In 2021, the American Cancer Society estimated that approximately 248,530 patients would be diagnosed with prostate cancer and 34,130 patients would die from the disease in the United States.1 Prostate cancer is the most common malignancy among US men, accounting for 26% of all new cases of cancer and 11% of deaths from all cancer types in US men. In recent years, early detection and more advanced treatments have decreased the incidence of prostate cancer and have stabilized the death rate from this malignancy.2
For a long time, prostate cancer has been managed by surgery, radiation, and androgen-deprivation therapy (ADT), which includes luteinizing hormone-releasing hormone (LHRH) agonists and antagonists, and androgen receptor blockers, such as flutamide, nilutamide, and bicalutamide, for the prevention of tumor flare from LHRH agonists.2,3 Patients typically have an excellent initial response to these treatments; however, eventually, the disease progresses in most cases as a result of androgen receptor mutations, intratumoral androgen production, or adrenal gland testosterone production.3
To overcome resistance, second-generation antiandrogens have been introduced for the treatment of prostate cancer in recent years, including enzalutamide, apalutamide, and darolutamide. The primary goal of this review is to compare the pharmacology, efficacy, safety, and drug interactions of these 3 antiandrogens in the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) and metastatic castration-sensitive prostate cancer (CSPC).
The US Food and Drug Administration has approved apalutamide and enzalutamide for the treatment of metastatic CSPC and enzalutamide, apalutamide, and darolutamide for the treatment of nonmetastatic CRPC.4-6 (Of note, enzalutamide is also approved for the treatment of metastatic CRPC,6 which is outside the scope of this article.) The National Comprehensive Cancer Network guidelines classified all 3 of these antiandrogens as category 1 treatment options for metastatic CSPC and has also recommended apalutamide and enzalutamide as category 1 treatment options for nonmetastatic CRPC.2
Mechanism of Action
Enzalutamide, apalutamide, and darolutamide are second-generation antiandrogens that were developed after the first-generation antiandrogens flutamide, nilutamide, and bicalutamide. The first-generation antiandrogens inhibit the binding of testosterone and dihydrotestosterone to androgen receptor and interrupt the androgen-dependent cellular cascade that promotes prostate cancer growth.4 However, in CRPC, androgen receptor overexpression and androgen receptor gene mutations can occur and can induce agonist activity from the first-generation antiandrogens.7 In addition, changes in co-regulatory complexes, the development of androgen receptor variants, and altered steroidogenesis contribute to mechanisms of resistance in CRPC.8
To overcome these shortfalls, newer agents have been developed. The second-generation antiandrogen, enzalutamide, inhibits androgen binding to androgen receptors by blocking nuclear translocation of activated androgen receptors and by impeding the connection between activated androgen receptors and DNA.6 Enzalutamide also has 5 to 8 times higher androgen binding affinity to the androgen receptor than bicalutamide.9
Similar to enzalutamide, apalutamide inhibits nuclear translocation of the androgen–androgen receptor complex and inhibits binding with DNA.10 When apalutamide binds to the androgen receptor, it binds to the same ligand-binding site as bicalutamide, but it has a 7- to 10-fold increased affinity for the androgen receptor.10 Similar to enzalutamide and apalutamide, darolutamide also inhibits androgen receptor translocation to the nucleus.11 However, darolutamide has much higher binding to the androgen receptor, which leads to stronger suppression of androgen-induced cell growth and androgen receptor mutations.11
Pharmacokinetics
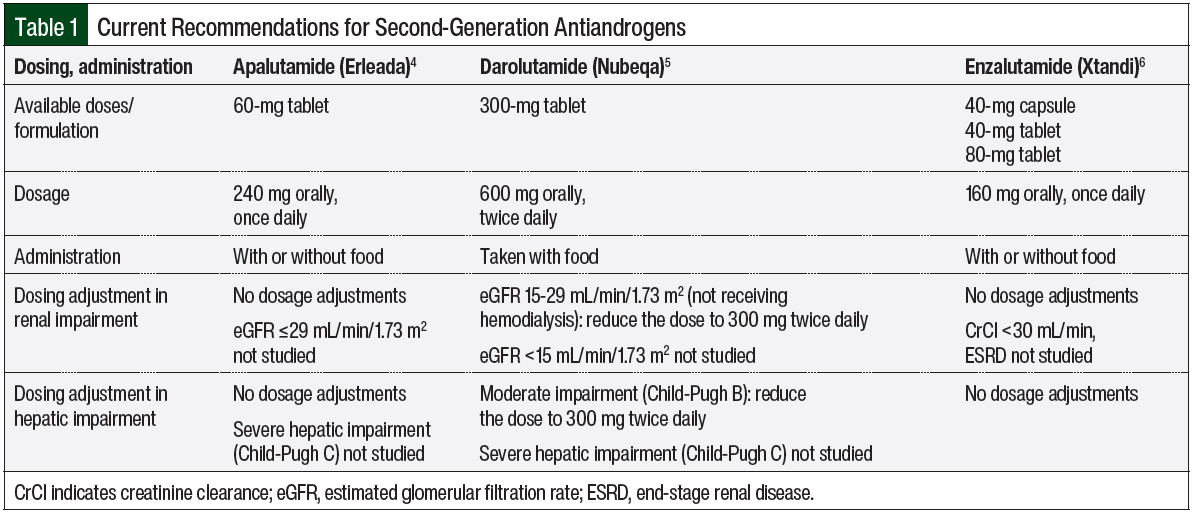
Clinical pharmacology comparison can be useful in selecting the appropriate antiandrogens for patients, because each agent has differences in absorption, metabolism, and elimination.2 Apalutamide is rapidly absorbed, with an oral bioavailability of 100%.4 Enzalutamide is also readily absorbed.6 By contrast, darolutamide has a bioavailability of approximately 30%, with a 2- to 2.5-fold increased bioavailability when taken with food.5 Consequently, enzalutamide and apalutamide can be administered with or without food, whereas darolutamide is recommended to be administered only with food.4-6
Enzalutamide and apalutamide are mainly eliminated by cytochrome (CY)P2C8 and CYP3A4 hepatic metabolism, with half-lives of 5.8 to 8.6 days and 3 days, respectively.4,6 Darolutamide is metabolized by CYP3A4, UDP-glucuronosyltransferase (UGT)1A9, and UGT1A1, with a much shorter half-life of approximately 20 hours.5 All agents are mainly excreted in urine and feces; Table 1 outlines the current recommendations for the administration of second-generation antiandrogens.4-6
For dose adjustment in patients with hepatic impairment, enzalutamide’s concentration for patients with mild (Child-Pugh A), moderate (Child-Pugh B), or severe (Child-Pugh C) baseline hepatic impairment is similar to that in patients with normal hepatic function.6 Apalutamide’s concentration in patients with mild or moderate hepatic impairment is similar to that in patients with normal hepatic function, but the pharmacokinetics of apalutamide are unknown in patients with severe hepatic impairment.4 Darolutamide has a higher concentration in patients with moderate hepatic impairment than in patients with normal hepatic function, and thus dose reduction is recommended; as in the case of apalutamide, the pharmacokinetics of darolutamide are unknown in patients with severe hepatic impairment.5
In patients with renal impairment, the clearance of enzalutamide and apalutamide are not affected by mild or moderate renal impairment, namely, the creatinine clearance (CrCl) is 30-<90 mL/min for enzalutamide, and the estimated glomerular filtration rate (eGFR) is 30-89 mL/min/1.73 m2 for apalutamide.4,6 For patients with severe renal impairment or end-stage renal disease (CrCl <30 mL/min for enzalutamide, and eGFR ≤29 mL/min/1.73 m2 for apalutamide), the pharmacokinetics are unknown.4,6
For darolutamide, a dose reduction is recommended for patients with severe renal impairment (eGFR, 15-29 mL/min/1.73 m2), because of a higher accumulation of the drug in this population.5 Otherwise, no dose reduction is recommended for darolutamide in patients with mild or moderate renal impairment, and the effect of end-stage renal disease on darolutamide’s pharmacokinetics is unknown.
It is also important to understand the differences in administration among the second-generation antiandrogens. Apalutamide and enzalutamide are dosed once daily, regardless of food intake, whereas darolutamide requires twice-daily dosing, with food.4-6 In addition, it is important to note that darolutamide has the smallest physical tablet size compared with apalutamide and enzalutamide. For patients with renal or hepatic impairment, darolutamide has dose adjustment recommendations.5
Drug Interactions
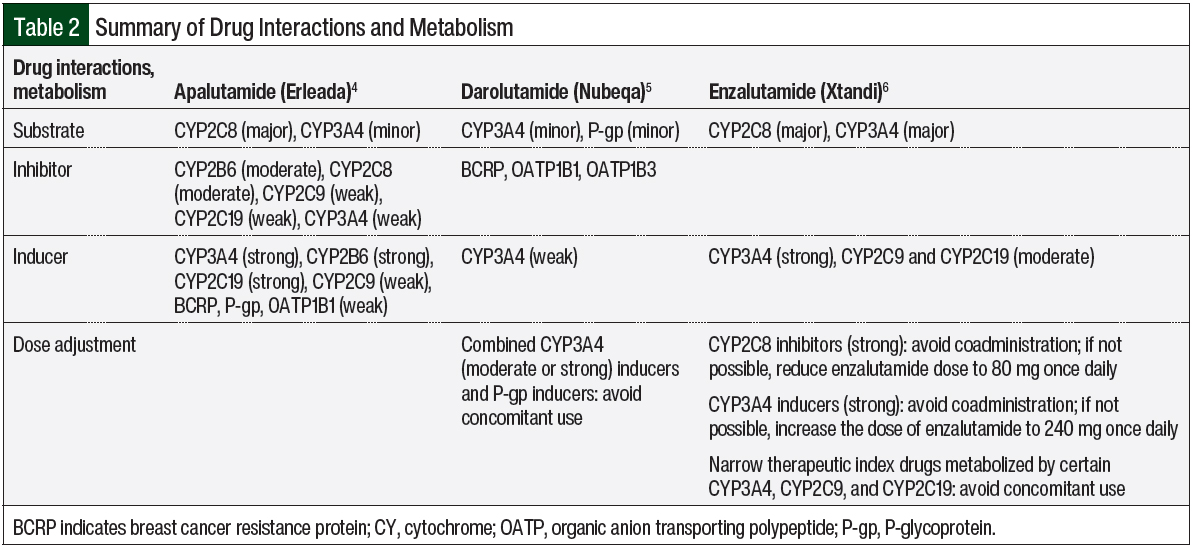
The data indicate that enzalutamide is a strong inducer of CYP3A4 and a moderate inducer of CYP2C9 and CYP2C19.6 Therefore, it is recommended to avoid the narrow therapeutic index of CYP3A4 (eg, cyclosporine, sirolimus), CYP2C9 (eg, phenytoin, warfarin), and CYP2C19 (eg, omeprazole) to prevent the decreased concentration of these substrates. Because enzalutamide is a major substrate of CYP3A4 and CYP2C8, a dose change of enzalutamide is recommended when strong CYP2C8 inhibitors or strong CYP3A4 inducers are used concomitantly with enzalutamide. A strong CYP2C8 inhibitor (eg, gemfibrozil) can increase the concentration of enzalutamide; therefore, reducing the dose of enzalutamide is recommended. In comparison, strong CYP3A4 inducers (eg, carbamazepine, phenytoin, rifampin) can decrease the concentration of enzalutamide; therefore, increasing the dose of enzalutamide is advised.6
Similar to enzalutamide, apalutamide is a substrate of CYP3A4 and CYP2C8.4 Despite these similarities, dose changes are not warranted for apalutamide because there was no major pharmacokinetic change with apalutamide when it was used with strong CYP3A4 or CYP2C8 inducers or inhibitors.4,12 The data indicate that apalutamide is a strong inducer of CYP3A4 and CYP2C19 and a weak inducer of CYP2C9, P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and organic anion transporting polypeptide 1B1 (OATP1B1).4 Apalutamide can lower the concentration of these substrates; therefore, close monitoring of patients is recommended. Similarly, apalutamide can decrease the concentration of UGT substrates. The same precautions should be applied for UGT substrates.4
Darolutamide is less affected by CYP450 drug–drug interactions than enzalutamide or apalutamide.5 The concomitant use of medications that are combined P-gp inducers and strong and moderate CYP3A4 inducers (eg, carbamazepine, phenytoin, rifampin, St John’s wort) should be avoided, because they can decrease the concentration of darolutamide. In contrast, combined P-gp and strong CYP3A4 inhibitors can increase the concentration of darolutamide; therefore, more frequent monitoring is recommended. In addition, darolutamide is an inhibitor of BCRP, OATP1B1, and OATP1B3. Consequently, the concomitant use of darolutamide with BCRP, OATP1B1, and OATP1B3 substrates can increase the concentration of these substrates. Therefore, more frequent monitoring for adverse events related to these substrates is recommended.5
When comparing drug–drug interactions, darolutamide has the least CYP metabolism versus apalutamide and enzalutamide; therefore, fewer drug interactions occur with darolutamide than with apalutamide or enzalutamide.4-6 Table 2 summarizes the drug interactions and the metabolism of apalutamide, darolutamide, and enzalutamide.4-6
Efficacy in Nonmetastatic CRPC
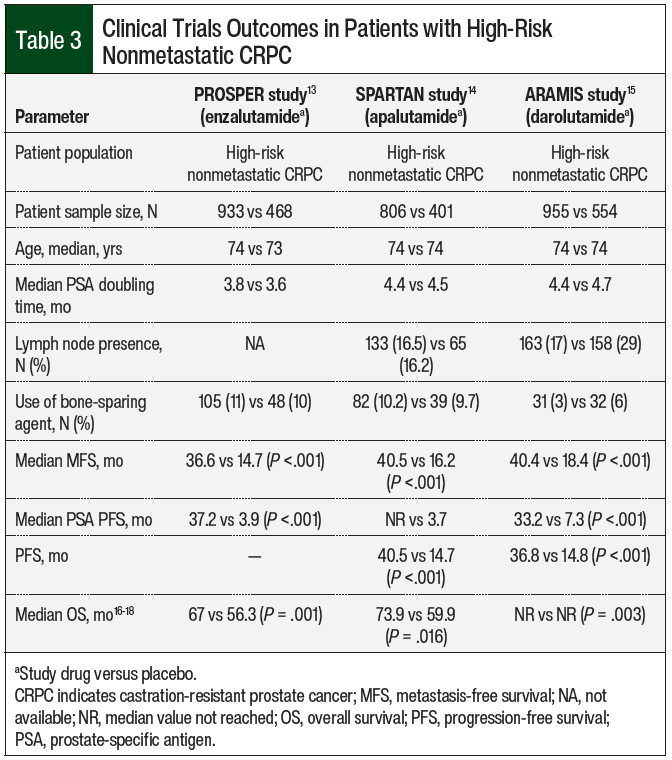
A randomized phase 3 clinical trial was conducted for each antiandrogen agent that demonstrated their efficacy in patients with nonmetastatic CRPC compared with patients receiving placebo, including the PROSPER trial for enzalutamide, the SPARTAN trial for apalutamide, and the ARAMIS trial for darolutamide (Table 3).13-15
All 3 studies were phase 3, international, double-blind, randomized, and placebo-controlled trials. The studies had similar patient populations with high-risk nonmetastatic CRPC, a median age of 74 years, and median prostate-specific antigen (PSA) doubling times of 3.8 to 4.4 months (Table 3). Of note, all 3 studies required a PSA doubling time of 10 months or less.
The primary end point for these studies was metastasis-free survival (MFS), and the secondary end points included the time to progression-free survival (PFS), PSA progression, and overall survival (OS).13-15 In the PROSPER study, the median MFS was 36.6 months in the enzalutamide group and 14.7 months in the placebo group at the first interim analysis (hazard ratio [HR], 0.29; 95% confidence interval [CI], 0.24-0.35).13 As for the secondary end points, the median time to PSA progression was 37.2 months in the enzalutamide arm versus 3.9 months in the placebo arm (HR, 0.07; 95% CI, 0.05-0.08).13 In the final analysis, the OS was 67 months and 56.3 months, respectively (HR, 0.73; 95% CI, 0.61-0.89).16
In the SPARTAN study, the median MFS was 40.5 months in the apalutamide group and 16.2 months in the placebo group (HR, 0.28; 95% CI, 0.23-0.35).14 The median time to metastasis was 40.5 months with apalutamide and 16.6 months with placebo (HR, 0.27; 95% CI, 0.22-0.34). The median time to PSA progression was not reached with apalutamide versus 3.7 months for the placebo group (HR, 0.06; 95% CI, 0.05-0.08).14 In the final analysis, the OS was 73.9 months in the apalutamide group and 59.9 months in the placebo group (HR, 0.78; 95% CI, 0.64-0.96).17
In the ARAMIS study, the median MFS was 40.4 months with darolutamide and 18.4 months with the placebo (HR, 0.41; 95% CI, 0.34-0.50).15 The median PFS was 36.8 months with darolutamide and 14.8 months with the placebo (HR, 0.38; 95% CI, 0.32-0.45).15 In the final analysis, OS was not reached in both groups (HR, 0.69; 95% CI, 0.53-0.88).18
For the nonmetastatic CRPC population, it is important to identify the PSA doubling time, which will determine the treatment decision.13-15 If the PSA doubling time is 10 months or less, the second-generation antiandrogens can be a good treatment option. In the PROSPER, SPARTAN, and ARAMIS clinical trials, the use of second-generation antiandrogens significantly increased the MFS and OS compared with placebo in patients with nonmetastatic CRPC.
Efficacy in Metastatic CSPC
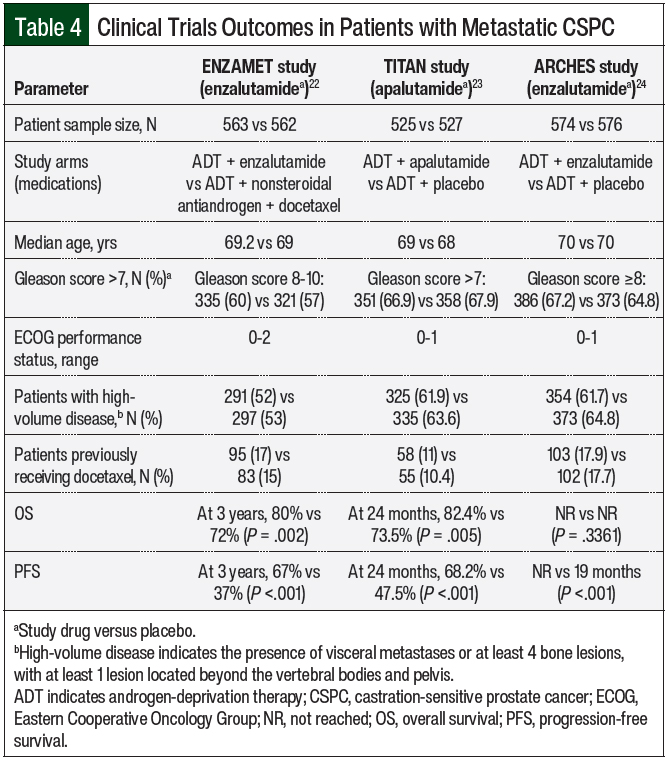
Various treatment options are available for metastatic CSPC in conjunction with ADT, including docetaxel, abiraterone, and antiandrogens, that have shown significant survival benefits.2,19-21 Among these options, 2 antiandrogens—enzalutamide and apalutamide—were studied for the treatment of metastatic CSPC (Table 4).22-24
In the ENZAMET trial, enzalutamide plus ADT was compared with ADT plus nonsteroidal antiandrogens (ie, flutamide, bicalutamide, or nilutamide).22 Early treatment with docetaxel was allowed per the physician’s discretion. Up to 2 cycles of docetaxel could be administered before randomization.22 The ARCHES trial evaluated treatment with enzalutamide plus ADT compared with placebo plus ADT.24 In the TITAN trial, treatment with apalutamide plus ADT was compared with ADT plus placebo.23
All of these studies were phase 3, multinational, randomized, and placebo-controlled trials.22-24 ENZAMET was an open-label trial, whereas TITAN and ARCHES were double-blind trials. These 3 studies had similar baseline patient characteristics in median age, Gleason score, percentage of high-volume disease—defined as the presence of visceral metastases or at least 4 bone lesions, with at least 1 lesion located beyond the vertebral bodies and the pelvis—and previous docetaxel use. Of note, the ENZAMET trial included Eastern Cooperative Oncology Group (ECOG) performance status scores of 0, 1, and 2, whereas the TITAN and ARCHES trials only included ECOG performance status scores of 0 and 1.22-24
The ENZAMET trial showed a significant improvement in OS with enzalutamide compared with placebo (HR, 0.67; 95% CI, 0.52-0.86) and a significant improvement in PFS (HR, 0.39; 95% CI, 0.33-0.47).22 Similarly, the ARCHES trial showed a significant improvement in PFS with enzalutamide compared with placebo (HR, 0.39; 95% CI, 0.3-0.5), regardless of disease volume and previous docetaxel use.24 At the time of analysis, the OS data were immature in the ARCHES trial and longer follow-up was needed.24
The TITAN trial showed significant benefits in OS (HR, 0.67; 95% CI, 0.51-0.89) and PFS (HR, 0.48; 95% CI, 0.39-0.6) with the use of apalutamide versus placebo.23 In the subgroup analyses, enzalutamide and apalutamide demonstrated significant improvements of PFS in patients with low- and high-volume disease.22,23
In patients with metastatic CSPC, a study of darolutamide was recently published, comparing the OS with the combination of darolutamide, docetaxel, and ADT versus the combination of ADT and docetaxel.25 In the primary analysis, the darolutamide group showed a significant improvement in OS. At 4 years, the OS was 62.7% in the darolutamide arm versus 50.4% in the placebo arm (P <.001).25
Overall, for patients with metastatic CSPC, ADT alone is no longer the standard-of-care therapy, as a result of the significant benefits seen with other treatment options, including second-generation antiandrogens.22-25 Antiandrogens can be a good treatment choice for patients with low- or high-volume diseases, whereas docetaxel is more appropriate for patients with high-volume disease.2 In the ENZAMET and TITAN trials, enzalutamide and apalutamide showed significant benefits in PFS and OS in patients with metastatic CSPC.22,23 The ARCHES trial also showed a significant PFS improvement with enzalutamide treatment in this population, but the OS data were still immature.24
Safety
A drug’s side-effect profile should also be considered when choosing a treatment. Although there are no significant differences in adverse events among apalutamide, darolutamide, and enzalutamide, darolutamide has lower risks for some events, such as fatigue, falls, rash, fracture, and seizure.13-15 In addition, drug discontinuation resulting from adverse events was the lowest with darolutamide, although this difference was not statistically significant.13-15
Adverse events and safety for these 3 medications have been assessed in clinical trials in comparison with placebo.16-18 In the PROSPER study of enzalutamide, the most common adverse events were fatigue (46%), musculoskeletal events (34%), fractures (18%), hypertension (18%), and falls (18%).16 In the SPARTAN study of apalutamide, the most common adverse events were fatigue (33%), hypertension (28%), rash (26%), falls (22%), and fracture (18%).17 In the ARAMIS study of darolutamide, the most common adverse event was fatigue (13.2%).18
In a retrospective clinical trial of apalutamide, darolutamide, and enzalutamide and central nervous system (CNS) adverse events, darolutamide had approximately 26 and 46 times lower blood–brain barrier penetration than apalutamide and enzalutamide, respectively, resulting in less CNS adverse events, including seizures.26 Although the incidence of seizures was not statistically significant, patients who received enzalutamide or apalutamide had an increased number of seizures compared with the placebo group, whereas patients who received darolutamide had the same incidence of seizures as the patients who received placebo.16-18 Of note, the ARAMIS study included patients with a history of seizures, whereas the PROSPER and SPARTAN trials did not.16-18
Although rare, the risk for cardiovascular events was increased by antiandrogen use, with relative risk of 2.125, 4.466, and 2.71 in the PROSPER, SPARTAN, and ARAMIS studies, respectively.27 Similarly, fall risk was increased significantly with antiandrogen use, with relative risk of 2, 3.139, and 1.161 in the PROSPER, SPARTAN, and ARAMIS trials, respectively. Fracture risk was not reported in the PROSPER trial; however, the SPARTAN and the ARAMIS trials showed a significant increase in fracture risk compared with placebo, with relative risk of 4.130 and 1.045, respectively. Rash was significantly higher in the SPARTAN trial than in the ARAMIS trial (relative risk, 20.817 vs 1.743, respectively).27 Posterior reversible encephalopathy syndrome has been reported with enzalutamide treatment; therefore, precaution should be taken when taking enzalutamide.6
In addition, all 3 antiandrogens may cause infertility in males, which is an important counseling point before patients start therapy with any of these drugs.4-6
Overall, the incidence of common adverse events (frequency >10%) and grade ≥3 adverse events was comparable for all 3 agents.4-6 The incidence of adverse events leading to drug discontinuation was also similar in all 3 antiandrogens, with darolutamide having the lowest incidence of events leading to treatment discontinuation.4-6
Conclusion
The treatment options for prostate cancer have evolved over the past decades. Newer antiandrogen therapies have broadened the spectrum of treatment choice for patients. For the metastatic CSPC population, ADT alone is no longer a standard-of-care therapy; antiandrogens can be a good treatment choice for patients with low- or high-volume disease, whereas docetaxel is more appropriate for patients with high-volume disease.
With the significant benefits seen with antiandrogen agents, it is important to understand the differences among the second-generation antiandrogens of apalutamide, darolutamide, and enzalutamide. The drug’s side-effect profile should also be considered when choosing a treatment. By comparing the properties and characteristics of the drug profiles of apalutamide, darolutamide, and enzalutamide, clinicians will be able to determine the efficacy and safety of these antiandrogens, and will be able to make individualized decisions based on the patient’s disease stage, comorbidities, performance status, concomitant medication use, drug cost, and compliance.
Author Disclosure Statement
Dr Park, Dr Yoder, and Dr Li have no conflicts of interest to report.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33. Erratum in: CA Cancer J Clin. 2021;71:359.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Prostate Cancer. Version 3.2022. January 10, 2022. www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed March 22, 2022.
- Rice MA, Malhotra SV, Stoyanova T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front Oncol. 2019;9:801. doi: 10.3389/fonc.2019.00801.
- Erleada (apalutamide) tablets, for oral use [prescribing information]. Janssen Pharmaceutical Companies; September 2021. www.janssenlabels.com/package-insert/product-monograph/prescribing-information/ERLEADA-pi.pdf. Accessed March 22, 2022.
- Nubeqa (darolutamide) tablets, for oral use [prescribing information]. Bayer HealthCare Pharmaceuticals; January 2021. https://labeling.bayerhealthcare.com/html/products/pi/Nubeqa_PI.pdf. Accessed March 22, 2022.
- Xtandi (enzalutamide) capsules/tablets, for oral use [prescribing information]. Astellas Pharma US; January 2022. www.astellas.us/docs/us/12A005-ENZ-WPI.pdf. Accessed March 22, 2022.
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276-308.
- Chandrasekar T, Yang JC, Gao AC, Evans CP. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl Androl Urol. 2015;4:365-380.
- Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787-790.
- Chong JT, Oh WK, Liaw BC. Profile of apalutamide in the treatment of metastatic castration-resistant prostate cancer: evidence to date. Onco Targets Ther. 2018;11:2141-2147.
- Crawford ED, Stanton W, Mandair D. Darolutamide: an evidenced-based review of its efficacy and safety in the treatment of prostate cancer. Cancer Manag Res. 2020;12:5667-5676.
- Van den Bergh A, Snoeys J, De Zwart L, et al. Pharmacokinetic drug–drug interaction of apalutamide, part 2: investigating interaction potential using a physiologically based pharmacokinetic model. Clin Pharmacokinet. 2020;59:1149-1160.
- Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465-2474.
- Smith MR, Saad F, Chowdhury S, et al; for the SPARTAN investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
- Fizazi K, Shore N, Tammela TL, et al; for the ARAMIS investigators. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235-1246.
- Sternberg CN, Fizazi K, Saad F, et al; for the PROSPER investigators. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197-2206.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79:150-158.
- Fizazi K, Shore N, Tammela TL, et al; for the ARAMIS investigators. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383:1040-1049.
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080-1087.
- Gravis G, Boher JM, Joly F, et al; for the GETUG. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70:256-262.
- Hoyle AP, Ali A, James ND, et al; for the STAMPEDE investigators. Abiraterone in “high-” and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol. 2019;76:719-728.
- Davis ID, Martin AJ, Stockler MR, et al; for the ENZAMET trial investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121-131.
- Chi KN, Agarwal N, Bjartell A, et al; for the TITAN investigators. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13-24.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974-2986.
- Smith MR, Hussain M, Saad F, et al; for the ARASENS trial investigators. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386:1132-1142.
- Zurth C, Sandman S, Trummel D, et al. Higher blood–brain barrier penetration of [14C]apalutamide and [14C]enzalutamide compared to [14C]darolutamide in rats using whole-body autoradiography. J Clin Oncol. 2019;37(7_suppl):Abstract 156.
- Di Nunno V, Mollica V, Santoni M, et al. New hormonal agents in patients with nonmetastatic castration-resistant prostate cancer: meta-analysis of efficacy and safety outcomes. Clin Genitourin Cancer. 2019;17:e871-e877.