Febrile neutropenia (FN) is an oncologic emergency that necessitates prompt treatment with antibiotics. National consensus guidelines, such as those from the Infectious Diseases Society of America, the National Comprehensive Cancer Network, and the American Society of Clinical Oncology, recommend monotherapy with an antipseudomonal β-lactam antibiotic for most cases of FN.1-3 These guidelines recommend the addition of a second antipseudomonal agent in patients with complications, such as hypotension and pulmonary sources of infection, or if treatment resistance is suspected.1-3 However, there is a paucity of clinical data to suggest that there are added benefits of dual therapy in patients with FN. Few studies have directly assessed the safety and efficacy of initial empiric dual therapy with an antipseudomonal β-lactam and aminoglycoside in patients with FN.4-7 With a lack of data supporting the efficacy of dual coverage, it is important to consider that the administration of unnecessary antibiotics could result in increased antibiotic resistance, adverse drug reactions, and an increased cost of care.
At our institution, Augusta University Medical Center, a 520-bed academic medical center, the FN protocol recommends treatment with cefepime and 48 hours of treatment with tobramycin as a result of a poor local susceptibility rate of Pseudomonas aeruginosa to cefepime (85% at the time of the protocol initiation).1 Per the institutional protocol, treatment with tobramycin is discontinued if no gram-negative organism is detected within 48 hours of a blood culture collection, for which our institution uses rapid diagnostic testing via the BioFire Blood Culture Identification Panel.
The purpose of this study was to evaluate adherence to the current treatment protocol and to compare the safety and efficacy outcomes of patients with cancer who received dual coverage with tobramycin and antipseudomonal β-lactam for the treatment of FN with patients with cancer and FN who did not receive dual coverage.
Methods
This retrospective chart review included adult patients with cancer who were admitted to Augusta University Medical Center with a diagnosis of FN between January 1, 2019, and July 31, 2020. Eligible patients were identified via a report for the International Classification of Diseases, Tenth Revision diagnosis code for FN (R50.81). Patients were included in the study if they received ≥1 dose of cefepime. Patients were excluded from the study if they received an antipseudomonal agent other than cefepime or tobramycin as their initial empiric therapy.
The patients were categorized based on their initial empiric treatment for FN, cefepime monotherapy (ie, they never received any aminoglycoside) or cefepime plus tobramycin (ie, dual therapy). The dual-therapy cohort was further divided into early- and delayed-treatment cohorts, depending on the timing of the initiation of tobramycin treatment in relation to the administration of cefepime. The patients who received tobramycin within 48 hours of receiving cefepime composed the early-treatment cohort, whereas the patients who received tobramycin more than 48 hours after receiving cefepime composed the delayed-treatment cohort.
Cefepime was given at 2 g via intravenous (IV) dosing every 8 hours, with renal adjustment per an automatic dose-adjustment protocol for creatinine clearance of <50 mL/min. Tobramycin was given per the pharmacy protocol at an extended-interval dose of 5 mg/kg IV every 24 hours or pulse dosing in patients with renal dysfunction.
The primary outcome was adherence to the institutional FN protocol. The secondary outcomes included the incidence of gram-negative microorganism growth on blood culture(s), the incidence of gram-negative organism resistant to cefepime, an escalation or change of antibiotic therapy, the incidence of acute kidney injury (AKI), hospital length of stay (LOS), the percentage of patients transferred to the intensive care unit (ICU), LOS in the ICU, and in-hospital mortality for patients receiving cefepime monotherapy compared with those who received early dual therapy and those who received delayed dual therapy.
Electronic medical records were reviewed to extract the patients’ demographic information, vital signs, culture results, LOS in the hospital and/or ICU, duration of antibiotic therapy, and mortality. FN was defined in our institutional policy as a single oral temperature of ≥38.3°C (101°F) or ≥38°C (100.4°F) for at least 1 hour, with a neutrophil count of <500 cells/mm3 or <1000 cells/mm3 that was anticipated to decline. AKI was defined as an increase in serum creatinine of ≥0.3 mg/dL in 48 hours or a ≥50% increase from baseline within 7 days.8 Sepsis was defined as patients meeting 2 of the criteria on the Quick Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA).9
The study was approved by the local institutional review board with a waiver of patient-informed consent and HIPAA (Health Insurance Portability and Accountability Act) authorization.
All statistical analyses were implemented in R software version 3.6.3 (R Foundation for Statistical Computing; Vienna, Austria). Logistic models were built to investigate the differences in mortality, incidence of gram-negative bacteria, ICU admission, and AKI among the patients receiving cefepime monotherapy, early dual therapy, or delayed dual therapy. The variables that were insignificant or highly correlated with a treatment group were removed before the final models were reached. Overall and pairwise comparisons were conducted among the cohorts that were receiving treatment to evaluate for differences in the clinical and safety outcomes. Moreover, chi-square and Fisher exact tests were performed to examine the association between treatment and variables such as persistent fever. To further analyze the impact of treatment on the duration of tobramycin, LOS, and LOS in the ICU, a Kruskal-Wallis test and a Mann-Whitney U test with Bonferroni adjustment were conducted to explore the overall and pairwise differences among the cefepime monotherapy, early dual-therapy, and delayed dual-therapy cohorts.
Results
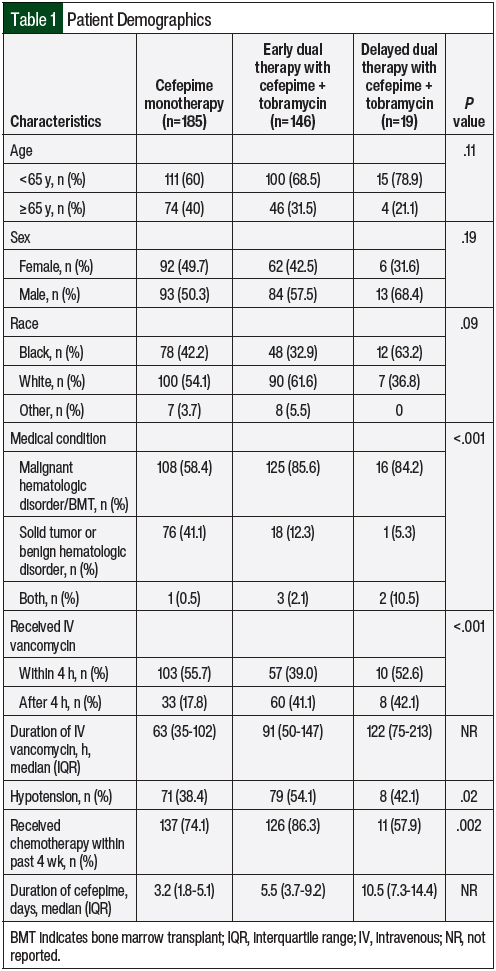
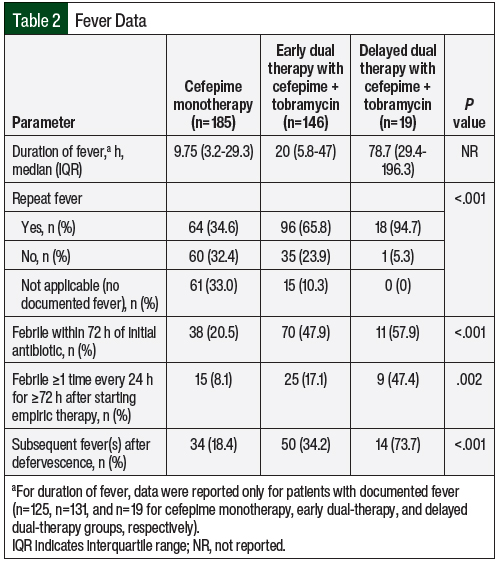
Between January 1, 2019, and July 31, 2020, a total of 350 patients who were diagnosed with FN in this study received cefepime. Among these patients, 185 (53%) received cefepime monotherapy, 146 (42%) received early dual therapy with cefepime plus tobramycin, and 19 (5%) received delayed dual therapy with cefepime plus tobramycin. The patients’ demographic information was similar among the cohorts. However, significant differences were seen in the patients’ baseline oncologic medical diagnoses, the concomitant use of IV vancomycin, and incidence of hypotension (Table 1). Data on fevers, such as duration of fever and repeat incidence of fever, are presented in Table 2.
In the patients who received dual therapy, a median of 1.9 hours and 77.4 hours were taken to add tobramycin treatment in the early- and delayed-therapy cohorts, respectively. The duration of tobramycin therapy in the early dual-therapy cohort was 43.2 hours versus 30 hours in the delayed dual-therapy cohort (P=.21). Treatment with tobramycin was discontinued after 2 doses (ie, 48 hours of coverage per institution’s extended interval dosing protocol) in 116 (79.5%) of the 146 patients in the early dual-therapy cohort and in 15 (78.9%) of the 19 patients in the delayed dual-therapy cohort.
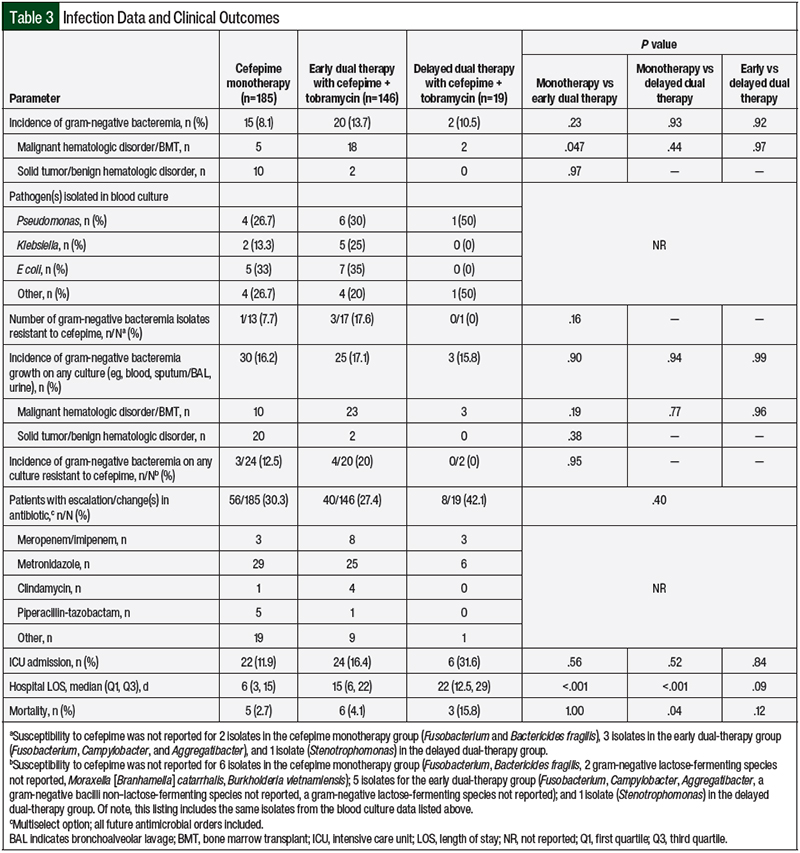
Of the 350 total study patients, 37 (10.6%) had gram-negative bacteremia (Table 3). Of the 37 patients with gram-negative bacteremia, 10 of the 12 patients who were diagnosed with a solid tumor or benign hematologic disorder were included in the cefepime monotherapy cohort, whereas 5 of the 25 patients who were diagnosed with a malignant hematologic disorder or who had a bone marrow transplant were included in the cefepime monotherapy cohort. Of the 37 total patients with gram-negative bacteremia, resistance to cefepime was documented in 4 (13%) of the 31 isolates that had susceptibility to cefepime. No significant differences were observed among the treatment cohorts with respect to the incidence of gram-negative bacteremia or the incidence of gram-negative bacteremia with documented resistance to cefepime (all P>.05; Table 3).
When all of the culture results were analyzed (eg, blood, sputum/bronchoalveolar lavage, fluid/abscess), neither the incidence of gram-negative pathogen growth nor the incidence of resistance to cefepime significantly differed among the cohorts (all P>.05; Table 3). There were no differences in the rate of escalation, change in antibiotic therapy, or rate of ICU admission among the 3 cohorts. Of the patients admitted to the ICU, the median LOS did not significantly differ among the cefepime monotherapy (n=22; 3.6±7.6), early dual-therapy (n=24; 2.0±7.9), and delayed dual-therapy groups (n=6; 4.1±4.5; all P>.05). Although no significant difference in the hospital LOS was found between the early and delayed dual-therapy cohorts (P>.05), the cefepime monotherapy cohort had a significantly shorter hospital LOS than the early and delayed dual-therapy cohorts (P<.05; Table 3).
When comparing the 3 cohorts using a 4-hour time point (ie, the initiation of tobramycin within 4 hours of receiving cefepime vs >4 hours after receiving cefepime) and controlling for differences between the groups, no significant differences were seen in the incidence of gram-negative bacteremia, transfer to the ICU, or mortality (all P>.05).
The incidence of AKI was similar in all 3 cohorts, at 7.4% in the cefepime cohort, 6.3% in the early dual-therapy cohort, and 6.6% in the delayed dual-therapy cohort (P>.05). The in-hospital mortality rates were 2.7%, 4.1%, and 15.8%, in the cefepime monotherapy, early dual-therapy, and delayed dual-therapy cohorts, respectively (P=.08). Mortality was significantly higher in the delayed dual-therapy cohort than in the cefepime-only cohort; however, there were no significant differences between the early dual-therapy cohort and the cefepime-only cohort or between the early and delayed dual-therapy cohorts (Table 3).
Discussion
In our review of these 350 patients with FN who were admitted to a large, academic medical center, adherence to institutional policy requiring dual coverage was low. The high rates of protocol deviation may be driven by prescriber familiarity with updated national guidelines1 to add the aminoglycoside to the treatment regimen only for specific indications. Patients with solid tumors or benign hematologic disorders were less likely than patients with malignant hematologic disorders or bone marrow transplant to receive dual therapy, which can indicate a perceived lower risk for severe FN. Notably, our institutional antibiogram data for the 2 years (2016-2017) preceding the implementation of the dual-coverage FN policy revealed susceptibility of P aeruginosa to cefepime of 82% and 85%, whereas the susceptibility for piperacillin-tazobactam was 96% and 83% in 2016 and 2017, respectively. During the period analyzed within this study of 2019 and 2020, the susceptibility rates of Pseudomonas to cefepime and piperacillin-tazobactam were consistent with the preprotocol period (79% and 87%, and 86% and 89% for each agent in 2019 and 2020, respectively).
Our institution uses cefepime as the preferred antipseudomonal β-lactam over piperacillin-tazobactam because of the increased risk for AKI with concomitant IV vancomycin, which is frequently used for the treatment of FN based on specific indications.1,10 Clinicians should consider the local antibiogram data when choosing antipseudomonal therapy.
There was no difference in mortality between the patients who received cefepime monotherapy and those who received initial early dual therapy with an aminoglycoside. However, the few patients who received aminoglycosides more than 48 hours after starting treatment with cefepime had a higher rate of in-hospital mortality than the other 2 cohorts. This difference could be attributed to variations in the patients’ baseline characteristics (eg, a diagnosis of malignant hematologic disorder/bone marrow transplant vs a solid tumor) or factors that were not captured in the scope of this study, such as a change in clinical status or other clinical factors. Therefore, the difference between the cefepime monotherapy and delayed dual-therapy cohorts cannot be extrapolated to imply the use of early dual antibiotic therapy, and these results should be interpreted with caution.
Supporting this assertion is that the incidence of gram-negative bacteremia was similar among all 3 of the cohorts, and there were no patients in the delayed dual-therapy cohort who had bacteremia caused by a gram-negative organism that was resistant to cefepime (Table 3). In addition, an escalation or change in antibiotic therapy occurred most frequently in the delayed dual-therapy cohort, which likely reflects interventions based on changing clinical status or updated microbiologic information. Further supporting this assertion, the most frequent antibiotic change among each cohort was the addition of metronidazole for anaerobic coverage rather than switching to another antipseudomonal agent.
The use of combination therapy for empiric antimicrobial coverage has been widely debated, but clinical data remain limited. An 11-year retrospective cohort study in France of 428 patients with cancer and neutropenia who were admitted to the ICU with sepsis showed that the combination of treatment with an aminoglycoside and early catheter removal improved survival.11 However, neither of these interventions were controlled nor randomized, which hindered the ability to draw conclusions from these results.11
The retrospective cohort study AMINOLACTAM evaluated patients with a hematologic malignancy and neutropenia who received monotherapy (with β-lactam, predominantly carbapenem) or dual combination therapy (β-lactam plus an aminoglycoside, predominantly amikacin) and showed an improved 7-day fatality rate with the combination therapy (13% vs approximately 6%, respectively).7 Notably, the rate of multidrug resistance in that study was high (approximately 28%), and the patients’ baseline demographics were not balanced between the groups: the monotherapy group had more comorbidities and were more likely to have diabetes, whereas the combination therapy group was more likely to present with septic shock.
Contrasting these results, a Cochrane review of 71 trials between 1983 and 2012 showed a trend toward improved survival, among other outcomes, for patients with FN who received monotherapy with an aminoglycoside as opposed to combination therapy with an aminoglycoside.12 Notably, this benefit was seen in subgroups with microbiologically documented infection, patients with bacteremia, patients with documented gram-negative infections, patients with infections caused by P aeruginosa, patients with hematologic cancer, and patients with severe neutropenia on admission.12 Despite these findings, it is common practice for patients with FN to receive dual therapy that includes an aminoglycoside. Our study adds to the literature supporting empiric antipseudomonal β-lactam monotherapy in most cases of FN.
In considering dual therapy with an aminoglycoside antibiotic for FN, safety must also be evaluated. One well-described adverse event of aminoglycosides is nephrotoxicity.13,14 Aminoglycoside-associated nephrotoxicity occurs via tubular necrosis that results from the retention of aminoglycosides in the epithelial lining of the proximal tubules. These events can be mitigated through extended-interval dosing and shortened durations of therapy.13,14
Our study showed no significant difference in the rates of AKI in patients who received aminoglycosides compared with those who did not receive aminoglycosides. This is likely a result of our institutional FN protocol calling for only 2 doses of tobramycin unless the growth of a gram-negative bacteria that is resistant to cefepime is determined on culture and because the protocol uses an extended-interval dosing strategy. Although some data suggest the suboptimal attainment of Cmax with first-dose aminoglycoside in this population,15 several studies support the once-daily administration of aminoglycosides,16-19 and practitioners should take into consideration that dual coverage is for synergy.
Despite the baseline differences among the cohorts with respect to the incidence of hypotension and the concomitant use of IV vancomycin, which are possible risk factors for AKI, there were no significant differences in the incidence of AKI among the cohorts in our study. This suggests that combination therapy with cefepime and tobramycin is safe, at least when performed according to a pharmacy-to-dose extended-interval protocol with a limited duration of aminoglycoside exposure, as is the standard of care at our institution.
Limitations
This study has several limitations. These include the use of self-reported fever at admission in some patients for the diagnosis of FN and reliance on the accuracy of documentation in the medical records. Occasionally, documentation was lacking for the rationale to use cefepime monotherapy off protocol, for the delayed addition of aminoglycoside treatment, or for an escalation or change in an antibiotic regimen.
Furthermore, the limited number of patients with gram-negative bacteremia, and therefore resistance to gram-negative bacteria, was low, which limited the data’s interpretation and application to institutions with higher rates of resistance. Therefore, generalizability to other institutions requires a comparison of the populations’ demographics and antibiogram data.
Conclusion
In conclusion, adherence to the institutional protocol for the empiric addition of tobramycin to cefepime treatment in adults at the onset of FN was low. Overall, dual therapy with tobramycin and cefepime did not improve patients’ clinical outcomes, and it is important to note that the cefepime monotherapy cohort did not have worse outcomes than the dual-therapy cohorts. Empiric monotherapy with an antipseudomonal β-lactam should remain the standard of care, with the addition of an aminoglycoside only in patients who meet specific guideline-recommended criteria of having a history or high suspicion of treatment resistance, hypotension, or pneumonia.
Based on these findings, our institution has changed back to monotherapy with cefepime for the empiric coverage of FN with the addition of an aminoglycoside for specific indications as stated above. The optimal antimicrobial therapy for FN at each institution should be dictated by local treatment resistance patterns and by the consideration of a risk-benefit analysis for the individual patient, as well as the long-term effects of increased antimicrobial use (eg, antimicrobial resistance). Further prospective and randomized studies that include specific patient populations should be encouraged to further elucidate the optimal empiric therapy for patients with FN.
Funding Source
The data have been generated as part of the routine work of Augusta University Medical Center, and internal funding from the University of Georgia College of Pharmacy was used for biostatistical analysis.
Author Disclosure Statement
Dr Clemmons, Dr Shelley, Dr Chittavong, Dr Hu, Dr Chen, Dr Eudy, Dr Chao, and Dr Anderson have no conflicts of interest to report.
References
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Prevention and Treatment of Cancer-Related Infections. Version 3.2022. October 28, 2022. www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed April 10, 2023.
- Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36:1443-1453.
- Lee NH, Kang JM, Lee JW, et al. Cefepime versus cefepime plus amikacin as an initial antibiotic choice for pediatric cancer patients with febrile neutropenia in an era of increasing cefepime resistance. Pediatr Infect Dis J. 2020;39(10):931-936.
- Zengin E, Sarper N, Çakı Kılıç S. Piperacillin/tazobactam monotherapy versus piperacillin/tazobactam plus amikacin as initial empirical therapy for febrile neutropenia in children with acute leukemia. Pediatr Hematol Oncol. 2011;28(4):311-320.
- Ponraj M, Dubashi B, Harish BH, et al. Cefepime vs. cefoperazone/sulbactam in combination with amikacin as empirical antibiotic therapy in febrile neutropenia. Support Care Cancer. 2018;26(11):3899-3908.
- Albasanz-Puig A, Gudiol C, Puerta-Alcalde P, et al. Impact of the inclusion of an aminoglycoside to the initial empirical antibiotic therapy for gram-negative bloodstream infections in hematological neutropenic patients: a propensity-matched cohort study (AMINOLACTAM Study). Antimicrob Agents Chemother. 2021;65(8):e00045-21.
- Kidney Disease Improving Global Outcomes. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl (2011). 2012;2(1):1-138.
- Marik PE, Taeb AM. SIRS, qSOFA and new sepsis definition. J Thorac Dis. 2017;9(4):943-945.
- Blair M, Côté JM, Cotter A, et al. Nephrotoxicity from vancomycin combined with piperacillin-tazobactam: a comprehensive review. Am J Nephrol. 2021;52(2):85-97.
- Legrand M, Max A, Peigne V, et al. Survival in neutropenic patients with severe sepsis or septic shock. Crit Care Med. 2012;40(1):43-49.
- Paul M, Dickstein Y, Schlesinger A, et al. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database Syst Rev. 2013(6):CD003038.
- Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43(5):1003-1012.
- Bailey TC, Little JR, Littenberg B, et al. A meta-analysis of extended-interval dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24(5):786-795.
- Blackburn LM, Tverdek FP, Hernandez M, Bruno JJ. First-dose pharmacokinetics of aminoglycosides in critically ill haematological malignancy patients. Int J Antimicrob Agents. 2015;45(1):46-53.
- Aiken SK, Wetzstein GA. Once-daily aminoglycosides in patients with neutropenic fever. Cancer Control. 2002;9(5):426-431.
- Preston SL, Briceland LL. Single daily dosing of aminoglycosides. Pharmacotherapy. 1995;15(3):297-316.
- Rice DAK. Once daily aminoglycosides. Clin Trends Pharm Pract. 1996;10:9-15.
- Freeman CD, Nicolau DP, Belliveau PP, Nightingale CH. Once-daily dosing of aminoglycosides: review and recommendations for clinical practice. J Antimicrob Chemother. 1997;39(6):677-686.