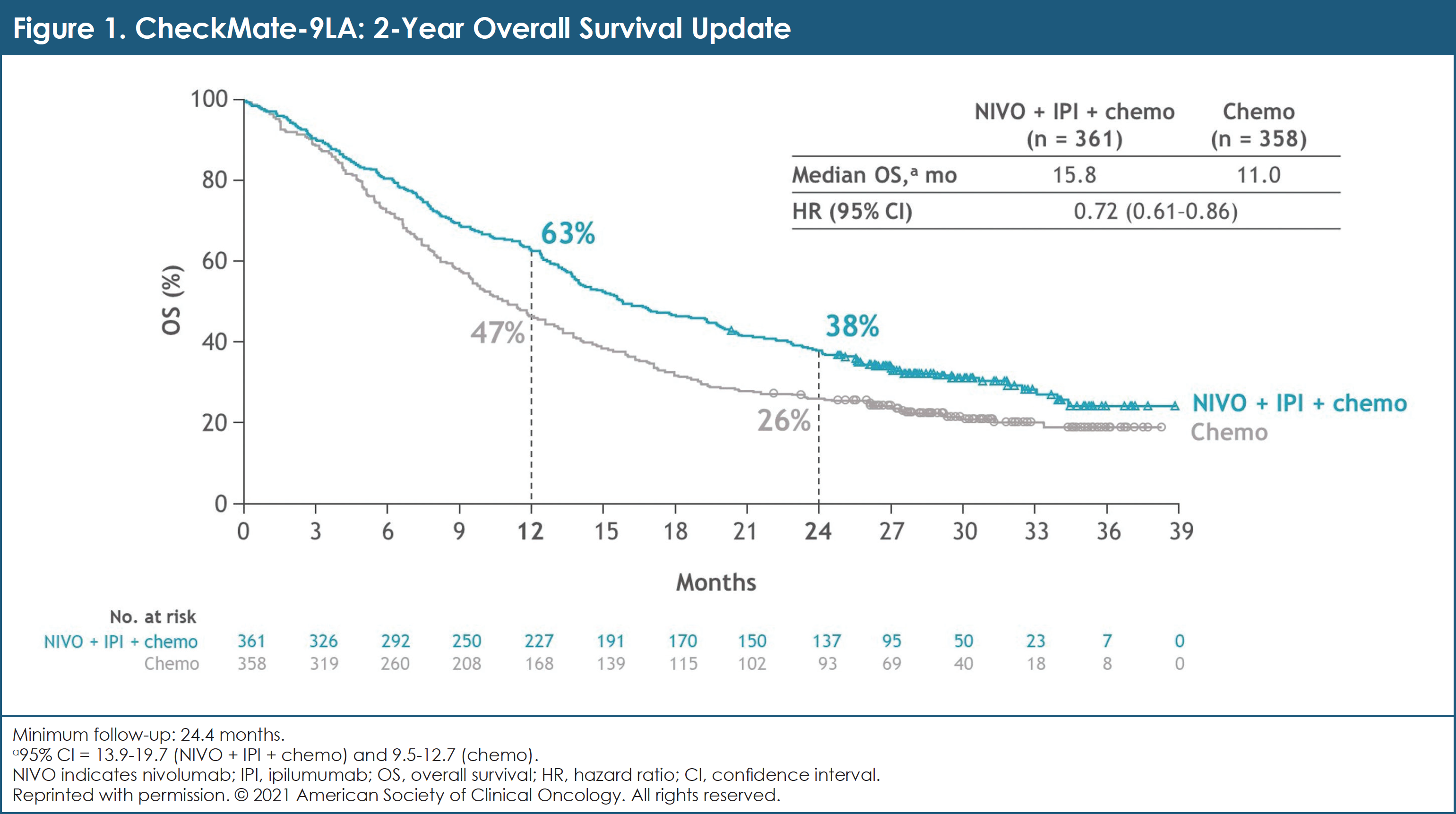
Median PFS with NIVO + IPI + chemotherapy versus chemotherapy was 6.7 months compared with 5.3 months for chemotherapy alone (HR, 0.67; 95% CI, 0.56-0.79); 8% and 37% of patients who had disease progression received subsequent immunotherapy, respectively.1 ORR was 38% with NIVO + IPI + chemotherapy compared with 25% with chemotherapy.1 Similar clinical benefit with NIVO + IPI + chemotherapy versus chemotherapy alone was observed in all randomized patients and across the majority of subgroups, including by PD-L1 expression level and histology.1
Any-grade and grade 3/4 treatment-related adverse events (AEs) were reported in 92% and 48% of patients in the NIVO + IPI + chemotherapy arm, respectively, compared with 88% and 38% in the chemotherapy arm.1
Researchers concluded that after 2 years’ minimum follow-up, first-line NIVO + IPI + chemotherapy demonstrated durable survival and other efficacy benefits relative to chemotherapy alone in patients with advanced NSCLC. No new safety signals were identified.1
Four-Year Update from CheckMate-227 Study of Nivolumab plus Ipilimumab in First-Line Advanced NSCLC
After 4 years, combination use of nivolumab and ipilimumab continued to provide a durable survival benefit compared with chemotherapy in patients with advanced NSCLC.
The combination of nivolumab (NIVO) and ipilimumab (IPI) was shown to provide durable long-term OS benefit compared with chemotherapy regardless of tumor PD-L1 expression in patients with advanced NSCLC in CheckMate-227 Part 1. In this study, the 3-year OS rates were 33% compared with 22% in patients with PD-L1 expression levels of ≥1% (HR, 0.79; 95% CI, 0.67-0.93).2 Three-year OS rates were 34% compared with 15% in patients with PD-L1 expression levels <1% (HR, 0.64; 95% CI, 0.51-0.81).2 At the 2021 ASCO Annual Meeting, researchers reported updated results from the CheckMate-227 study with 4 years’ minimum follow-up.2
Adults with previously untreated stage IV or recurrent NSCLC, no known EGFR or ALK alterations, and ECOG performance status of 0 or 1 were enrolled in CheckMate-227. Patients were stratified by histology: squamous and nonsquamous. A total of 1189 patients with PD-L1 ≥1% were randomized to receive NIVO at a dose of 3 mg/kg every 2 weeks plus IPI at a dose of 1 mg/kg every 6 weeks, NIVO alone (240 mg every 2 weeks), or chemotherapy.2 Another 550 patients with PD-L1 <1% were randomized to receive NIVO + IPI, NIVO (360 mg every 3 weeks) + chemotherapy, or chemotherapy alone.2 The primary end point was OS with NIVO + IPI compared with chemotherapy in patients with PD-L1 ≥1%.2
After minimum follow-up of 49.4 months (database lock, February 18, 2021), patients were at least 2 years beyond the protocol-specified end of immunotherapy treatment.2 Patients with PD-L1 ≥1% continued to show a durable OS benefit with NIVO + IPI compared with chemotherapy (HR, 0.76; 95% CI, 0.65-0.90).2 Four-year OS rates were 29% (NIVO + IPI), 21% (NIVO), and 18% (chemotherapy).2 At 4 years, 14% (NIVO + IPI), 10% (NIVO), and 4% (chemotherapy) remained progression-free.2 Among responders, 34%, 30%, and 7% remained in response, respectively.2
In an exploratory analysis in patients with PD-L1 ≥50%, 4-year OS rates were 37% (NIVO + IPI), 26% (NIVO), and 20% (chemotherapy).2 In patients with PD-L1 <1%, OS HR for NIVO + IPI versus chemotherapy was 0.64 (95% CI, 0.51-0.81) and 4-year OS rates were 24% (NIVO + IPI), 13% (NIVO + chemotherapy), and 10% (chemotherapy).2 At 4 years, 12% (NIVO + IPI), 7% (NIVO + chemotherapy), and 0% (chemotherapy) remained progression-free.2 Among responders, 31%, 13%, and 0% remained in response, respectively.2
Among patients who progressed on NIVO + IPI versus chemotherapy, 7% versus 40% (PD-L1 ≥1%), and 9% versus 33% (PD-L1 <1%), received subsequent immunotherapy.2 Benefit with NIVO + IPI compared with chemotherapy was observed regardless of NSCLC histology.2 With long-term follow-up, no new safety signals were identified.2
Researchers concluded that after 4 years’ minimum follow-up, first-line NIVO + IPI continued to provide durable, long-term OS benefit compared with chemotherapy in patients with advanced NSCLC regardless of PD-L1 expression or histology.2
Pooled Analyses of Immune-Related Adverse Events and Efficacy from IMpower130, IMpower132, and IMpower150
Patients who received atezolizumab and who experienced immune-related adverse events had longer OS compared with patients who did not experience immune-related adverse events in the IMpower150 study.
Inhibitors of PD-L1 and PD-1 have transformed the treatment of advanced NSCLC. Evidence suggests that the occurrence of immune-related AEs (irAEs) with these agents may predict improved outcomes in cancers, including NSCLC. Atezolizumab, an anti–PD-L1 agent, has shown efficacy and tolerability in NSCLC and is currently approved in first- and later-line settings. The phase 3 IMpower130, IMpower132, and IMpower150 trials evaluated atezolizumab + chemotherapy ± bevacizumab as first-line therapy for patients with NSCLC. At the 2021 ASCO Annual Meeting, researchers evaluated the nature and magnitude of the association between irAEs and efficacy in these trials.3
Each of the 3 trials enrolled treatment-naïve patients with nonsquamous stage IV NSCLC. Patients were randomized to carboplatin + nab-paclitaxel alone or with atezolizumab in IMpower130; carboplatin or cisplatin alone or with atezolizumab in IMpower132; atezolizumab + bevacizumab + carboplatin + paclitaxel, atezolizumab + bevacizumab + paclitaxel, or bevacizumab + carboplatin + paclitaxel in IMpower150. Data were pooled (data cutoffs: March 15, 2018 [IMpower130]; May 22, 2018 [IMpower132]; September 13, 2019 [IMpower150]) and analyzed by treatment (atezolizumab-containing vs control) and irAE status. A time-dependent Cox model and landmark analyses at 1, 3, 6, and 12 months were used to control for immortal bias. Study protocols required atezolizumab treatment interruption or discontinuation for grade ≥3 irAEs.3
A total of 2503 patients were included in the analysis: 1577 who received atezolizumab and 926 in the control group.3 In both arms, baseline characteristics were generally balanced between patients with irAEs (atezolizumab, n = 753; control, n = 289) and without irAEs (atezolizumab, n = 824; control, n = 637).3 Any-grade irAEs occurred in 48% of atezolizumab patients and 32% of patients in the control group; grade 3 to 5 irAEs occurred in 11% of atezolizumab patients and in 5% of patients in the control group.3
The most common irAEs with atezolizumab compared with control were rash (28% vs 18%), hepatitis (lab abnormalities; 15% vs 10%), and hypothyroidism (12% vs 4%).3 Median time to onset of first irAE was 1.7 months (atezolizumab) versus 1.4 months (control).3 Hazard ratios for OS from the time-dependent Cox model between patients with or without irAEs were 0.69 (95% CI, 0.60-0.78) in the atezolizumab arm and 0.82 (95% CI, 0.68-0.99) in the control arm.3 Excluding rash (perceived as the least specific irAE), OS HRs were 0.75 (95% CI, 0.65-0.87) and 0.90 (95% CI, 0.71-1.12), respectively.3
In this exploratory pooled analysis, patients with irAEs had longer OS compared with patients who did not experience irAEs in the atezolizumab-containing and control arms per the time-dependent Cox model and landmark analyses (for patients receiving atezolizumab who experienced irAEs, median OS was 25.7 months; for patients receiving atezolizumab who did not experience irAEs, median OS was 13.0 months).3 This trend remained for patients in the atezolizumab arm after excluding rash from the list of irAEs.3 Landmark analyses show that, in the atezolizumab arm, patients with grade 1/2 irAEs had longer OS compared with patients with grade ≥3 irAEs, potentially due to treatment interruption and/or discontinuation.3
These data suggest an association between irAEs and efficacy in patients with NSCLC and further support treatment with atezolizumab combined with chemotherapy, with or without bevacizumab, in the first-line setting.3
Atezolizumab versus Best Supportive Care After Adjuvant Chemotherapy for Resected NSCLC (IMpower010)
In patients with resected stage II and stage IIIA NSCLC, adjuvant use of atezolizumab after adjuvant chemotherapy offered superior disease-free survival compared with best supportive care after adjuvant chemotherapy.
In patients with fully resected, high-risk, early-stage NSCLC, adjuvant platinum-based chemotherapy provides only a modest 5-year survival benefit. At the 2021 ASCO Annual Meeting, researchers reported disease-free survival (DFS) results from the preplanned interim analysis of IMpower010, a randomized, phase 3, open-label trial of adjuvant atezolizumab (anti–PD-L1) compared with best supportive care (BSC) after adjuvant chemotherapy in patients with early-stage resected NSCLC.4
Patients who were eligible for enrollment in IMpower010 had completely resected (4-12 weeks prior to enrollment) stage IB (≥4 cm) to IIIA NSCLC (based on American Joint Committee on Cancer/International Union for Cancer Control, 7th edition) and ECOG performance status 0 or 1. Of the 1280 patients who were enrolled, 1269 patients received up to four 21-day cycles of cisplatin-based chemotherapy plus pemetrexed, docetaxel, gemcitabine, or vinorelbine. Of these 1269 patients, 1005 were subsequently randomized to either 16 cycles of atezolizumab 1200 mg every 3 weeks or BSC.4
The primary end point of IMpower010 was investigator-assessed DFS, while a key secondary end point was OS. Efficacy assessments were based on randomized patients. Safety was assessed in the safety-evaluable population, defined as patients who received at least 1 dose of atezolizumab or who had at least 1 post-baseline safety assessment if randomized to the BSC arm.4
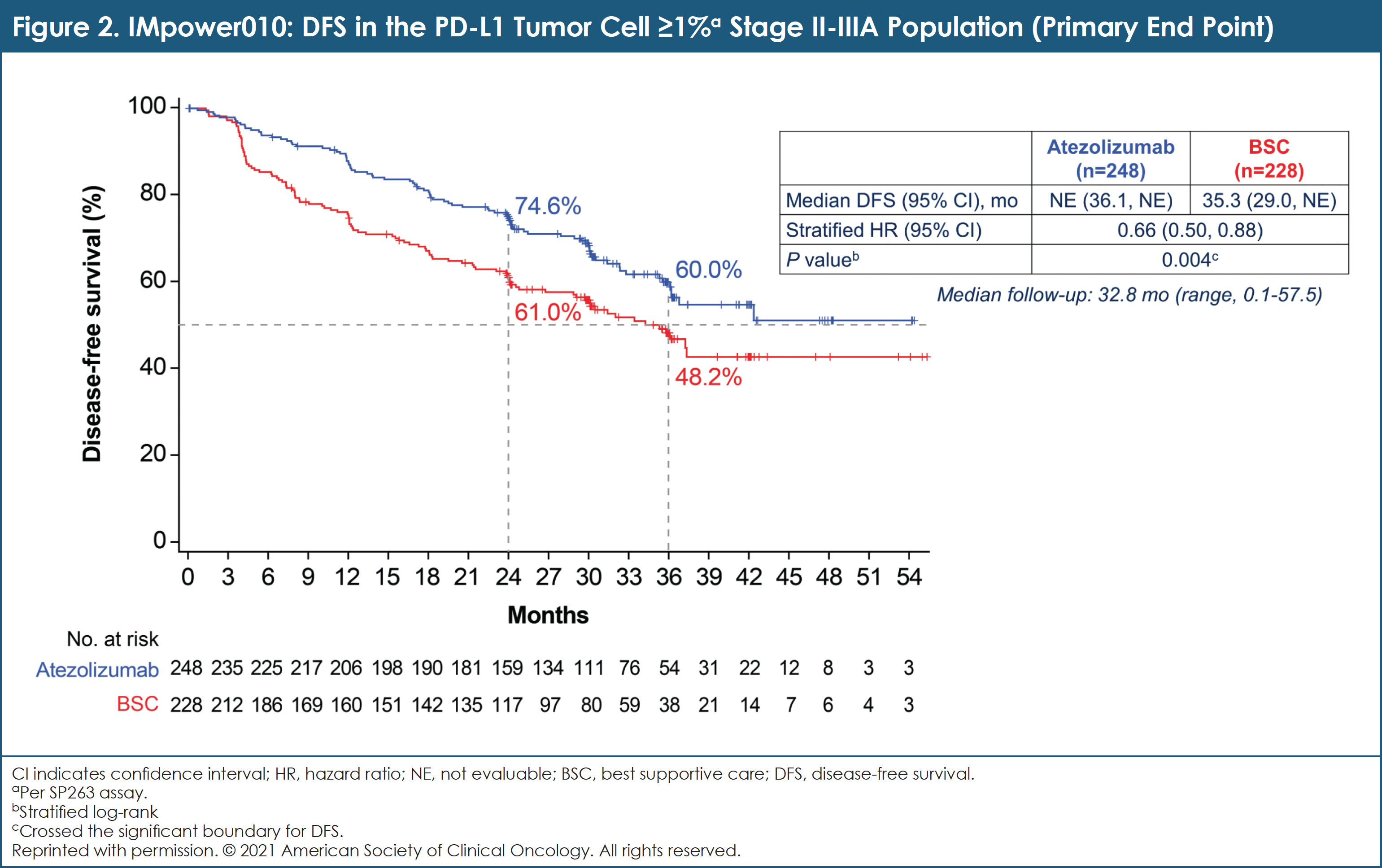
At data cutoff on January 21, 2021, median follow-up was 32.8 months in the intent-to-treat (ITT) population.4 Baseline characteristics were generally balanced between arms.4 Atezolizumab showed statistically significant DFS benefit compared with BSC in the PD-L1 tumor cell ≥1% stage II to IIIA group and in all randomized stage II to IIIA patients; median DFS was not reached for atezolizumab compared with 35.3 months for BSC (HR, 0.66; 95% CI, 0.50-0.88; P = .004).4 (See Figure 2.) At this interim analysis, the significance boundary was not crossed for DFS in the ITT population; testing will continue to the final DFS analysis.4 OS data were immature and not formally tested.4
Patients in the atezolizumab arm received a median of 16 doses (range, 1-16) of atezolizumab.4 Any-grade AEs occurred in 93% (atezolizumab) and 71% (BSC) of patients.4 Events were grade 3/4 in 22% and 12%, respectively.4 Grade 5 treatment-related AEs (TRAEs) occurred in 0.8% of patients in the atezolizumab arm.4 Adverse events leading to treatment discontinuation occurred in 18% of atezolizumab-treated patients.4
Researchers concluded that IMpower010 met its primary end point: adjuvant atezolizumab showed a DFS benefit compared with BSC after adjuvant chemotherapy in patients with resected stage II/IIIA NSCLC. The benefit was particularly pronounced in the PD-L1 tumor cell ≥1% subgroup. Toxicities seen with atezolizumab were consistent with prior experience of atezolizumab monotherapy across indications and lines of therapy.4
Neoadjuvant and Adjuvant Atezolizumab and Chemoradiation for Stage III NSCLC (AFT-16)
Atezolizumab given prior to and following chemoradiation for stage III unresectable NSCLC was well-tolerated and offered encouraging efficacy outcomes.
In the United States, a minority of the approximately 40,000 patients diagnosed annually with stage III NSCLC can be cured by concurrent chemoradiation therapy (CRT). Standard adjuvant immune checkpoint inhibitors (ICIs) improve outcomes for those patients who complete CRT with good performance status and without disease progression, but most patients diagnosed with unresectable stage III NSCLC will not meet the criteria for adjuvant ICIs. The phase 2 AFT-16 study investigated the safety and efficacy of neoadjuvant and adjuvant atezolizumab as a strategy that may allow more patients with stage III NSCLC to benefit from ICIs.5
Eligible patients received 4 cycles of atezolizumab 1200 mg intravenously every 21 days followed by CRT with 60 Gy plus weekly carboplatin and paclitaxel (chemotherapy), chemotherapy consolidation, and adjuvant atezolizumab to complete 1 year of therapy (17 cycles). The primary end point of disease control rate (DCR) at 12 weeks has been reported elsewhere. At the 2021 ASCO Annual Meeting, secondary end points were reported, including overall response rate, safety, and PFS and OS measured from the start of induction therapy.5
From November 2017 to July 2019, a total of 64 patients with unresectable stage III NSCLC, performance status of 0 or 1, and no active autoimmune disease or significant organ dysfunction were enrolled at 13 Alliance for Clinical Trials in Oncology sites.5 Of these, 62 patients received at least 1 dose of atezolizumab and were included in this analysis.5 Their median age was 64 years (range, 38-87); approximately half were female (52%), most were white (77%), and most were current or former smokers (89%).5 Slightly more than half had performance status of 0 (57%).5
All patients are now off study treatment.5 The mean number of cycles of treatment received was 9 (range, 1-17).5 Forty-six patients were alive at a median follow-up of 24 months (range, 3-34).5 The rates of PFS at 12 and 18 months from start of induction atezolizumab were 66% (95% CI, 55%-79%) and 57% (95% CI, 45%-71%), respectively.5 Median PFS was 23.7 months (95% CI, 13.2-not evaluable).5 OS at 18 months was 84% (95% CI, 75%-94%). Median OS is not yet estimable.5
Atezolizumab was well-tolerated.5 One report of grade 4 Guillain-Barre syndrome and 1 report each of grade 3 pneumonia, pneumonitis, and colitis were attributable to neoadjuvant atezolizumab.5 The remaining 9 severe AEs were deemed unrelated to ICIs.5
Researchers concluded that treatment with atezolizumab prior to and following CRT for stage III unresectable NSCLC was well-tolerated and offered encouraging PFS and OS without unexpected safety signals. They suggest that further study of induction atezolizumab in patients with unresectable stage III NSCLC is warranted.5
Phase 3 Study of Tislelizumab Compared with Docetaxel in Advanced NSCLC (RATIONALE 303)
Tislelizumab given as second- or third-line therapy in patients with advanced NSCLC demonstrates superior efficacy compared with docetaxel regardless of histology or PD-L1 expression level.
In patients with advanced NSCLC who progressed after treatment with platinum-based chemotherapy regimens, anti–PD-1 and anti–PD-L1 therapies have been shown to improve OS by 2 to 4 months compared with docetaxel. Tislelizumab is an anti–PD-1 antibody engineered to minimize FcγR binding on macrophages, which is a mechanism of T-cell clearance and potential anti–PD-1 resistance.6
The phase 3 RATIONALE 303 study compared the efficacy and safety of tislelizumab with docetaxel as second- or third-line therapy for patients with advanced NSCLC. Patients without oncogenic driver mutations who failed at least 1 prior systemic therapy including a platinum regimen were randomized 2:1 to receive intravenous (IV) tislelizumab 200 mg every 3 weeks (arm A) or IV docetaxel 75 mg/m2 every 3 weeks (arm B).6
The primary end points were OS in the ITT analysis set and OS in the PD-L1 high (≥25% in tumor cells) analysis set. A prespecified interim analysis was conducted after approximately 426 deaths or 76% of planned events. In the interim analysis, formal OS superiority testing was conducted only in the ITT population.6
A total of 805 patients were randomized in the RATIONALE 303 study, 535 to tislelizumab and 270 to docetaxel.6 Patient demographics were generally balanced between arms.6 After 19 months of median follow-up or 441 OS events in the ITT population, median OS was significantly longer for tislelizumab compared with docetaxel: 17.2 months compared with 11.9 months (HR, 0.64; 95% CI, 0.53-0.78; P <.0001).6
The OS benefit seen with tislelizumab was also observed in the PD-L1 high analysis group: 19.1 months versus 11.9 months (HR, 0.52; 95% CI, 0.38-0.71) and across most subgroups including histology.6 Progression-free survival, overall response rate, and duration of response (DOR) were also improved in the tislelizumab arm compared with the docetaxel arm.6
The most commonly reported AEs were anemia and increased alanine aminotransferase for tislelizumab, and alopecia and neutropenia for docetaxel.6 Pneumonia and neutropenia were the most common grade ≥3 AEs in the tislelizumab and docetaxel arms, respectively.6 Treatment-related AEs leading to death were observed in 1.5% of tislelizumab patients and 1.6% of docetaxel recipients.6
Researchers concluded that the RATIONALE 303 study demonstrated that tislelizumab given as second- or third-line therapy in patients with advanced NSCLC prolonged OS, improved PFS, and increased ORR compared with docetaxel regardless of NSCLC histology or PD-L1 expression level. The safety profile of tislelizumab was deemed tolerable and manageable.6
Randomized Phase 3 Study of Sintilimab versus Docetaxel in Advanced Squamous NSCLC (ORIENT-3)
Compared with docetaxel used as second-line treatment for advanced and metastatic squamous NSCLC, sintilimab offers superior OS and PFS benefits.
Patients with advanced squamous NSCLC have few treatment options after failure of a platinum-based doublet chemotherapy regimen. ORIENT-3, a randomized phase 3 study, was conducted to compare the efficacy and safety of sintilimab, an anti–PD-1 antibody, with docetaxel in the second-line treatment of patients with squamous NSCLC.7
Patients with squamous NSCLC (stage IIIB, stage IIIC, or stage IV and ineligible for radical chemo-radiotherapy) who experienced disease progression during or after first-line platinum-based chemotherapy were enrolled in ORIENT-3. They were randomized 1:1 to receive IV sintilimab 200 mg or docetaxel 75 mg/m2 every 3 weeks until disease progression or intolerable toxicity. Patient stratification was based on ECOG performance status score (0 vs 1). The primary end point of ORIENT-3 was OS.7
As of July 31, 2020, 290 patients were enrolled in the study, with 145 patients in each treatment group. The efficacy analysis was based on the final analysis set of 280 patients.7 The safety analysis was based on the safety set of 274 patients.7
Baseline characteristics were well-balanced between the 2 treatment groups. Most patients in the efficacy analysis had an ECOG score of 1: 76% in the sintilimab arm, 77% in the docetaxel arm.7 Patients received a median of 8 cycles (range, 1-45) of sintilimab, and a median of 2 cycles of docetaxel (range, 1-15).7
After a median follow-up of 23.6 months, sintilimab significantly improved OS versus docetaxel: median 11.8 months (95% CI, 10.3-15.6) versus 8.3 months (95% CI, 6.5-9.8) (HR, 0.74; 95% CI, 0.56-0.96; P = .025).7 The median PFS was also significantly superior in the sintilimab arm compared with the docetaxel arm: 4.3 months (95% CI, 4.0-5.8) versus 2.8 months (95% CI, 1.9-3.2) (HR, 0.52; 95% CI, 0.39-0.68; P <.00001).7 The confirmed ORR was 25.5% (95% CI, 18.6%-33.4%) in the sintilimab arm and 2.2% (95% CI, 0.5%-6.4%) in the docetaxel arm.7
Treatment-related AEs were reported in 85% of patients in the sintilimab arm and in 83% of patients in the docetaxel arm.7 The most common TRAEs were hypothyroidism (18%) and alopecia (35%) for sintilimab and docetaxel, respectively.7 Severe TRAEs (grade ≥3) were less frequent in the sintilimab arm (18%) than in the docetaxel arm (36%).7 Five patient deaths were related to sintilimab and 1 death was reported as related to docetaxel.7
Researchers concluded that sintilimab used as second-line treatment for advanced and metastatic squamous NSCLC offers superior OS and PFS benefits compared with docetaxel.7
Targeted Therapy
Acquired Resistance Mutations After Treatment of EGFR-Mutated Metastatic NSCLC with Osimertinib plus Savolitinib (TATTON)
Approximately half of patients who progressed on osimertinib combined with savolitinib had an identifiable acquired resistance mechanism, most often mediated by MET, EGFR, or KRAS.
Up to 22% of patients with EGFR mutation–positive NSCLC after progression on osimertinib have a MET amplification as a resistance mechanism.8 In the expansion cohorts (parts B and D) of the phase 1b TATTON study, patients with MET-amplified EGFR-mutated advanced NSCLC who progressed on a prior EGFR tyrosine kinase inhibitor (TKI) received osimertinib (80 mg) plus savolitinib (300 mg or 600 mg once daily). Osimertinib is a third-generation, irreversible, oral EGFR TKI. Savolitinib is an oral highly selective MET TKI.8
The part B group of TATTON was split into 3 cohorts by prior therapy and tumor T790M status. Patients in part D had not received a third-generation EGFR TKI and were T790M negative. For patients who eventually develop resistance to the osimertinib plus savolitinib combination, it is not known which driver mutation(s) mediate this resistance.8
In the analysis presented at the 2021 AACR Annual Meeting, researchers assessed paired plasma samples (collected at baseline) upon disease progression and/or treatment discontinuation up to a data cutoff date (March 4, 2020). Next-generation sequencing (NGS) using either Guardant Health (Guardant360 73-gene panel) or Omni 500-gene panel was used to analyze the plasma ctDNA samples. All 73 genes on the Guardant360 panel were included in the Omni 500-gene panel. Analyses from each patient were reported only for genes included across the panels used. Genomic alterations were identified using Guardant Health’s pipeline, which included mutations and amplifications of EGFR and MET.8
Disease progression was assessed by the investigator, according to RECIST version 1.1. Assessments were completed for patients with PFS of >2 months.8
Of 180 patients who received treatment with osimertinib plus savolitinib, 70 provided plasma samples at baseline and at progression or discontinuation.8 Of these, 45 patients were used for the analysis: 18 of 70 were not evaluable for ctDNA detection and 7 of 70 had a PFS of ≤2 months.8
Among evaluable samples, the following acquired mutations were recorded with exclusivity between genes in most patients: MET D1228X, Y1230X, L1212X – 20% (9 of 45); EGFR C797X – 16% (7 of 45); KRAS G12X, G13X – 11% (5 of 45); PIK3CA E545K – 4% (2 of 45).8
Seven of 9 patients who developed MET-based resistance developed more than 1 MET mutation, suggesting polyclonal resistance.8 Across both parts B and D, the resistance profiles appeared similar by prior EGFR-TKI status and by savolitinib dose.8
In this analysis, approximately half of all evaluable patients had an identifiable acquired resistance mechanism.8 Resistance to osimertinib plus savolitinib appeared to be predominantly mediated by MET, EGFR, or KRAS.8 Co-occurring mutations across multiple genes were rare.8 However, multiple acquired mutations were often detected in a specific gene, particularly MET, which suggests to researchers that individual tumors showed inherent resistance dependencies.8
Phase 2 Study of Osimertinib + Oleclumab in Progressive T790M-Negative EGFR-Mutated NSCLC
The combination of oleclumab, a CD73 inhibitor, and osimertinib was well-tolerated at the recommended phase 2 dose.
Patients with EGFR-mutated T790M-negative NSCLC who experience progressive disease after treatment with an EGFR TKI have few therapy options. CD73, which may promote immune evasion, is overexpressed in EGFR-mutated NSCLC, suggesting the potential for combining CD73 blockers with EGFR TKIs. Oleclumab (MEDI9447) disrupts immune evasion by binding to CD73. To evaluate the safety and efficacy of oleclumab plus osimertinib in locally advanced/metastatic EGFR-mutated NSCLC, a phase 1b/2, multicenter, dose-escalation, dose-expansion study was conducted.9
Patients who enrolled in this clinical trial had histologically or cytologically confirmed T790M-negative EGFR-mutated NSCLC by local testing, a TKI-sensitive EGFR mutation (ie, exon 19 del, L858R), ECOG performance status score of 0 or 1, disease progression on or after a first- or second-generation TKI, and no prior osimertinib. Intravenous oleclumab 1500 mg (dose level 1) or 3000 mg (recommended phase 2 dose) every 2 weeks was combined with daily oral osimertinib 80 mg until disease progression or intolerable AEs.9
The primary study end points included AEs, serious AEs, and overall response rate by RECIST version 1.1. Dose-limiting toxicities were predefined and reviewed by a dose-escalation committee. Secondary end points included DOR, DCR, PFS, and OS.9
As of November 9, 2020, 5 patients received oleclumab at dose level 1 and 21 patients received the recommended phase 2 dose.9 Minimum patient follow-up was 44 weeks.9 For the 5 patients receiving dose level 1, the safety profile and response rate were generally similar to the recommended phase 2 dose. No dose-limiting toxicities (DLTs) occurred.9
All 21 patients who received the recommended phase 2 dose of oleclumab had at least 1 treatment-emergent AEs, with 38% experiencing severe (grade 3/4) treatment-emergent AEs.9 Treatment-related AEs occurred in 81% (19% grade 3/4); none was deemed serious, and there were no patient deaths.9 The most common TRAEs were rash (33%), stomatitis (29%), diarrhea (24%), and paronychia (24%).9
At data cutoff, 38% of patients remained on treatment; 48% discontinued due to progressive disease, with death, patient decision, and AE (pneumonitis) as other causes.9 The ORR for patients receiving the recommended phase 2 dose of oleclumab plus osimertinib was 19% (all partial responses), while median PFS was 11 months (95% CI, 3.7 months-not evaluable).9 The DCR at the recommended phase 2 dose was 81%.9
Researchers concluded that the combination of oleclumab, a CD73 inhibitor, and osimertinib was well-tolerated at the recommended phase 2 dose. Compared with T790M-negative EGFR-mutated NSCLC patients in a prior study of osimertinib monotherapy, the ORR was similar while median PFS was longer (11 months [oleclumab + osimertinib] vs 2.8 months [osimertinib monotherapy]).9
Phase 2 Study of D-0316 in Patients with Advanced NSCLC and EGFR T790M Mutation
D-0316, a third-generation EGFR inhibitor, shows antitumor activity and an acceptable toxicity profile in patients with EGFR T790M–positive NSCLC who progressed.
Although they may have an initial response to treatment with an EGFR TKI, most patients develop resistance. The EGFR T790M mutation is detectable in approximately 50% of patients treated with first- and second-generation EGFR TKIs. D-0316 is a third-generation EGFR TKI that is selective for both EGFR TKI sensitizing and T790M resistance mutations in patients with NSCLC. At the 2021 AACR Annual Meeting, researchers reported results from a single-arm phase 2 study of D-0316 in NSCLC patients with EGFR T790M who progressed on previous treatment with first-line EGFR TKIs.10
In this phase 2, open-label, single-arm study, eligible patients had confirmed locally advanced or metastatic NSCLC with disease progression after first-line EGFR TKI and with a T790M mutation. Patients were initially treated with oral D-0316 50 mg. However, upon considering the benefits and risks, the dose of D-0316 was modified to 100 mg once daily with a 21-day lead-in at 75 mg once daily. The primary end point was ORR based on independent review committee (IRC) according to RECIST version 1.1.10
As of October 31, 2019, 176 patients were enrolled in the 50-mg phase of the D-0316 trial.10 In this phase, 90 patients experienced a partial response, achieving an ORR of 51% (95% CI, 43.5-58.7).10 Disease progression or death occurred in 60 (34%) patients and the median PFS was 8.4 months (95% CI, 8.0-not evaluable).10
Between September 12, 2019, and July 29, 2020, another 689 patients were screened and 290 patients were enrolled in China.10 Their median age was 62 years.10 Patients received 100 mg D-0316 with a 21-day lead-in at 75 mg. At data cutoff (October 18, 2020), the median duration of follow-up was 5.5 months.10 A total of 188 of the 290 patients achieved confirmed partial responses by IRC, such that the ORR was 64.8% (95% CI, 59.0-70.3).10 The DCR was 95.2% (95% CI, 92.0-97.3).10 ORR was consistent across most subgroups.10 Among 34 patients with brain metastases at baseline, 18 patients achieved confirmed partial responses, such that the intracranial ORR was 53% (95% CI, 35.1-70.2).10 At the data cutoff, PFS, DOR, and OS data were premature.10
The most common TRAEs were thrombocytopenia (57%), headache (28%), leukopenia (23%), anemia (22%), and rash (21%).10 The most common grade ≥3 TRAE was thrombocytopenia (12%).10 One death was attributed to TRAE, specifically interstitial lung disease.10 Six cases of interstitial lung disease (2.1%) were observed during the study.10
Researchers concluded that D-0316 demonstrated antitumor activity and an acceptable toxicity profile in patients with EGFR T790M–positive NSCLC who have progressed after EGFR-TKI treatment.10
Comparison of Aumolertinib and Gefitinib in First-Line Treatment of EGFR-Mutated NSCLC
Aumolertinib, a novel EGFR inhibitor, shows a longer duration of benefit compared with gefitinib as first-line therapy for patients with advanced NSCLC who have an EGFR exon 19 del or L858R mutation.
Aumolertinib is a novel, irreversible inhibitor of EGFR TKI with favorable pharmacologic properties that selectively inhibit both EGFR sensitizing and resistance mutations. This TKI has been approved in China for the treatment of patients with EGFR-mutated NSCLC with EGFR T790M who have progressive disease after treatment with an EGFR TKI. The phase 3 trial reported at the 2021 ASCO Annual Meeting assessed the efficacy and safety of aumolertinib compared with gefitinib as an initial treatment of patients with advanced EGFR-mutated NSCLC.11
Patients with previously untreated metastatic or locally advanced NSCLC and EGFR exon 19 deletion or L858R were randomly assigned in a 1:1 ratio to receive either aumolertinib (110 mg once daily) or gefitinib (250 mg once daily). The primary end point of this study was PFS by RECIST version 1.1 per investigator assessment. At 262 PFS events, the study had 90% power to detect a PFS HR of 0.67. Secondary end points included OS, ORR, DOR, and safety.11
Between November 30, 2018, and September 6, 2019, 429 patients across 53 sites in China were enrolled and randomized.11 Patient characteristics were well-balanced.11 At the planned final analysis of event-driven PFS, aumolertinib significantly prolonged PFS compared with gefitinib (median PFS, 19.3 vs 9.9 months; HR, 0.46 [95% CI, 0.36-0.60]; P <.0001).11 DOR was also significantly prolonged with aumolertinib.11 Median OS has not been reached.11 (See Figure 3.)
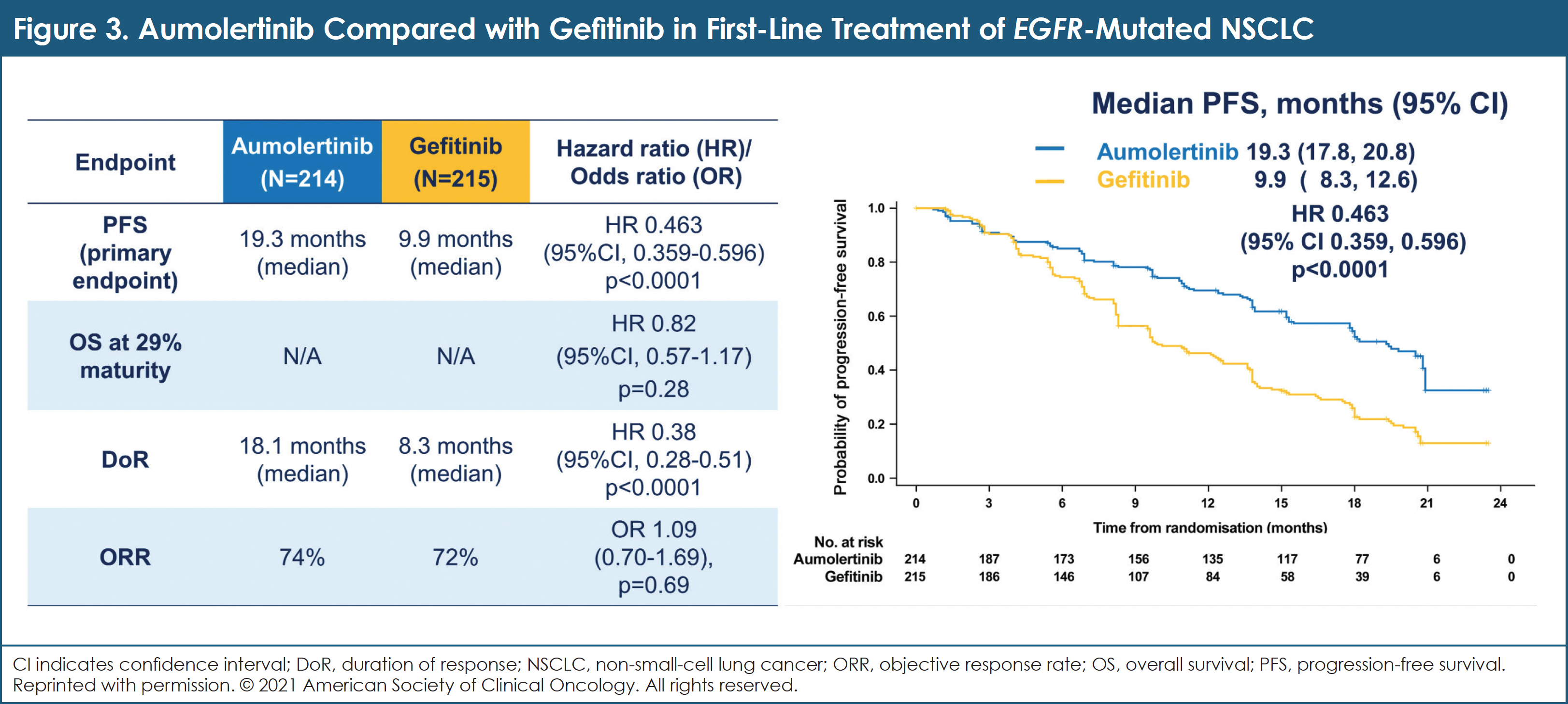
Despite a significantly longer duration of treatment (median, 463 vs 254 days) compared with gefitinib, aumolertinib was associated with a lower incidence of rash, diarrhea, aspartate aminotransferase/alanine aminotransferase increase, and treatment-related serious AEs (4.2% vs 11.2%).11 Aumolertinib was associated with higher rates of creatine phosphokinase increase, platelet count decrease, and neutrophil count decrease; these toxicities were predominantly low grade in terms of severity.11
Researchers concluded that aumolertinib, a novel EGFR TKI, significantly prolonged PFS and DOR compared with gefitinib as first-line therapy in patients with advanced NSCLC who have an EGFR exon 19 del or L858R mutation. In addition, aumolertinib has a favorable safety profile, especially related to toxicities that are mediated by wild-type EGFR. These results establish aumolertinib as a promising option for these patients.11
Mobocertinib in EGFR Exon 20 Insertion–Positive Metastatic NSCLC
Mobocertinib shows manageable safety and clinically meaningful efficacy benefits in patients with EGFR exon 20 insertion–positive metastatic NSCLC.
Currently, there are no targeted therapies for patients with EGFR exon 20 insertion–positive metastatic NSCLC. Mobocertinib is a first-in-class oral TKI that targets EGFR exon 20 insertion mutations. This drug has received breakthrough therapy designation in the United States and China for patients who were treated with platinum-based chemotherapy and who have EGFR exon 20 insertion–positive metastatic NSCLC.12
Researchers reported results from a 3-part, open-label, multicenter study that included dose-escalation, expansion, and extension (EXCLAIM) cohorts. Patients with EGFR exon 20 insertion–positive metastatic NSCLC, ECOG status 0 or 1, and ≥1 prior lines of therapy for locally advanced and/or metastatic disease received mobocertinib at a dose of 160 mg daily. The study’s primary end point was confirmed ORR (RECIST version 1.1) as assessed by IRC. Additional efficacy and safety data were presented for 114 platinum-pretreated patients and 96 patients from the EXCLAIM safety cohort.12
The date of data cutoff was November 1, 2020. The 114 platinum-pretreated patients’ average age was 60 years (median, 27-84), mostly female (66%), and mostly Asian (60%).12 The majority (59%) had received ≥2 prior systemic therapies for NSCLC.12 The confirmed ORR associated with mobocertinib based on the IRC was 28%, including 1 complete response (CR).12 The DCR was 78% (95% CI, 69%-85%).12 Median DOR was 17.5 months.12
Among 96 EXCLAIM patients, the median age was 59 years (range, 27-80), 65% were female, 69% were Asian, and 49% had ≥2 prior treatments.12 The confirmed ORR was 25%, with 1 CR, while the DCR was 76% (95% CI, 66%-84%).12 Median DOR was not reached in this population.12 Baseline brain metastases were present in 33 of the 96 patients (34%) in the EXCLAIM cohort.12 The first site of disease progression by IRC was the brain in 38% of all EXCLAIM patients and 68% of the 33 patients with baseline brain metastases.12
Confirmed responses were seen in all prespecified subgroups in the platinum-pretreated and EXCLAIM patient cohorts.12 More than 30 independent EGFR exon 20 insertion variants were identified among 95 patients with exact insertions known.12 Responses to mobocertinib occurred across all mutation subtypes, regardless of their frequency or position from the C-helix.12
Treatment-related AEs that were observed in ≥20% of the patients who were pretreated with platinum-based chemotherapy were diarrhea (93%), rash (45%), paronychia (39%), decreased appetite (32%), dry skin (31%), nausea (30%), increased blood creatinine (28%), stomatitis (27%), vomiting (26%), dermatitis acneiform (21%), amylase increased (20%), and pruritus (20%).12 Diarrhea was the only grade ≥3 TRAE that occurred in ≥5% of these patients (16%).12 Adverse events that led to discontinuation in ≥2% of the platinum-pretreated patients were diarrhea (2%) and nausea (2%).12 A similar safety profile was observed in the EXCLAIM cohort.12
Researchers concluded that mobocertinib demonstrated clinically meaningful benefit for patients with EGFR exon 20 insertion–positive metastatic NSCLC in both patient cohorts, those who progressed after platinum-based chemotherapy, and those in the phase 3 study extension, with a manageable safety profile.12
Adjuvant Gefitinib versus Cisplatin/Vinorelbine in Japanese Patients with Completely Resected EGFR-Mutated Stage II and III NSCLC (IMPACT)
Adjuvant gefitinib prevents early relapse but did not significantly prolong survival in patients with completely resected stage II or III EGFR-mutated NSCLC.
Treatment with EGFR TKI is a standard of care for patients with EGFR mutation–positive, untreated metastatic NSCLC. However, the efficacy and safety of adjuvant gefitinib for patients with completely resected lung cancer that harbors an EGFR mutation over cisplatin-based adjuvant chemotherapy were not known when this study was initiated in 2011.13
From September 2011 to December 2015, we randomly assigned 234 patients with completely resected, EGFR mutation–positive (exon 19 deletion or L858R), stage II or III NSCLC to receive either gefitinib (250 mg once daily) for 24 months or cisplatin (80 mg/m2 on day 1) plus vinorelbine (25 mg/m2 on days 1 and 8) every 3 weeks for 4 cycles. The primary end point was DFS according to central review in the ITT population.13
Two patients in the gefitinib arm withdrew consent and were excluded from the ITT population.13 No treatment-related deaths were seen in the gefitinib arm, but 3 treatment-related deaths were reported in the cohort receiving cisplatin plus vinorelbine.13 Median duration of follow-up was 71 months.13
Median DFS was numerically longer in the gefitinib arm (36 months) than in the cisplatin plus vinorelbine arm (25 months).13 However, Kaplan–Meier curves began to overlap at approximately 5 years after surgery and no significant difference in DFS was seen.13 The DFS HR was 0.92 (95% CI, 0.67-1.28; P = .63).13
Overall survival was also not significantly different; the median was not reached in either arm.13 Five-year survival rates for the gefitinib and cisplatin plus vinorelbine arms were 78.0% and 74.6%, respectively (HR, 1.03; 95% CI, 0.65-1.65; P = .89).13
Exploratory subset analysis revealed that patients aged ≥70 years in the gefitinib arm survived longer than their counterparts in the cisplatin plus vinorelbine arm (HR, 0.31; 95% CI, 0.10-0.98; P = .018).13
Researchers concluded that adjuvant gefitinib prevents early relapse but did not significantly prolong DFS or OS in patients with completely resected stage II or III EGFR-mutated NSCLC. Noninferiority of DFS and OS justifies use of adjuvant gefitinib in a selected subset of EGFR-mutated patients, especially patients who are deemed unsuitable for cisplatin plus vinorelbine adjuvant therapy.13
Capmatinib in MET Exon 14–Mutated Advanced NSCLC (GEOMETRY Mono-1 Study)
Capmatinib is a promising new treatment for patients with MET exon 14–mutated advanced NSCLC regardless of the line of therapy based on evidence of deep responses and manageable toxicity.
Capmatinib is a highly potent and selective MET inhibitor. Previous data from the GEOMETRY mono-1 study showed a clinically meaningful overall response rate and manageable toxicity profile for capmatinib in patients with MET exon 14–mutated NSCLC who had received 1 to 2 prior lines of treatment (cohort 4) and in treatment-naïve patients (cohort 5b). At the 2021 ASCO Annual Meeting, researchers reported additional efficacy results for capmatinib in MET exon 14–mutated NSCLC, including DOR and PFS, as well as the updated overall response rate results.14
GEOMETRY mono-1 was a phase 2, multicohort, multicenter study evaluating capmatinib in patients with MET exon 14–mutated or MET-amplified advanced NSCLC across 6 cohorts. Adult patients with ECOG performance status 0 or 1, wild-type ALK and EGFR, and stage IIIB or stage IV NSCLC were eligible. Patients with MET exon 14–mutated NSCLC (centrally confirmed) were assigned (regardless of MET amplification status/gene copy number) to cohorts 4 and 5b. These patients received capmatinib tablets 400 mg twice daily. The primary efficacy end point was overall response rate by blinded independent review committee (BIRC) per RECIST version 1.1. A key secondary end point was DOR by BIRC.14
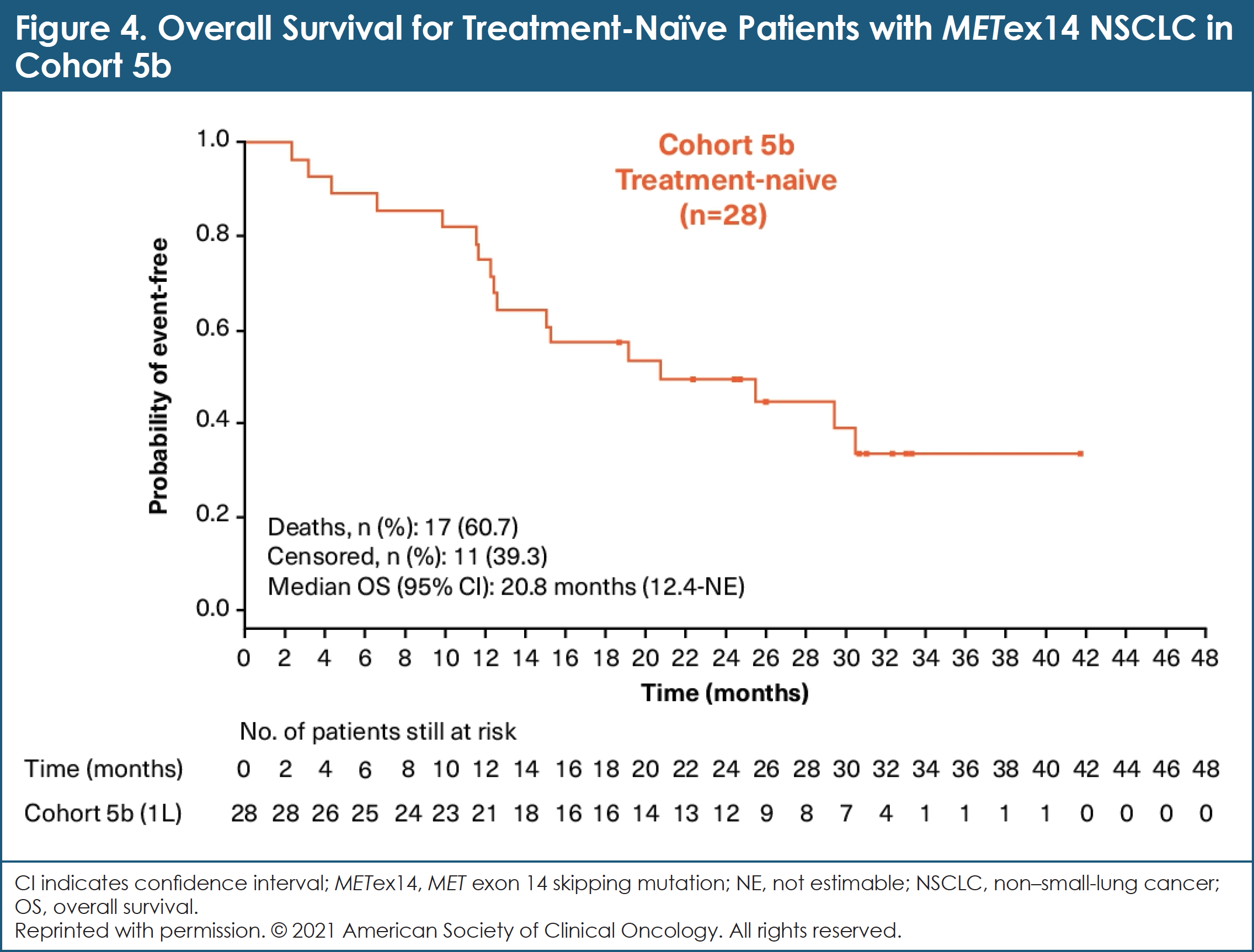
As of November 8, 2018, 97 patients with MET exon 14–mutated NSCLC were evaluable for efficacy: 69 patients in cohort 4; 28 patients in cohort 5b.14 In cohort 4, the overall response rate by BIRC was 40.6% (95% CI, 28.9%-53.1%).14 In cohort 5b, the overall response by BIRC was 67.9% (95% CI, 47.6%-84.1%).14 While still immature at the time of this analysis, data regarding DOR are promising: median DOR (95% CI) by BIRC was 9.7 months (5.6-13.0) and 8.4 months (12.6-not estimable) for cohorts 4 and 5b, respectively.14 Median PFS (95% CI) by BIRC was 5.4 months (4.2-7.0) and 12.4 months (8.2-23.4) for cohorts 4 and 5b, respectively.14 Mature median OS was 20.8 months (95% CI, 12.4-not estimable) in cohort 5b and 13.6 months (95% CI, 8.6-22.2) in cohort 4.14 (See Figure 4.)
The safety profile of capmatinib remains favorable and unchanged.14 The most common AEs (≥25% all grades) seen across all cohorts (n = 373) were peripheral edema (54%), nausea (45%), vomiting (28%), and increased blood creatinine (27%).14 The majority of these AEs were grade 1 or 2.14
Researchers concluded that these data confirm the status of capmatinib as a promising new treatment for patients with MET exon 14–mutated advanced NSCLC regardless of the line of therapy, offering deep and durable responses with a manageable toxicity profile.14
Final OS Analysis of ALK Inhibitors Crizotinib and Alectinib in the Phase 3 J-ALEX Study
Crossover of most crizotinib patients to alectinib as their next therapy may contribute to the lack of prolonged OS for alectinib versus crizotinib in ALK inhibitor–naïve ALK-positive NSCLC.
The primary analysis of the J-ALEX (JapicCTI-132316) study for patients who are ALK inhibitor–naïve with ALK-positive NSCLC demonstrated superior PFS of alectinib compared with crizotinib (HR, 0.34; 99.7% CI, 0.17-0.71; stratified log-rank P <.0001) by the independent review facility (IRF) (data cutoff, December 3, 2015).15 At the 2021 ASCO Annual Meeting, researchers reported the final analysis of OS.16
Patients with ALK-positive NSCLC by immunohistochemistry (IHC) and fluorescence in situ hybridization or reverse transcription polymerase chain reaction were randomized to receive either alectinib (300 mg twice daily, n = 103) or crizotinib (250 mg twice daily, n = 104).16 Stratification factors included ECOG performance status, treatment line, and clinical stage. The primary end point of the J-ALEX study was PFS according to the blinded IRF. Secondary end points included OS, ORR, and safety.16
After a median follow-up of 68.6 months in the alectinib arm and 68.0 months in the crizotinib arm, death occurred in 41% of patients in the alectinib arm and in 39% of patients in the crizotinib arm, respectively.16 The final HR for OS was 1.03 (95% CI, 0.67-1.58).16 The median OS was not reached in either arm of the study.16 The 5-year OS rate with alectinib was 60.9% (95% CI, 51.4-70.3) compared with 64.1% with crizotinib (95% CI, 54.9-73.4).16
Most patients (79%) in the crizotinib arm received alectinib after crossover was permitted, whereas only 11% of patients in the alectinib arm crossed over to crizotinib.16 ALK inhibitors were administered to 83% of the crizotinib patients as their first subsequent treatment, while only 25% of the alectinib patients received a subsequent ALK inhibitor.16 The most common subsequent treatment received in the alectinib arm was chemotherapy (18%), primarily pemetrexed (13%).16
Patients in the crizotinib arm tended to change their treatment earlier than patients in the alectinib arm. Median time from randomization in the J-ALEX study to first change of treatment was 12.3 months for crizotinib patients (95% CI, 8.7-14.6) and not estimable for alectinib patients (95% CI, 42.8-not estimable).16
Researchers concluded that, in the final OS analysis, prolongation of OS was not observed for alectinib compared with crizotinib for ALK inhibitor–naïve ALK-positive NSCLC. This OS result might have been confounded by crossover of most patients in the crizotinib arm to alectinib as their next therapy.16
Brigatinib in ALK-Positive Crizotinib-Refractory NSCLC: Final Results of 2 Trials
Brigatinib demonstrates sustained efficacy and manageable safety in patients with crizotinib-refractory ALK-positive NSCLC.
Brigatinib is a kinase inhibitor approved for the treatment of patients with ALK-positive metastatic NSCLC. At the 2021 ASCO Annual Meeting, researchers reported long-term efficacy and safety results of 2 trials of brigatinib, a phase 1/2 trial and a phase 2 trial (ALTA).17
The phase 1/2 study was a single-arm, open-label trial of brigatinib given at doses ranging from 30 mg/day to 300 mg/day in patients with advanced malignancies. The ALTA study randomized patients with crizotinib-refractory ALK-positive NSCLC to receive brigatinib at a dose of 90 mg daily (arm A) or 180 mg once daily with a 7-day lead-in at 90 mg (arm B). Investigator assessments of confirmed ORR (RECIST version 1.1), DOR, PFS, OS, and safety in patients with ALK-positive NSCLC are reported. The primary end point of ALTA was confirmed ORR per investigators, while secondary end points included confirmed ORR per IRC, DOR, PFS, and OS.17
A total of 137 patients received brigatinib in the phase 1/2 trial.17 Of these, 79 patients had ALK-positive NSCLC and most (71 of 79) had progressed after treatment with crizotinib.17 A total of 28 of 79 patients received brigatinib at a dose of 180 mg once daily with a 7-day lead-in at 90 mg.17 Another 14 of 79 received brigatinib at a dose of 90 mg once daily.17 At the end of the phase 1/2 study (February 18, 2020), with median 27.7-month follow-up (67 months after last patient enrolled), 4 patients remained on brigatinib.17
In the ALTA trial, a total of 222 patients with crizotinib-refractory ALK-positive NSCLC were randomized (n = 112 to arm A, 110 to arm B).17 At the end of ALTA (February 27, 2020), with a median of 19.6 months and 28.3 months of follow-up in arm A and arm B, respectively (53 months after last patient enrolled), 10 patients in arm A and 17 patients in arm B were still on treatment.17
In ALTA, the IRC-assessed intracranial confirmed ORR in patients with measurable baseline brain metastases was 50% (13 of 26) in arm A and 67% (12 of 18) in arm B.17 Kaplan–Meier estimated median intracranial DOR was 9.4 months (95% CI, 3.7-not reached [NR]) in arm A and 16.6 months (3.7-NR) in arm B.17
With long-term follow-up, no new safety signals were identified for brigatinib.17 TRAEs led to dose interruption (phase 1/2, 59%; ALTA arm A, 49%; ALTA arm B, 61%), dose reduction (13%; 8%; 33%), or brigatinib discontinuation (10%; 4%; 13%).17
Researchers concluded that brigatinib showed sustained long-term activity, PFS, and manageable safety in patients with crizotinib-refractory ALK-positive NSCLC. The 180-mg once-daily dose after a 7-day lead-in resulted in numerically higher median PFS and OS. These results are similar to those reported for other ALK TKIs in this patient population.17
Efficacy Data from Phase 2 CodeBreaK 100 in Pretreated KRAS G12C–Mutated NSCLC
Sotorasib is active and well-tolerated in patients with pretreated KRAS G12C–mutated NSCLC.
In the registrational phase 2 CodeBreaK 100 trial, sotorasib demonstrated an ORR of 37% (95% Cl, 29%-46%) and a median PFS of 6.8 months (95% Cl, 5.1-8.2) in 126 patients with pretreated KRAS G12C–mutated NSCLC.18 Tumor response was observed in patients bearing co-mutations in STK11, a driver of poor clinical outcomes with standard of care. At the 2021 ASCO Annual Meeting, researchers reported efficacy data for an extended set of patient subgroups described by key baseline characteristics and biomarkers.18
In the CodeBreaK 100 trial, sotorasib was given orally at 960 mg once daily to eligible patients who had advanced NSCLC harboring KRAS G12C and who had received prior standard therapies. The study’s primary end point was ORR assessed by central review. Key secondary end points included PFS, OS, and safety.18
KRAS G12C mutant allele frequency and tumor mutational burden (TMB) were analyzed by NGS using tissue samples. Mutational status of individual genes was determined by NGS using tissue and/or plasma samples. Correlations between response and KRAS G12C mutant allele frequency, TMB, or co-mutations were analyzed in subsets of patients who had available respective results.18
The median age of the 126 patients with pretreated KRAS G12C–mutated NSCLC enrolled in CodeBreaK 100 was 63.5 years (range, 37-80).18 Most had received 2 or 3 prior lines of treatment (57%), and most were previously treated with both platinum-based chemotherapy and PD-L1 inhibitors (81%).18
Data analysis showed an ORR of 37.1% (95% CI, 28.6-46.2) for sotorasib, including 4 CRs (3.2%).18 Median DOR was 11.1 months (95% CI, 6.9-NE).18 ORR associated with sotorasib was independent of KRAS G12C mutant allele frequency in the study population (odds ratio, 1.11; 95% CI, 0.88-1.39).18 The DCR was 80.6% (95% CI, 72.6-87.2).18
Median PFS was 6.8 months (95% CI, 5.1-8.2), and median OS was 12.5 months (95% CI, 10.0-NE).18 (See Figure 5.)
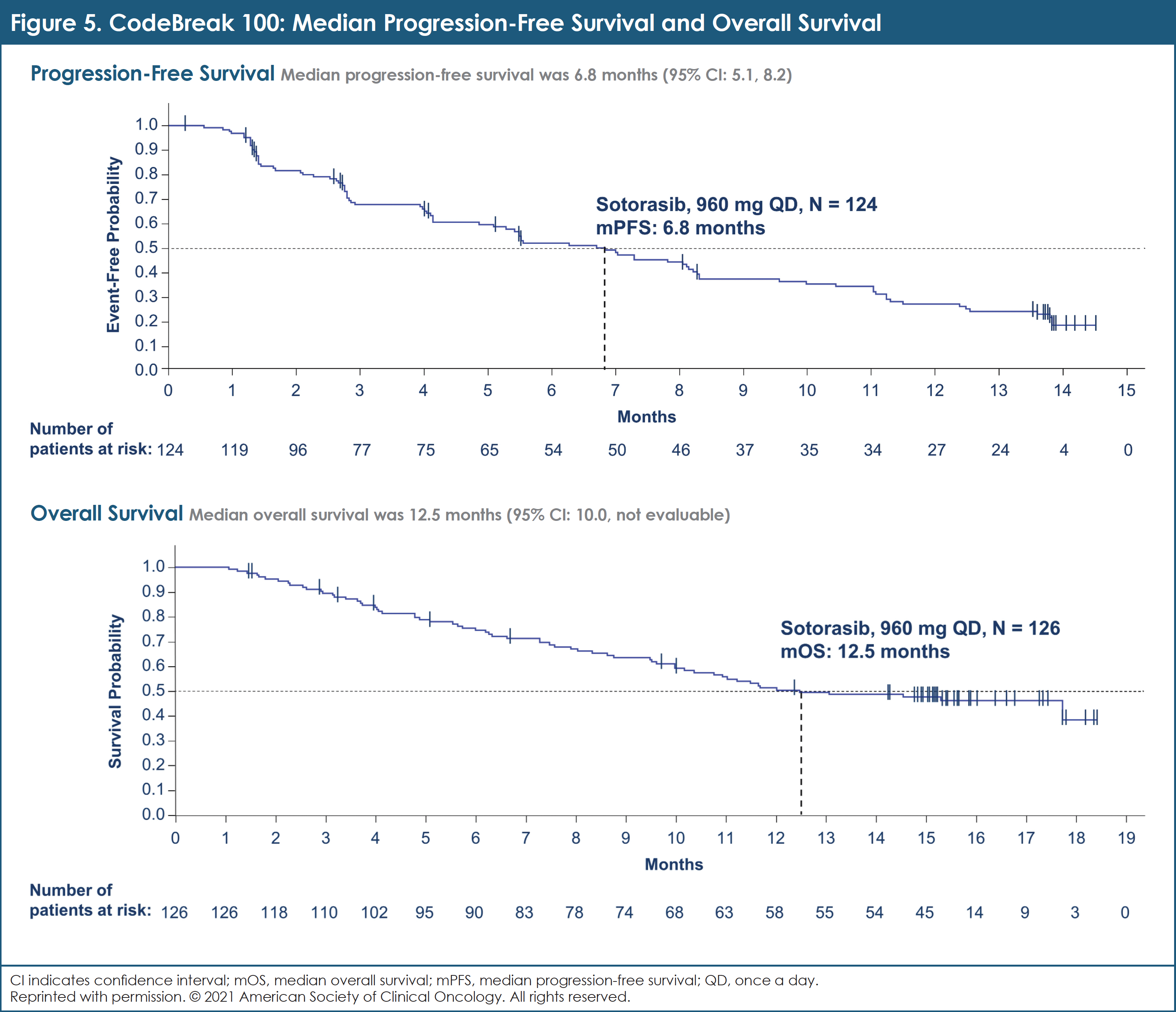
In this analysis of the phase 2 CodeBreaK 100 trial, the clinical benefit of sotorasib was observed across KRAS G12C patient subgroups. Efficacy was seen in subgroups with STK11 or KEAP1, which are molecular indicators of suboptimal outcomes with standard-of-care systemic therapies.18 A confirmatory trial comparing sotorasib and docetaxel in patients with pretreated KRAS G12C–mutated NSCLC is underway.
Rucaparib in High Genomic Loss of Heterozygosity and/or BRCA1/2-Mutated NSCLC (Lung-MAP Sub-Study, S1900A)
Rucaparib failed to show the requisite level of efficacy in advanced NSCLC patients with high genomic loss of heterozygosity and/or a BRCA1/2 mutation.
While prior studies have shown robust efficacy leading to US Food and Drug Administration approval of poly (ADP-ribose) polymerase (PARP) inhibitors in BRCA-associated cancers, data in NSCLC are much less clear. S1900A, a Lung-MAP substudy, evaluated the PARP inhibitor rucaparib in advanced-stage NSCLC harboring BRCA1/2 mutations or genomic loss of heterozygosity as a phenotypic marker of homologous recombination deficiency.19
Eligible patients were required to have a deleterious mutation in BRCA1 or BRCA2 and/or high (≥21%) genomic loss of heterozygosity. Other key eligibility criteria included advanced NSCLC that progressed on or after platinum-based chemotherapy and/or PD-L1 antibody and that progressed after the most recent line of systemic therapy, Zubrod performance status of 0 or 1, adequate organ function, no grade ≥3 hypercholesterolemia, no previous PARP inhibitor exposure, and no systemic therapy within 21 days of registration. Patients were stratified by histology into 2 cohorts: squamous and nonsquamous or mixed histology. With 40 eligible patients per cohort, the design had 91% power to rule out an overall response rate of 15% if the true rate was at least 35% at the 1-sided 5% level. A planned interim analysis on the first 20 patients evaluable for response per cohort required ≥3 responses to proceed to full study enrollment.19
A total of 64 patients were enrolled in the Lung-MAP Sub-Study, S1900A: 27 squamous NSCLC and 37 nonsquamous, of whom 59 were eligible for the study.19 Their median age was 66 years and most were male (56%).19 Almost all patients (98%) received 1 or 2 prior lines of treatment for stage IV disease.19 Biomarker selection included 36 (61%) patients with loss of heterozygosity only, 8 (14%) patients BRCA1 mutated, and 15 (26%) patients BRCA2 mutated.19
Both cohorts were closed for futility with insufficient responses in the interim analysis populations.19 In the full study, 6 responses (4 nonsquamous and 2 squamous) were reported, such that the overall response rate was 10% (95% CI, 2%-18%).19 The DCR was 63% (95% CI, 50%-75%).19 The 6-month PFS rate was 25% (95% CI, 4-46) in BRCA1/2-mutated patients and 50% (95% CI, 10-90) in patients with BRCA1/2-mutated homozygous NSCLC.19 Median OS was 7.8 months in the nonsquamous cohort and 8.2 months in the squamous cohort.19 The most frequent grade ≥3 AEs were anemia (22%), lymphopenia (8%), fatigue (8%), transaminitis (5%), and thrombocytopenia (5%).19
Researchers concluded that the S1900A substudy failed to show the requisite level of efficacy for rucaparib in advanced NSCLC patients with high genomic loss of heterozygosity and/or a BRCA1/2 mutation. Genomic loss of heterozygosity as a phenotypic marker of homologous recombination deficiency does not predict sufficient activity of rucaparib in NSCLC. These results contrast with the high level of efficacy of PARP inhibitors in patients with BRCA-associated or high loss of heterozygosity in other tumor types. Underlying biologic differences in the genomic characteristics of these cancers compared with NSCLC may be responsible. Ongoing studies are examining this premise.19
Combination Therapies
Combination of Nivolumab and Chemotherapy in Nonmetastatic Resectable NSCLC (CheckMate-816)
CheckMate-816 is the first phase 3 trial demonstrating a significant improvement in pathologic response rate with neoadjuvant nivolumab plus chemotherapy in patients with resectable NSCLC.
In patients with nonmetastatic NSCLC, surgery can be curative. However, 30% to 80% of patients who undergo resection experience disease recurrence.20 Neoadjuvant or adjuvant chemotherapy is currently recommended for patients who are at high risk of recurrence, but its benefits are modest and the rate of pathologic complete response (pCR) is low.20
Immunotherapy that targets the PD-1 pathway has demonstrated survival benefits in metastatic NSCLC, but robust data in resectable disease have not yet been shared. Recently, neoadjuvant nivolumab (NIVO), alone or in combination with chemotherapy, has shown encouraging rates of pCR in single-arm phase 2 studies.20 In April 2021 at the AACR Annual Meeting, researchers reported the final analysis of pCR rates in the CheckMate-816 study, a randomized, phase 3, open-label study comparing NIVO plus chemotherapy with chemotherapy alone as neoadjuvant therapy for resectable NSCLC.20
CheckMate-816 enrolled adults with clinical stage IB (≥4 cm) to stage IIIA resectable NSCLC, good performance status (ECOG performance status 0 or 1), and no known EGFR or ALK alterations. Patients were randomized to either IV NIVO at a dose of 360 mg plus platinum-doublet chemotherapy every 3 weeks or chemotherapy alone every 3 weeks for 3 cycles followed by surgery. Stratification parameters included disease stage (IB/II vs IIIA), PD-L1 status (≥1% or <1%), and sex.20
Primary end points of the study include pCR by blinded independent pathological review (BIPR) and event-free survival by BICR. The definition of pCR was 0% viable tumor cells in resected lung and lymph nodes. Patients who did not undergo surgery were counted as nonresponders. Overall survival, major pathologic response (MPR; ≤10% viable tumor in both lung and lymph nodes) per BIPR, and time to death or distant metastases are secondary end points of the CheckMate-816 trial. Exploratory end points include ORR per BICR and potential predictive biomarkers including PD-L1 and TMB.20
Researchers reported that characteristics of patients were balanced between the study arms at baseline; there were 179 patients in each arm.20 The study met its pCR end point: neoadjuvant NIVO + chemotherapy significantly increased pCR rates compared with chemotherapy in the ITT population: 24.0% versus 2.2%.20 The odds ratio was 13.94 (99% CI, 3.49-55.75); P <.0001.20 The improvement in rate of pCR with NIVO + chemotherapy compared with chemotherapy was consistent across key subgroups, including disease stage (IB/II [26% vs 5%]; ≥IIIA [23% vs 1%]), PD-L1 status (<1% [17% vs 3%]; ≥1% [33% vs 2%]), and TMB (low [22% vs 2%]; high [31% vs 3%]).20
In the ITT population, use of neoadjuvant NIVO + chemotherapy also improved MPR rates compared with chemotherapy (37% vs 9%), as well as ORR (54% vs 37%) and radiographic downstaging rates (31% vs 24%).20 Definitive surgery occurred in 83% of patients treated with NIVO + chemotherapy and 75% with chemotherapy alone.20 Surgery was canceled rarely due to AEs (2 patients per arm) and due to disease progression in 12 and 17 patients, respectively.20
Grade 3 and 4 therapy-related AEs and grade 3 and 4 surgery-related AEs were reported in 34% and 37% and 11% versus 15% of patients in the NIVO + chemotherapy versus chemotherapy arms, respectively.20
The CheckMate-816 investigators concluded that the trial met its first primary end point with a statistically significant improvement in the rate of pCR with neoadjuvant NIVO + chemotherapy compared with chemotherapy alone based on independent review. They also noted that the safety profile of NIVO + chemotherapy was consistent with known information about this combination regimen and that the addition of NIVO did not affect the ability to perform surgery. CheckMate-816 is the first phase 3 trial to demonstrate a significant improvement in the rate of pathologic response with neoadjuvant immunotherapy plus chemotherapy in resectable NSCLC.20
Anti–PD-L1 Therapy in Combination with Chemotherapy versus Immunotherapy Alone in First-Line NSCLC with PD-L1 Score 1% to 49%
Chemotherapy combined with immunotherapy improves efficacy outcomes compared with immunotherapy alone in most patients who have PD-L1 scores between 1% and 49%.
Approved regimens for the first-line treatment of metastatic NSCLC include immunotherapy (IO) plus chemotherapy ± anti-angiogenesis inhibitors. IO-only therapy is approved only for PD-L1–positive NSCLC. Patients with PD-L1 scores between 1% and 49% have many therapeutic options, and little is known about how subgroups of patients in this cohort benefit from available treatment regimens.21
To investigate this, data were pooled from 8 randomized controlled trials that investigated anti–PD-L1 therapy as IO-only or in chemotherapy plus IO regimens for the first-line treatment of patients with advanced NSCLC. PD-L1 score was defined as the proportion of tumor cells stained by the assay, and analysis was conducted for patients whose tumors had PD-L1 scores between 1% and 49%. Tumor-infiltrating immune cell staining was not considered. Overall survival and PFS were compared between chemo-IO and IO alone via a pooled analysis. Median survival times were estimated using Kaplan–Meier methods. Hazard ratios were estimated using Cox proportional hazards models stratified by trial and adjusted for age, sex, race, ECOG score, histology, and smoking status.21
A total of 2108 patients with advanced NSCLC and PD-L1 scores between 1% and 49% were identified for this analysis.21 At baseline, 37% of patients were between the ages of 65 and 74 years, while 12% were aged ≥75 years.21 Most were male (67%) and white (79%) with an ECOG performance status of ≥1 (65%).21 Most of these patients were also smokers (85%).21 Median follow-up time was 12.1 months.21
This pooled analysis showed that the 639 patients with advanced NSCLC and PD-L1 scores between 1% and 49% who received chemo-IO had longer PFS and OS compared with 529 patients treated with IO alone.21 Median PFS results for the 2 groups were 7.7 months and 4.2 months, respectively (HR, 0.60; 95% CI, 0.48-0.76).21 Median OS was 21.4 months compared with 14.5 months (HR, 0.68; 95% CI, 0.52-0.90).21 Researchers noted that results should be considered exploratory and hypothesis-generating.21
Researchers believe that this exploratory pooled analysis suggests that chemo-IO may improve efficacy outcomes compared with IO alone in most subgroups of patients with advanced NSCLC who have PD-L1 scores between 1% and 49%. Patients who were aged ≥75 years experienced similar outcomes across therapeutic options.21
Amivantamab Combined with Lazertinib in Osimertinib-Relapsed, Chemotherapy-Naïve EGFR-Mutated NSCLC (CHRYSALIS)
The combination of amivantamab and lazertinib yields responses in chemotherapy-naïve patients with advanced NSCLC who progressed on osimertinib.
The combination of amivantamab, an EGFR-MET bispecific antibody, and lazertinib, a third-generation TKI, has been shown to be effective in treatment-naïve and osimertinib-relapsed patients with EGFR-mutated NSCLC.22 At the 2021 ASCO Annual Meeting, researchers presented updated results of a study of this combination in osimertinib-relapsed patients, including an analysis of potential biomarkers of response.23
Patients with EGFR exon 19 deletion or L858R mutation NSCLC who had progressed on osimertinib without intervening chemotherapy, were enrolled in the combination cohort of the ongoing CHRYSALIS study. With pretreatment tumor biopsies and circulating tumor DNA collected prospectively, patients received the combination dose of 1050/1400 mg amivantamab plus 240 mg lazertinib to assess safety and efficacy in the osimertinib-relapsed population. Response was assessed by investigator per RECIST version 1.1. Osimertinib-resistance mutations or amplifications in EGFR and MET that were identified by NGS using either circulating tumor DNA or tumor biopsy (biomarker-positive) were evaluated for enriching response. Immunohistochemistry staining for EGFR and MET expression was also explored as a potential biomarker for response.23
Of the 45 osimertinib-relapsed patients, 36% had a confirmed response (95% CI, 22%-51%; 1 CR and 15 partial responses [PRs]).23 The clinical benefit rate was 64%.23 After median follow-up of 11.0 months (1.0-15.0 months), 20 of 45 patients (44%) remained on treatment.23 With 11 of 16 patients (69%) continuing in response (2.6-9.6+ months), median DOR has not been reached (NR).23 The median PFS (mPFS) was 4.9 months (95% CI, 3.7-9.5).23
In total, 44 of 45 patients were evaluable by circulating tumor DNA and 29 of 45 were evaluable by tumor NGS.23 Genetic testing identified 17 biomarker-positive patients, of whom 8 (47%) responded.23 Of the remaining 28 patients, 8 (29%) responded.23 Among these 28 patients, 18 had unknown mechanisms of osimertinib-resistance (8 PR) and 10 had non-EGFR, non-MET mechanisms of resistance identified (none responded).23 The mPFS (95% CI) for biomarker-positive and remaining patients was 6.7 months (3.4-NR) and 4.1 months (1.4-9.5), respectively.23 Adequate tissue was available for 20 patients to perform IHC testing for EGFR and MET.23 Nine of 10 (90%) IHC-high (combined EGFR + MET H-score >400) patients responded to treatment, while 1 in 10 IHC-low patients responded to treatment.23
Summarizing this study, researchers stated that treatment with amivantamab plus lazertinib yielded responses in chemotherapy-naïve patients who progressed on osimertinib. Genetic EGFR- and MET-based biomarkers of resistance could be used to identify a subgroup of patients that was more likely to respond to amivantamab and lazertinib, but additional patients who lacked the identified resistance markers also responded. IHC testing may identify patients most likely to benefit from the combination of amivantamab and lazertinib.23
Real-World Study of Combined Inhibition of EGFR and MET in EGFR-Mutant Advanced NSCLC
Combination use of EGFR and MET inhibitors could improve clinical outcomes for patients with EGFR-mutant NSCLC who acquire MET amplification after prior EGFR inhibitor therapy.
MET amplification is an important mechanism mediating acquired resistance to EGFR-TKI therapy. Until now, no consensus exists on the standard treatment strategy for this subset of patients due to the lack of clinical data from large cohort or controlled trials. In the researchers’ clinical experience, 3 regimens were commonly administered to patients after MET amplification–mediated EGFR-TKI progression: EGFR-TKI and MET-TKI combination therapy, MET-TKI monotherapy, or chemotherapy. At the 2021 ASCO Annual Meeting, researchers presented data comparing the effectiveness of these 3 regimens.24
A total of 70 patients with EGFR-mutant advanced NSCLC who progressed from prior EGFR-TKI therapy through the acquisition of MET amplification and received treatment were included in this study.24 Treatment was received between March 2015 and March 2020.24 Of the 70 patients, 38 received EGFR-TKI therapy plus crizotinib, 10 received crizotinib monotherapy, and 22 received platinum-based doublet chemotherapy.24 Somatic mutation profiling was performed on blood and tissue biopsy samples. Resistance mechanisms to the combination targeted therapy were also explored in 12 patients.24
The ORR and DCR were 49% and 83% for the EGFR-TKI + crizotinib group, 40% and 70% for the crizotinib monotherapy group, and 18% and 50% for the chemotherapy group, respectively.24 The EGFR-TKI + crizotinib group had significantly better ORR (P = .03) and DCR (P = .02) compared with the chemotherapy group, but these rates were not statistically different from the crizotinib monotherapy group (ORR, P = .73; DCR, P = .39).24 Progression-free survival was significantly longer for the EGFR-TKI + crizotinib group than those who received crizotinib monotherapy (5.0 vs 2.3 months; P = .004) or chemotherapy (5.0 vs 2.9 months; P = .04), but OS was comparable (10.0 vs 4.1 vs 8.5 months; P = .09).24
TP53 mutation (59%) and EGFR amplifications (43%) were 2 common concurrent mutations in the 3 cohorts.24 The PFS was significantly longer for patients with either concurrent TP53 mutation (n = 17) (6.0 vs 2.3 vs 2.9 months; P = .01) or concurrent EGFR amplification (n = 13) (5.0 vs 1.2 vs 2.4 months; P = .02) who received EGFR-TKI + crizotinib.24 Potential molecular mechanisms of acquired resistance to EGFR-TKI + crizotinib therapy included EGFR T790M (n = 2), EGFR L718Q (n = 1), EGFR S645C (n = 1), MET D1228H (n = 1), BRAF V600E (n = 1), NRAS Q61H (n = 1), and amplifications in KRAS (n = 2), ERBB2 (n = 1), CDK4 (n = 1), and MYC (n = 2).24
This study provides real-world clinical evidence in the largest cohort to date that simultaneous inhibition of EGFR and MET improves clinical outcomes of patients with EGFR-mutant NSCLC who acquired MET amplification from prior EGFR-TKI therapy, indicating that the combination regimen of EGFR-TKI and MET-TKI could be more effective in this patient subset.24
Trastuzumab, Pertuzumab, and Docetaxel in HER2-Mutated Advanced NSCLC (IFCT-1703 R2D2)
The triplet combination of trastuzumab, pertuzumab, and docetaxel is active in pretreated patients with HER2-mutated advanced NSCLC.
Human epidermal growth factor receptor 2 (HER2) exon 20 insertions and mutations are oncogenic drivers found in 1% to 2% of patients with NSCLC.25 However, there are no approved therapies for these patients. Studies suggest that use of HER2 inhibitors that were initially developed for patients with breast cancer might be of value for these patients with NSCLC. At the 2021 ASCO Annual Meeting, researchers reported findings from a trial evaluating the combination of 2 antibodies against HER2, trastuzumab and pertuzumab, and docetaxel.25
The IFCT-1703 R2D2 trial was a multicenter, nonrandomized, phase 2 study with a 2-stage design. Patients’ HER2 mutational status was assessed locally in certified molecular genetic centers. Other key inclusion criteria included advanced NSCLC, progression after ≥1 platinum-based chemotherapy regimens, asymptomatic brain metastases, left ventricular ejection fraction (LVEF) of ≥50%, and performance status of 0, 1, or 2. Patients were treated every 3 weeks with pertuzumab (loading dose of 840 mg, 420 mg thereafter) plus trastuzumab (loading dose of 8 mg/kg, 6 mg/kg thereafter), and docetaxel at 75 mg/m2. Treatment was given until toxicity or disease progression. The primary outcome was overall response rate. Other end points included DOR, PFS, and safety.25
From May 2019 to October 2020, 46 patients were enrolled in 17 centers and received study treatment.25 Their median age was 64.5 years (range, 31-84); 72% were female, 65% were never smokers, and 100% had adenocarcinoma histology.25 A minority of patients (15%) had an ECOG performance status of 2.25 Approximately one-third (30%) of patients had brain metastases.25 PD-L1 was expressed ≥1% and ≥50% in 36% and 7% of patients, respectively.25 No other oncogene driver was found associated with HER2 exon 20 mutation.25
With a median follow-up of 12 months, 44 of 46 patients were evaluable for the primary end point.25 Median duration of treatment in the 13 patients with confirmed response was 10 months (95% CI, 2.7-14.9).25 The ORR was 29%, and the stable disease rate was 56%.25 Median PFS was 6.8 months (95% CI, 4.0-8.5).25 Median OS was 17.6 months.25 At the time of data cutoff, 15 (33%) patients were still under treatment.25
Grade 3/4 TRAEs were observed in 64% of patients.25 No patient required treatment discontinuation because of toxicity.25 One sudden death was possibly related to treatment.25 The most frequent grade ≥3 AEs were neutropenia (33%), diarrhea (13%), and anemia (9%).25 Grade 1/2 dyspnea was observed in 3 (6.7%) patients.25 No interstitial lung disease events were reported.25 The variation in LVEF was –1.7% on average (minimum: –18%; maximum: 10%).25
Researchers concluded that the triplet regimen of trastuzumab, pertuzumab, and docetaxel is feasible and active in pretreated patients with HER2-mutated advanced NSCLC. Based on this activity, HER2 antibody-based strategies should be considered in these patients.25
Early Data
Phase 1 Trial of VS-6766, a Dual RAF-MEK Inhibitor, and Defactinib, an FAK Inhibitor
VS-6766 combined with defactinib is active in pretreated patients with non-G12C KRAS-mutated NSCLC, particularly patients who have KRAS G12V mutations.
KRAS is a known oncogenic driver in NSCLC, with KRAS G12C and KRAS G12V mutations occurring in approximately 13% and approximately 7% of NSCLC adenocarcinomas, respectively.26 As reported by Guo et al, VS-6766, a dual RAF-MEK inhibitor, has shown single-agent activity against KRAS G12V–mutated NSCLC.27
Based on preclinical data, researchers hypothesized that augmented focal adhesion kinase (FAK) signaling is a mechanism of resistance to MEK inhibition. They then devised the current clinical trial.26 These scientists had previously reported safety data for an intermittent schedule of the combination of VS-6766 and defactinib, as well as its efficacy in low-grade serous ovarian cancer.28 At the 2021 AACR Annual Meeting, researchers reported the activity of the combination in KRAS-mutated NSCLC.26
In the dose-escalation and expansion cohorts of the study, patients were treated with an intermittent dose of VS-6766 (3.2-4 mg twice a week) and defactinib (200 mg twice daily). Both drugs were administered 3 weeks on, 1 week off in 28-day cycles. The study aims to recruit 20 patients with KRAS-mutated NSCLC in an expansion cohort.26
As of data cutoff, 19 patients with KRAS-mutated NSCLC were treated in the dose-escalation and expansion cohorts.26 All patients had been previously treated with a PD-1 or PD-L1 targeting ICI. Their median age was 64 years (range, 22-73), and the median number of prior lines of treatment received was 3.26 Seventeen of 19 patients have had at least 1 restaging assessment, 2 of 17 (12%) patients had a PR, and 10 of 17 (59%) had a best response of stable disease.26 The researchers reported that 11 of 17 (65%) patients had a degree of reduction in the size of their tumors, while 5 of 17 (29%) patients have been treated for at least 6 months.26 Both patients with KRAS G12V–mutated NSCLC achieved a PR.26
The combination of VS-6766 and defactinib treatment in patients with non-G12C KRAS-mutated NSCLC who were pretreated with chemotherapy and immunotherapy has shown antitumor activity, particularly in patients whose tumors harbor KRAS G12V mutations.26 A registration-directed study of VS-6766 with or without defactinib in patients with recurrent NSCLC with KRAS G12V mutation is underway.
Patritumab Deruxtecan (HER3-DXd) in EGFR-Mutated NSCLC
In heavily pretreated patients with metastatic or locally advanced EGFR-mutated NSCLC, patritumab deruxtecan has antitumor activity in various EGFR-TKI resistance mechanisms.
Patients with advanced EGFR-mutated NSCLC have few treatment options after treatment with EGFR TKI and platinum-based chemotherapy. Patritumab deruxtecan (HER3-DXd) is an antibody–drug conjugate consisting of a fully human monoclonal antibody to HER3 attached to a topoisomerase I inhibitor payload using a tetrapeptide-based cleavable linker. Efficacy and safety data have been presented previously from an ongoing study of HER3-DXd in patients with EGFR-mutated NSCLC after failure of EGFR-TKI therapy (median follow-up, 5.4 months).29 At the 2021 ASCO Annual Meeting, researchers presented extended follow-up of patients who received the recommended dose of HER3-DXd; 5.6 mg/kg intravenously every 3 weeks.29
This study of HER3-DXd is a phase 1 dose-escalation and expansion study that included patients with locally advanced or metastatic EGFR-mutated NSCLC who progressed after prior EGFR-TKI therapy. Patients with stable brain metastases were included. The primary end point was confirmed ORR by BICR per RECIST version 1.1, while secondary end points included DOR, PFS, and safety.29
At data cutoff on September 24, 2020, 57 patients had been treated with HER3-DXd (5.6 mg/kg intravenously every 3 weeks).29 Median follow-up was 10.2 months (range, 5.2-19.9).29 Their median number of prior anticancer regimens was 4 (range, 1-9).29 All patients had received prior EGFR TKI (86% prior osimertinib), and 91% had progressed after platinum-based chemotherapy.29 Nearly half (47%) of these 57 patients had a history of brain metastases.29
Median duration of treatment with HER3-DXd was 5.7 months (range, 0.7-28.3), and treatment with HER3-DXd was ongoing in 18 (32%) patients.29 The confirmed ORR for HER3-DXd by BICR was 39% (95% CI, 26%-52%), including 1 CR and 21 PRs.29 Sixteen of 22 responses occurred within 3 months of starting HER3-DXd.29 The DCR was 72% (95% CI, 59%-83%).29 Median DOR was 6.9 months (95% CI, 3.1 months-not evaluable).29 Median PFS was 8.2 months (95% CI, 4.4-8.3).29 Among patients who received platinum-based chemotherapy and osimertinib, the ORR was 39% (95% CI, 24%-55%).29 (See Table.)
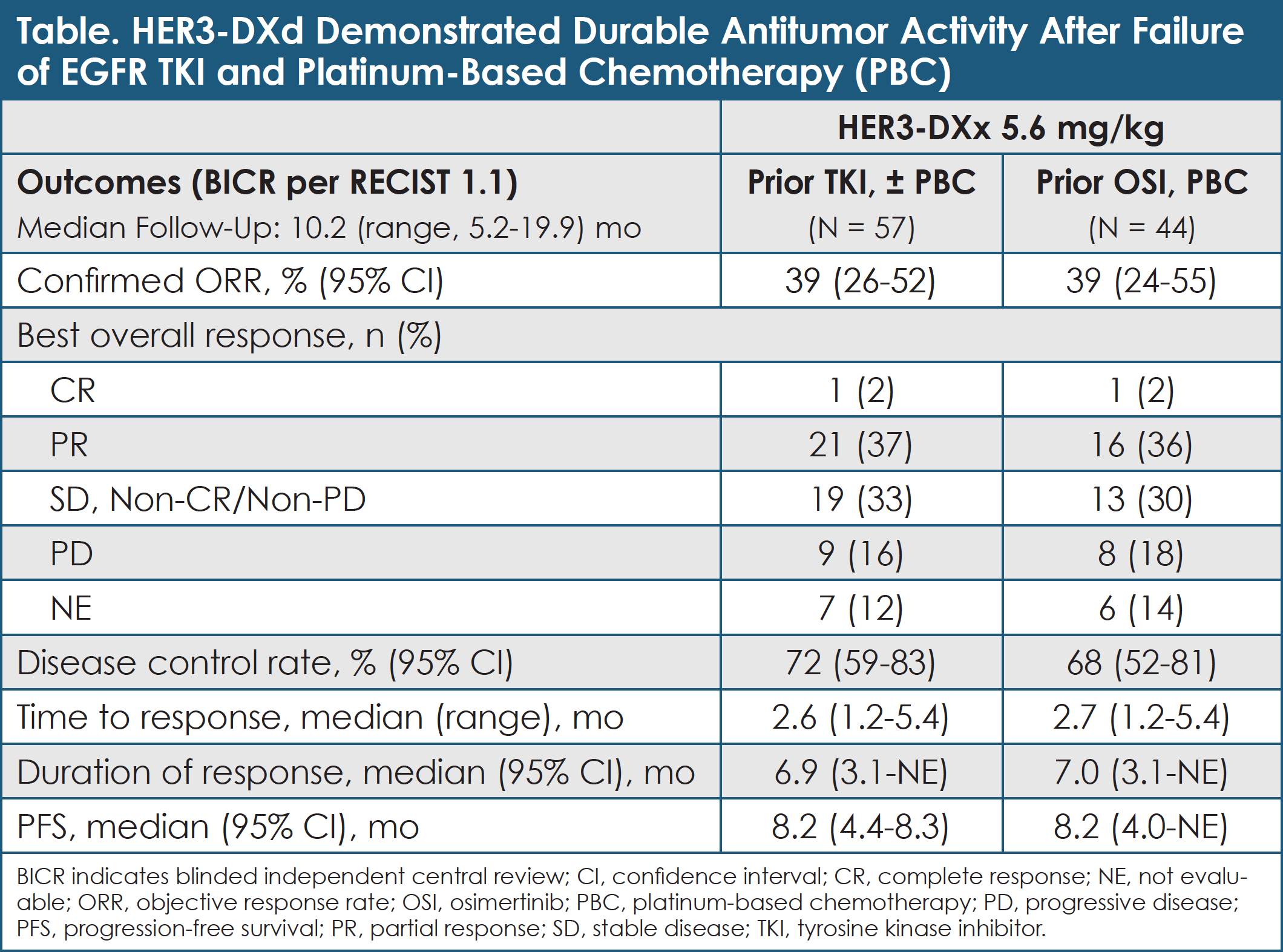
The antitumor activity of HER3-DXd was observed across diverse mechanisms of EGFR-TKI resistance, including those not directly related to HER3 (EGFR C797S, MET or HER2 amplification, and BRAF fusion).29
Among 43 patients evaluable for HER3 expression, nearly all expressed HER3; median membrane H-score by IHC was 180 (range, 2-280).29 Median H-score (range; N) was 195 (92-268; 15) in patients with CR/PR, 180 (4-280; 15) with stable disease, 126.5 (2-251; 6) with progressive disease, and 180 (36-180; 7) in patients unevaluable for best overall response.29
The most common grade ≥3 AEs were thrombocytopenia (26%), neutropenia (19%), and fatigue (14%).29 Drug-related interstitial lung disease by central adjudication occurred in 5% of patients; none was grade 4 or 5.29 Six of 57 patients (11%) had AEs associated with treatment discontinuation (none were due to thrombocytopenia).29
Researchers concluded that HER3-DXd has antitumor activity across various EGFR-TKI resistance mechanisms in heavily pretreated patients with metastatic or locally advanced EGFR-mutated NSCLC. The safety profile of HER3-DXd was consistent with previous reports. A phase 2 study of HER3-DXd is underway in patients with EGFR-mutated NSCLC who relapsed after EGFR-TKI and platinum-based chemotherapy.29
Phase 1 Study of Telisotuzumab Vedotin in Patients with c-Met–Mutated Advanced NSCLC
Telisotuzumab vedotin demonstrates an encouraging overall response rate in patients with nonsquamous EGFR wild-type NSCLC.
Telisotuzumab vedotin (teliso-V) is an anti–c-Met antibody conjugated with a tubulin inhibitor cytotoxic agent. The aim of this phase 2 trial was to explore safety and efficacy of teliso-V in cohorts of patients with c-Met–positive advanced NSCLC based on their histopathology and EGFR mutation status, as well as patient subgroups based on c-Met expression (stage 1), followed by expansion into an appropriately selected population for further evaluation of safety and efficacy (stage 2).30
Patients enrolled in this study of teliso-V had an ECOG performance status score of 0 or 1 and received 1 or 2 prior lines of therapy for NSCLC, including cytotoxic chemotherapy, immunotherapy, and targeted therapy. Their c-Met status was determined centrally by IHC (SP44 antibody). Membrane staining ≥25% 3+ or ≥75% 1+ was considered positive for nonsquamous and squamous NSCLC, respectively. The dose of teliso-V was 1.9 mg/kg every 2 weeks. The study’s primary end point was ORR per central review in patients with at least 12 weeks of follow-up. Secondary end points were DOR, DCR, PFS, and OS.30
In the nonsquamous EGFR wild-type cohort, the ORR associated with teliso-V was 35.1%.30 In the c-Met high group, ORR was 53.8%, while in the c-Met intermediate group, ORR was 25.0%.30 The ORR was deemed “modest” in the squamous and EGFR-mutated cohorts.30
Grade ≥3 AEs occurred in 44% of patients receiving teliso-V.23 The most common events (≥2%) were malignant neoplasm progression (6.2%), pneumonia (5.3%), hyponatremia (4.4%), anemia (2.7%), dyspnea (2.7%), fatigue (2.7%), increased GGT (2.7%), peripheral sensory neuropathy (2.7%), and pneumonitis (2.7%).30 Grade 5 AEs that investigators considered possibly related to teliso-V were sudden death, dyspnea, and pneumonitis (1 event each).30
Researchers summarized by sharing that the ORR in nonsquamous EGFR wild-type NSCLC was encouraging enough to move this patient cohort to stage 2 of the teliso-V trial. In this cohort, ORR was highest in the c-Met high group, although also clinically meaningful in the c-Met intermediate group. Enrollment in the squamous NSCLC cohort was stopped, while enrollment into the EGFR-mutated cohort continues until the next interim analysis.30
Novel Agents
Safety Results of Phase 2 Study of Bintrafusp plus Chemotherapy in Advanced NSCLC (INTR@PID LUNG 024)
Bintrafusp alfa given every 3 weeks via intravenous infusion is well-tolerated in patients with advanced NSCLC.
Bintrafusp alfa is a first-in-class bifunctional fusion protein that is composed of the extracellular domain of the TGF-βRII receptor, a TGF-β “trap,” fused to a human IgG1 monoclonal antibody blocking PD-L1. The global, phase 1b/2 INTR@PID LUNG 024 study combines bintrafusp alfa with chemotherapy in patients with stage IV NSCLC. At the 2021 AACR Annual Meeting, researchers reported cumulative safety data. This was the first report of safety results from a clinical study with bintrafusp alfa given at a dose of 2400 mg every 3 weeks.31
Adults with stage IV nonsquamous or squamous NSCLC and an ECOG performance status score of 0 or 1 were included in INTR@PID LUNG 024. In cohorts A, B, and C of this study, patients must not have received prior systemic therapy. In cohort D, patients had progressed on previous anti–PD-(L)1 therapy.31
Patients received IV bintrafusp alfa 2400 mg every 3 weeks in combination with chemotherapy for 4 cycles. In cohort A (nonsquamous only), chemotherapy consisted of cisplatin or carboplatin + pemetrexed. In cohort B, chemotherapy consisted of carboplatin + nab-paclitaxel or paclitaxel. In cohort C, chemotherapy consisted of cisplatin or carboplatin + gemcitabine. In cohort D, docetaxel was used. Treatment was then followed by bintrafusp alfa maintenance (monotherapy or combination with pemetrexed in cohort A) for up to 31 cycles.31
The primary objective of this study was to evaluate the safety of bintrafusp alfa in combination with chemotherapy. The occurrence of DLTs was assessed during a 3-week observation period.31
As of October 7, 2020, 64 patients received bintrafusp alfa in combination with chemotherapy.31 Of the 35 patients included in the DLT analysis, 4 experienced 1 DLT according to the safety monitoring committee (cohort A: N = 1 of 8; cohort B: N = 1 of 8; cohort C: N = 0 of 8; cohort D: N = 2 of 11).31 The most common (≥50%) treatment-emergent AEs in any cohort were anemia (cohort B: 56%; cohort C: 100%; cohort D: 75%), nausea (cohort A: 53%, cohort C: 67%), pruritus (cohort C: 67%), asthenia (cohort C: 56%; cohort D: 58%), neutropenia (cohort C: 56%), decreased appetite (cohort D: 50%), and diarrhea (cohort D: 50%).31 Skin lesions, including actinic keratosis, hyperkeratosis, and keratoacanthoma, were reported in this study (cohort A: 6%; cohort C: 44%; cohort D: 8%).31
Researchers concluded that bintrafusp alfa 2400 mg every 3 weeks in combination with chemotherapy was well-tolerated. No new safety signals were identified.31
Phase 2 Study of DM-CHOC-PEN in Patients with NSCLC with Central Nervous System Involvement
DM-CHOC-PEN, a bis-alkylator of DNA, produces long-term objective responses with manageable toxicities in patients with NSCLC and CNS involvement.
DM-CHOC-PEN (4-demethyl-4-cholesteryloxycarbonylpenclomedine) is a poly-chlorinated pyridine cholesteryl carbonate that acts via bis-alkylation of DNA at N7-guanine and N4-cytosine. DM-CHOC-PEN has completed phase 1 and 2 trials, including trials in patients with NSCLC and central nervous system (CNS) involvement. The primary purpose of the previously reported phase 1/2 DM-CHOC-PEN trials was to assess clinical response, monitor toxicities and safety, and verify the maximum tolerated doses (MTDs) for IV dosing. At the 2021 AACR Annual Meeting, researchers reported responses and toxicities observed with DM-CHOC-PEN in patients with NSCLC who had CNS involvement and whose tumors lacked genetic rearrangements and tumor targets, and/or whose disease had relapsed after treatment with standard therapies.32
DM-CHOC-PEN was administered to adults with cancer, including NSCLC, as a 3-hour IV infusion once every 21 days employing a verified 2-tier MTD schedule: 85.8 mg/m2 for patients with liver involvement and 98.7 mg/m2 for patients with normal liver function.32
So far, a total of 52 adults with cancer have received treatment with DM-CHOC-PEN, including 16 patients with lung cancer.32 Among these patients, 11 had NSCLC, specifically adenocarcinoma or large-cell carcinomas, that involved the CNS.32 These tumors lacked genetic rearrangements and had no tumor targets, and/or had failed standard therapies.32 Seven of the 11 patients with NSCLC involving the CNS also had brain metastases.32
DM-CHOC-PEN was well-tolerated.32 The most common AEs included fatigue (17%), nausea (11%), and reversible liver dysfunction (9%).32 No neurologic, psychological, hematologic, cardiac, or renal toxicities were observed, and there were no drug-associated deaths.32
Eight of 16 patients with NSCLC who received DM-CHOC-PEN had a CR or PR based on RECIST version 1.1 criteria.32 Overall survival and PFS based on Kaplan–Meier analyses ranged from 8+ to 70+ months.32
Researchers concluded that DM-CHOC-PEN, a bis-alkylator of DNA, is safe at the dose levels described. It has produced long-term objective responses with manageable toxicities in patients with NSCLC and CNS involvement who lack genetic rearrangements or tumor targets and/or whose disease progressed after standard therapies.32
Diagnostic Testing
Clinical Outcomes for Plasma-Based Genomic Profiling versus Tissue Testing in Advanced NSCLC (NILE)
This prospective community-based study shows that cell-free circulating tumor DNA detects guideline-recommended biomarkers at a rate similar to tissue genotyping for patients with lung cancer.
Expert guidelines recommend somatic genomic testing to inform targeted therapy treatment for patients with advanced lung adenocarcinoma. The NILE study was a prospective observational study that demonstrated noninferiority of cell-free circulating tumor DNA-based tumor genotyping compared with tissue-based genotyping to find targetable genomic alterations in patients with newly diagnosed advanced lung adenocarcinoma. At the 2021 ASCO Annual Meeting, researchers reported clinical outcomes data now that the NILE patient cohort has matured.33
NILE, a prospective multicenter North American study, enrolled patients with previously untreated advanced lung adenocarcinoma who underwent standard-of-care tissue genotyping and concurrent comprehensive cell-free circulating tumor DNA analysis using the commercially available Guardant360 assay (Guardant Health). After 12 months of study enrollment, data regarding ORR, DCR, and time to treatment were collected for patients with targetable genomic alterations as defined by National Comprehensive Cancer Network guidelines who were treated with community-based physician’s choice of therapy.33
Among 282 patients with NSCLC in the study, 32% had an actionable biomarker detected by tissue (21%) and/or cell-free circulating tumor DNA (27%) analysis.33 Sixty-one (69%) of these patients were treated with a US Food and Drug Administration–approved targeted therapy based on somatic genotyping results (ie, EGFR, ALK, ROS1 inhibitor).33 Thirty-three patients were eligible for clinical response evaluation and demonstrated an ORR of 58% and a DCR of 94%.33
Twenty-five patients in the evaluable cohort (76%) achieved a durable response of ≥6 months.33 Seventeen (52%) patients achieved a durable response of >12 months.33 Patients responded to targeted therapy regardless of the variant allele frequency of the target alteration.33 Time to treatment was significantly faster for cell-free circulating tumor DNA-informed biomarker detection compared with tissue genotyping (median, 18 vs 31 days, respectively; P = .0008).33
This is the first prospective community-based study to find that cell-free circulating tumor DNA detects guideline-recommended biomarkers at a rate similar to tissue genotyping, and that therapeutic outcomes based on plasma-based comprehensive genomic profiling are comparable to published tissue-based targeted therapy clinical outcomes. The NILE study complements and confirms findings in the prospective FLAURA and SLLIP studies, which exclusively enrolled patients from academic sites.33
Healthcare Disparities
Racial Disparities in Biomarker Testing and Clinical Trial Enrollment in NSCLC
Use of NGS-based testing for patients with advanced or metastatic NSCLC is the most notable disparity when comparing management patterns for black and white patients.
Racial disparities exist at many levels in the healthcare system, including cancer care screening to timely diagnosis and treatments received, as well as clinical trial enrollment. This study investigated differences in black versus white race among patients with NSCLC who underwent biomarker testing and enrolled in clinical trials in the United States.34
This retrospective observational study reviewed data from the Flatiron Health database, which includes longitudinal data of patients with advanced and metastatic NSCLC. Patient data were considered if the patient had received systemic therapy between January 1, 2017, and October 30, 2020. Descriptive analyses summarized differences by race in biomarker testing and trial enrollment. Multivariable regression examined the relationship between these factors.34
Data for a total of 14,768 patients were evaluated: 9793 (66.3%) of the patients were white and 1288 (8.7%) were black.34 Most white patients (76%) and black patients (74%) underwent at least a single molecular test or comprehensive genomic analysis (P = .03).34 Next-generation sequencing was performed among 50% of white patients and 40% of black patients (P <.0001).34 Clinical trial participation was observed among 4% of white patients and 2% of black patients (P = .0002).34
There was a statistically significant association between race (white vs black) and both biomarker testing (ever vs never) and trial participation (yes vs no) (both P <.001, unadjusted chi-square).34 Differences in NGS testing, baseline biomarker testing, and race remained statistically significant (P <.01) in adjusted regression analyses.34
Receipt of first-line targeted therapy was comparable between white and black patients (10% and 9%, respectively; P = .24).34 First-line use of pembrolizumab plus carboplatin plus pemetrexed was observed among 20% of white patients and 20% of black patients; carboplatin plus paclitaxel was observed among 16% and 17%, and single-agent pembrolizumab was observed among 14% and 15%, respectively.34
Use of NGS-based testing, which is recommended by the National Comprehensive Cancer Network Clinical Guidelines in Oncology for patients with advanced or metastatic NSCLC, is the most notable disparity among black patients, with a greater than 10% difference in receipt of this testing versus white counterparts. This may contribute in part to more than double the rate of participation in clinical trials observed among white patients since many second-line and later-line trials use molecular targets as inclusion criteria. While multiple factors are known to affect healthcare disparities, access to and receipt of appropriate biomarker testing may be an attainable goal to ensure equal access to quality care.34
References
- Reck M, Ciuleanu T-E, Cobo M, et al. First-line nivolumab (NIVO) plus ipilimumab (IPI) plus two cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients with advanced non-small cell lung cancer (NSCLC): two-year update from CheckMate 9LA. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9000.
- Paz-Ares LG, Ciuleanu T-E, Lee J-S, et al. Nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for advanced non-small cell lung cancer (NSCLC): 4-year update from CheckMate 227. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9016.
- Socinski MA, Jotte RM, Cappuzzo F, et al. Pooled analyses of immune-related adverse events (irAEs) and efficacy from the phase 3 trials IMpower130, IMpower132, and IMpower150. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9002.
- Wakelee HA, Altorki NK, Zhou C, et al. IMpower010: primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 8500.
- Ross HJ, Kozono DE, Urbanic JJ, et al. AFT-16: phase II trial of neoadjuvant and adjuvant atezolizumab and chemoradiation (CRT) for stage III non-small cell lung cancer (NSCLC). Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 8513.
- Zhou C, Huang D, Yu X, et al. Results from RATIONALE 303: a global phase 3 study of tislelizumab (TIS) vs docetaxel (TAX) as second- or third-line therapy for patients with locally advanced or metastatic NSCLC. Presented at: American Association of Cancer Research Annual Meeting 2021; April 10-15, 2021. Abstract CT039.
- Shi Y, Wu L, Yu X, et al. ORIENT-3: a randomized, open-label, phase 3 study of sintilimab versus docetaxel in previously treated advanced/metastatic squamous non-small-cell lung cancer (sqNSCLC). Presented at: American Association of Cancer Research Annual Meeting 2021; April 10-15, 2021. Abstract CT041.
- Markovets A, Han J-Y, Cho BC, et al. Acquired resistance in patients with EGFRm NSCLC following treatment with osimertinib plus savolitinib in the Ph1b TATTON study Parts B and D. Presented at: American Association of Cancer Research Annual Meeting 2021; April 10-15, 2021. Abstract CT024.
- Kim D-W, Kim S-W, Camidge DR, et al. CD73 inhibitor oleclumab plus osimertinib for advanced EGFRm NSCLC: first report of a phase 1b/2 study. Presented at: American Association of Cancer Research Annual Meeting 2021; April 10-15, 2021. Abstract CT163.
- Lu S, Zhang Y, Zhang G, et al. D-0316 in patients with advanced T790M-positive EGFR-mutant non-small cell lung cancer who progressed on prior EGFR-TKI therapy: results from a phase II study (NCT03861156). Presented at: American Association of Cancer Research Annual Meeting 2021; April 10-15, 2021. Abstract CT170.
- Lu S, Dong X, Jian H, et al. Randomized phase III trial of aumolertinib (HS-10296, Au) versus gefitinib (G) as first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) and EGFR exon 19 del or L858R mutations (EGFRm). Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9013.
- Ramalingam SS, Zhou C, Kim TM, et al. Mobocertinib (TAK-788) in EGFR exon 20 insertion (ex20ins)+ metastatic NSCLC (mNSCLC): additional results from platinum-pretreated patients and EXCLAIM cohort of phase 1/2 study. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9014.
- Tada H, Mitsudomi T, Yamanaka T, et al. Adjuvant gefitinib versus cisplatin/vinorelbine in Japanese patients with completely resected, EGFR-mutated, stage II-III non-small cell lung cancer (IMPACT, WJOG6410L): a randomized phase 3 trial. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 8501.
- Wolf J, Seto T, Han J-Y, et al. Capmatinib in MET exon 14-mutated, advanced NSCLC: updated results from the GEOMETRY mono-1 study. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9020.
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29-39.
- Seto T, Nishio M, Hida T, et al. Final OS analysis from the phase III J-ALEX study of alectinib versus crizotinib in Japanese ALK-inhibitor naïve ALK-positive non-small cell lung cancer (ALK+ NSCLC). Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9022.
- Gettinger SN, Huber RM, Kim D-W, et al. Brigatinib in ALK+ crizotinib-refractory non-small cell lung cancer (NSCLC): final results of the phase 1/2 and phase 2 (ALTA) trials. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9071.
- Skoulidis F, Li BT, Govindan R, et al. Overall survival and exploratory subgroup analyses from the phase 2 CodeBreaK 100 trial evaluating sotorasib in pretreated KRAS p.G12C mutated non-small cell lung cancer. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9003.
- Riess JW, Redman MW, Wheatley-Price P, et al. A phase II study of rucaparib in patients with high genomic LOH and/or BRCA1/2 mutated stage IV non-small cell lung cancer (Lung-MAP Sub-Study, S1900A). Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9024.
- Forde PM, Spicer J, Lu S, et al. Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the phase 3 CheckMate 816 trial. Presented at: American Association of Cancer Research Annual Meeting 2021; April 10-15, 2021. Abstract CT003.
- Akinboro O, Vallejo JJ, Mishra-Kalyani PS, et al. Outcomes of anti-PD-(L1) therapy in combination with chemotherapy versus immunotherapy (IO) alone for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC) with PD-L1 score 1-49%: FDA pooled analysis. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9001.
- Cho BC, Lee KH, Cho EK, et al. Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in combination with lazertinib, a third-generation tyrosine kinase inhibitor (TKI), in advanced EGFR NSCLC. Ann Oncol. 2020;31:S813.
- Bauml J, Cho BC, Park K, et al. Amivantamab in combination with lazertinib for the treatment of osimertinib-relapsed, chemotherapy-naïve EGFR mutant (EGFRm) non-small cell lung cancer (NSCLC) and potential biomarkers for response. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9006.
- Li L, Qu J, Heng J, et al. A large real-world study on the effectiveness of the combined inhibition of EGFR and MET in EGFR-mutant advanced non-small cell lung cancer (NSCLC). Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9043.
- Mazieres J, Lafitte C, Ricordel C, et al. Combination of trastuzumab, pertuzumab and docetaxel in patients with advanced non-small cell lung cancer (NSCLC) harboring HER2 mutation: final results from the IFCT-1703 R2D2 trial. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9015.
- Krebs MG, Shinde R, Rahman RA, et al. A phase I trial of the combination of the dual RAF-MEK inhibitor VS-6766 and the FAK inhibitor defactinib: evaluation of efficacy in KRAS mutated NSCLC. Presented at: American Association of Cancer Research Annual Meeting 2021; April 10-15, 2021. Abstract CT019.
- Guo C, Chénard-Poirier M, Roda D, et al. Intermittent schedules of the oral RAF-MEK inhibitor CH5126766/VS-6766 in patients with RAS/RAF-mutant solid tumors and multiple myeloma: a single-centre, open-label, phase 1 dose-escalation and basket dose-expansion study. Lancet Oncol. 2020;21:1478-1488.
- Shinde R, Terbuch A, Little M, et al. Phase I study of the combination of a RAF-MEK inhibitor CH5126766 and FAK inhibitor defactinib in an intermittent dosing schedule with expansions in KRAS mutant cancers. Presented at: American Association of Cancer Research 2020; April 24-29, 2020. Abstract CT143.
- Janne PA, Baik CS, Su W-C, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated (EGFRm) non-small cell lung cancer (NSCLC). Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9007.
- Camidge DR, Moiseenko F, Cicin I, et al. Telisotuzumab vedotin (teliso-v) monotherapy in patients with previously treated c-Met+ advanced non-small cell lung cancer. Presented at: American Association of Cancer Research Annual Meeting 2021; April 10-15, 2021. Abstract CT179.
- Rolfo C, Greillier L, Veillon R, et al. Bintrafusp alfa in combination with chemotherapy in patients with stage IV NSCLC: safety results of the INTR@PID LUNG 024 study. Presented at: American Association of Cancer Research Annual Meeting 2021; April 10-15, 2021. Abstract CT104.
- Weiner RS, Morgan LR, Ware M, et al. Phase II clinical trial results for 4-demethyl-4-cholesteryloxycarbonyl-penclomedine (DM-CHOC-PEN) in advanced non-small cell lung cancer (NSCLC) involving the CNS. Presented at: American Association of Cancer Research Annual Meeting 2021; April 10-15, 2021. Abstract CT152.
- Page RD, Drusbosky L, Dada HI, et al. Clinical outcomes for plasma-based comprehensive genomic profiling versus tissue testing in advanced lung adenocarcinoma. Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9027.
- Bruno DS, Hess LM, Li X, et al. Racial disparities in biomarker testing and clinical trial enrollment in non-small cell lung cancer (NSCLC). Presented at: 2021 American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021. Abstract 9005.

