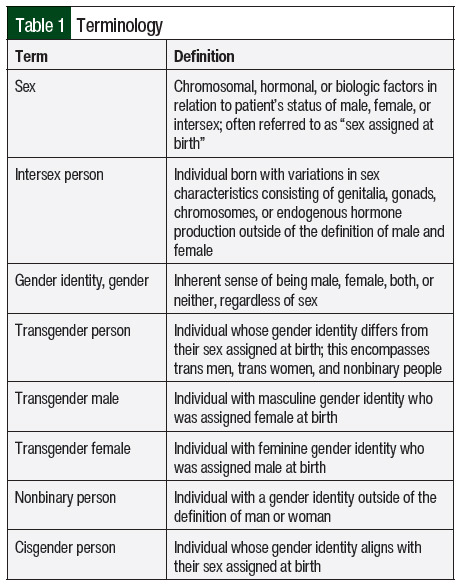
The worldwide transgender population is estimated to be 25 million people, with the United States having an estimated 1.6 million people identifying as transgender.1,2 Transgender people aged between 13 and 17 years make up 1.4% of the US population, whereas those aged ≥18 years make up 0.5% of the US population.2 Transgender patients face multiple barriers in accessing appropriate and culturally competent healthcare, which can result in poor health outcomes and higher rates of complications, such as substance abuse, infections, mental health conditions, and cancer.3,4 In addition, transgender adults have more chronic and comorbid conditions that may require medication therapy compared with cisgender adults.3 Various reports have shown that medical professionals have minimal training evaluating and treating transgender patients.3,5 Furthermore, there are often misconceptions regarding the appropriate terminology to use when interacting with transgender patients (Table 1).
As transgender individuals age, there is a growing initiative to understand the incidence and documentation of chronic diseases, such as cancer.2,6,7 In addition, there is an underrepresentation or nonrepresentation of transgender people in population surveys and clinical trials, which hinders our ability to fully understand the health disparities and the medical safety and efficacy outcomes that transgender patients face.8 This lack of representation in real-world data and clinical trials that include transgender patients receiving chemotherapy and undergoing hematopoietic stem-cell transplantation (HSCT) limits our understanding for effective medical management in this population. Medications used for HSCT, including chemotherapy and immunosuppression therapy, require precise dosing and therapeutic drug monitoring. Dosing and therapeutic drug monitoring can be impacted by gender-affirming therapy because it produces changes in body composition, drug metabolizing enzyme activity, and renal function, which may influence sex-related differences in medications.9 This review aims to increase the knowledge about medication therapy management, drug metabolism changes, drug interactions, and adverse events with medications used in HSCT and gender-affirming therapy.
Hormone Therapy
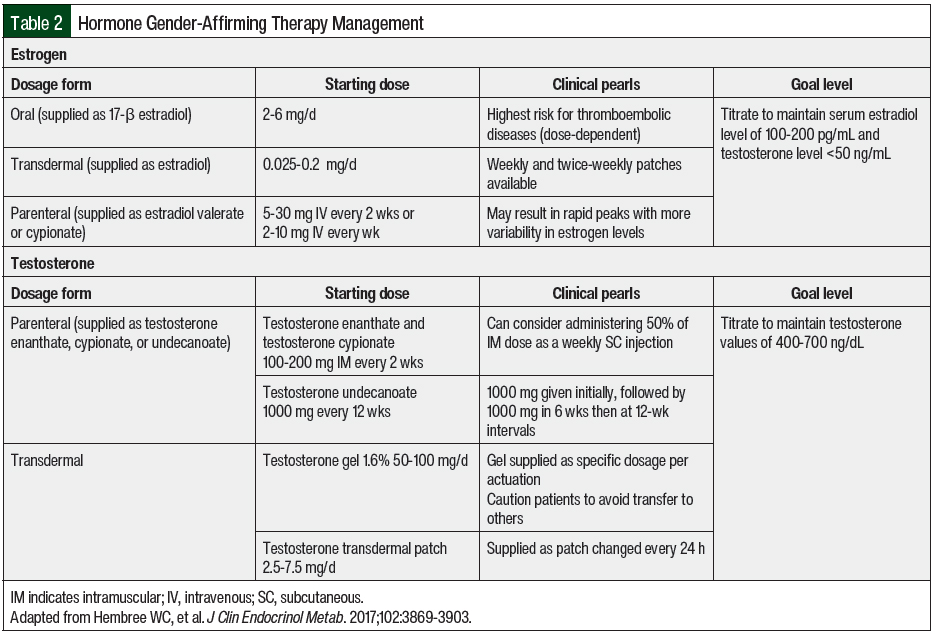
Hormone therapy is considered a medically necessary treatment for transgender adults seeking gender-affirming therapy. Although individual targets of hormone therapy may differ among individuals, the overall goals of therapy are to decrease endogenous sex hormones, reduce secondary sex characteristics, and increase exogenous sex hormones to maintain hormone levels consistent with gender identity.10-12 In adolescents, goals may also include suppressing pubertal development to minimize secondary sex characteristics. Hormone therapy is often initiated after an individual has undergone a psychosocial assessment and screening for medical issues, as well as an assessment for social barriers that could impair adherence to treatment.10 Specific dosing recommendations, formulations, and the desired target levels for hormone therapy are shown in Table 2.
Estrogen therapy is frequently used by transgender females to increase serum estradiol concentrations. In addition to estrogen, antiandrogen medications are also used to suppress endogenous testosterone levels. Estrogen administered as 17β-estradiol has a lower risk for venous thromboembolism compared with other estrogen preparations, such as ethinyl estradiol, and can be administered orally, topically, or parenterally.10,13 In addition to estradiol, which frequently cannot suppress endogenous testosterone significantly, other medications are concomitantly used for antiandrogen activity. Spironolactone and gonadotropin-releasing hormone analogs can be used to reduce testosterone levels in these patients.11 Transgender males may receive androgen therapy, frequently in the form of testosterone, to increase serum testosterone to a value that is similar to cisgender males. Testosterone can be administered parenterally or topically.10 Unlike estrogen therapy in transgender females, testosterone alone is enough to suppress endogenous estrogen production.14 Therefore, antiestrogen medications are not routinely used in transgender males.
During the first year of hormone therapy, patients should be monitored every 3 months to assess serum testosterone and estradiol levels, as well as advancing signs of feminization or masculinization. After 1 year of therapy, laboratory monitoring may be spaced every 6 to 12 months. Goal serum testosterone values in transgender men are levels in the normal physiologic male range (400-700 ng/dL). Sustained supraphysiologic concentrations of serum testosterone (>1000 ng/dL) may increase the risk for adverse events, such as hypertension, hyperlipidemia, and erythrocytosis.10 The goal levels for transgender females are serum testosterone levels of <50 ng/dL and serum estradiol levels of 100 to 200 pg/mL.10
The onset of feminizing or masculinizing effects of hormone therapy differs among patients and specific physical changes, with most patients seeing some effect in as early as 1 to 6 months of therapy.10 Physical changes for transgender males, such as oily skin, facial hair growth, fat redistribution, and the cessation of menses can occur as quickly as 1 month. In general, the effects of estrogen in transgender females happen within 3 months and include breast growth, decrease in muscle mass, and decreased testicular volume. Optimal physical changes can range from 1 to 5 years, depending on the effect.10 Some effects of hormone therapy, such as breast growth or altered fertility, may not be reversible, whereas other effects may be reversible depending on the duration of treatment.
Hormone therapy with estrogen or testosterone carries risks that should be considered in individual patients before initiation. Transgender females who are receiving estrogen therapy have an increased risk for thromboembolic events, which should be weighed carefully in patients with other risk factors or a history of thrombus.10,11 Estrogen can also increase the risk for breast cancer, cardiovascular disease, hypertriglyceridemia, and prolactinomas.10,12 Transgender males who initiate testosterone therapy are also at risk for significant side effects. Erythrocytosis is a very common side effect of testosterone, but it may not be relevant in patients who have had an HSCT, for whom anemia is common.10-12
Considerations for the HSCT Process
Body Composition and Metabolism
With the use of gender-affirming therapy, significant changes in drug metabolism and drug disposition can occur.9 Physiologic differences between cisgender men and women are demonstrated in height, weight, and body mass index (BMI), as well as in nonhormonal laboratory values, including serum creatinine (SCr), hemoglobin, and uric acid.3 In addition, there are pharmacokinetic and pharmacodynamic differences between cisgender women and men, including body composition, protein binding, and metabolism.3,9
Body composition can result in differences in drug dispositions based on sex and gender.3 Cisgender women typically have more total body fat, which increases the volume of distribution of lipophilic drugs.3 Cisgender males typically have more lean body mass (LBM), which increases the volume of distribution of hydrophilic drugs.3 Hormonal gender-affirming therapy has been shown to alter body composition. In a meta-analysis by Klaver and colleagues, there was an 1.8-kg increase in total body weight (95% confidence interval [CI], 0.2-3.4), a 3-kg increase in fat (95% CI, 2-3.9; P<.05), and a 2.4-kg decrease in LBM (95% CI, 2.1-2.8, P<.01) in transgender women receiving estrogen therapy.15 Visceral (17.5%) and abdominal subcutaneous fat (57%) increased (P=.01) along with BMI (6%) compared with baseline.9 In transgender men receiving testosterone therapy, the LBM increased by 3.9 kg (95% CI, 3.2-4.5) whereas the total body fat decreased by 2.6 kg (95% CI, 1.4-3.9; P<.01).9,15
Alterations in body composition occurred within 12 months of treatment initiation.15 Although body composition changes with gender-affirming hormone therapy, the clinical implications in drug distribution are not known to be significant.9 It is noted that women have more severe adverse drug events to medications, which is thought to result from differences between the pharmacokinetics of women and men.3 It is unclear whether these variations are directly related to hormone production or genetic variation.3 Additional studies in pharmacokinetics and pharmacodynamics in transgender individuals are needed to determine the significance of these differences in gender and their impact on dosing. For weight-based dosing considerations with chemotherapy and supportive care medications, the individual’s current weight regardless of hormonal therapy should be used except in obese individuals where specific chemotherapy dose modifications are recommended based on BMI, as per the American Society for Transplantation and Cellular Therapy guidelines.3,16
Serum protein-binding proteins, albumin, alpha 1-acid glycoprotein, and globulins can influence free drug exposure when their concentrations are altered.9,17 Clinically significant changes in protein-binding interactions can contribute to an increase in free drug exposure. In transgender men receiving testosterone therapy, serum albumin concentrations were unchanged whereas corticosteroid-binding globulin and sex hormone–binding globulin concentrations decreased.9 In transgender women receiving estrogen therapy, serum albumin slightly decreased, corticosteroid-binding globulin concentrations remained similar to those before estrogen therapy, and sex hormone–binding globulin increased by 3-fold.9 Although changes in plasma drug binding can increase or decrease with the introduction of hormonal gender-affirming therapy, such changes do not result in a significant difference in the available free drug.9
Kidney Elimination
Kidney function uses glomerular filtration, tubular secretion, and tubular reabsorption for the clearance of medications.9 Overall kidney function can be measured using a glomerular filtration rate (GFR) or an SCr-based equation such as the Modification of Diet in Renal Disease (MDRD) or the Cockcroft-Gault formula for creatinine clearance (CrCl). GFR in cisgender women is slightly lower than in cisgender men, often by approximately 10% after adjusting for body surface area.9 In addition, during pregnancy GFR can increase 5-fold compared with postpartum levels.9 There are few studies that focus on kidney function in transgender adults. A retrospective cohort of transgender adults receiving estrogen therapy for at least 1 year showed similar GFR before and during estrogen therapy.18 In transgender males receiving testosterone, the GFR decreased during testosterone therapy compared with baseline.18 Using the MDRD and Cockcroft-Gault formulas, which include sex assignment at birth, the SCr increased with testosterone therapy and decreased with estrogen therapy (P=.001).18 The GFR and MDRD demonstrate limitations in transgender patients that result from the significant changes in kidney function that result from long-term hormonal therapy, which impacts drug clearance.9
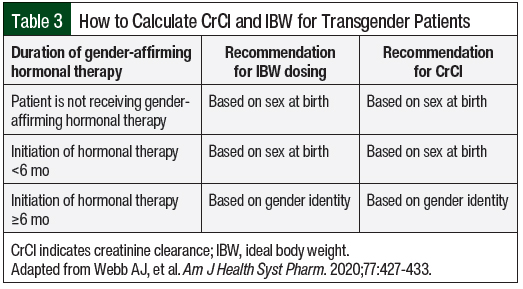
The Cockcroft-Gault equation is frequently used to dose medications, using an empiric reduction for women based on gender differences in fat and muscle distribution. Ideal body weight (IBW) is incorporated into the Cockcroft-Gault equation, which also adjusts for the sex of the patient.19 IBW is frequently used for dosing hydrophilic medications for a more precise measurement of the volume of distribution.20 In transgender individuals, the effect of hormonal gender-affirming therapy can influence muscle and fat distribution, changing CrCl and IBW.3 A recent review article examined LBM, BMI, and SCr at different time points of hormonal therapy to determine the appropriate time frame to use in these calculations.3 This article, like the ones above, show that SCr more closely reflects the affirmed gender as early as 4 months after the initiation of hormonal therapy.3 In LBM, there is not a significant difference in SCr in transgender women, but there is an increase in SCr in transgender men reflecting a higher IBW.21
This article using GFR, MDRD, and CrCl show that after hormone therapy is initiated, transgender patients more closely reflect their gender identity than their sex at birth.3 When calculating CrCl and IBW, the recommended time interval to incorporate gender identity versus sex at birth after the initiation of hormonal gender-affirming therapy is as early as 4 months, but ideally after 6 months.3 This time interval mimics the physiologic transition in transgender men, with amenorrhea frequently occurring at 6 months.22 The guideline in Table 3 was created to determine IBW and CrCl based on the duration of hormonal gender-affirming therapy.3 The use of this guideline for IBW and CrCl calculations can then be incorporated into dose recommendations for chemotherapy and supportive care from the drug’s prescribing information and institutional renal dose adjustment standards. Electronic medical record software should be optimized for transgender patients by providing alerts for a gender mismatch to allow for appropriate pharmacist assessment. This information can then be incorporated into the rules for calculating IBW and CrCl, which then allows for appropriate renal dose adjustments in real time.3
Phase I Metabolism
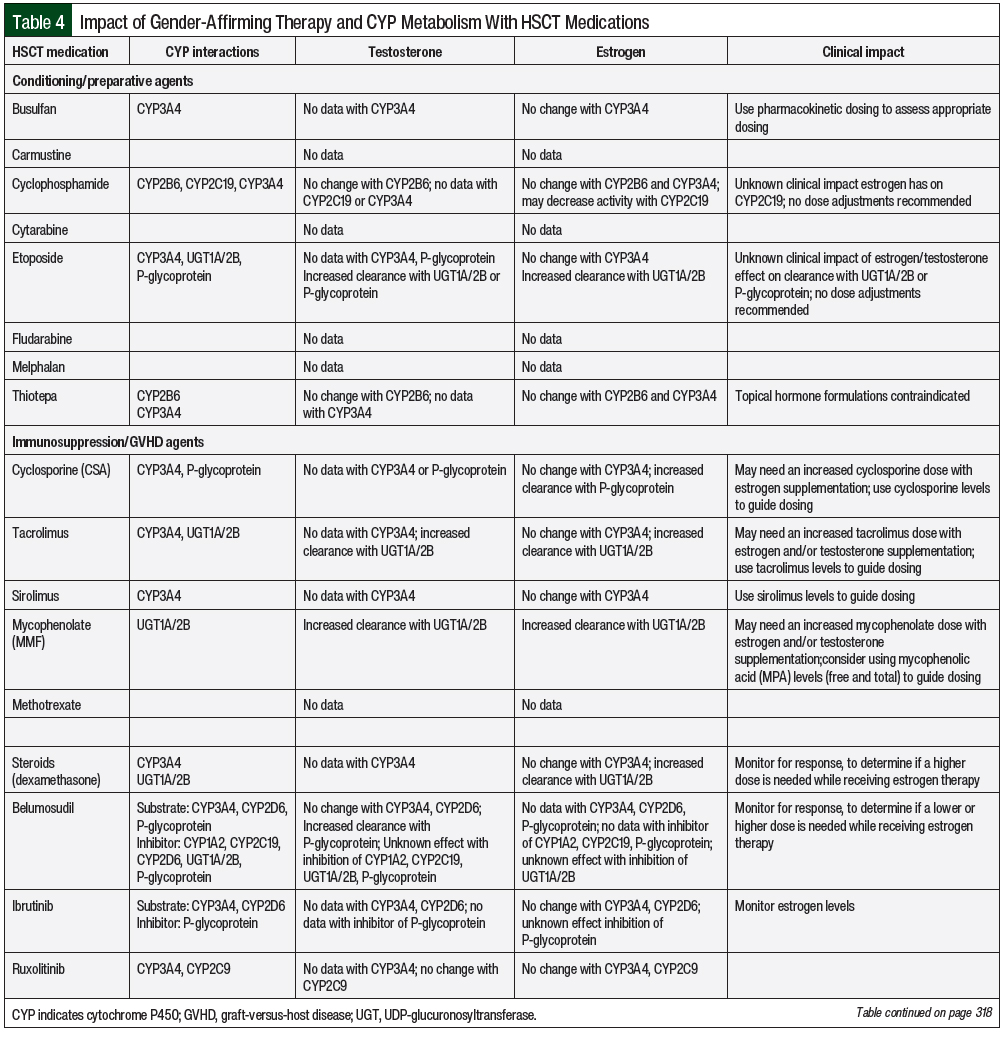
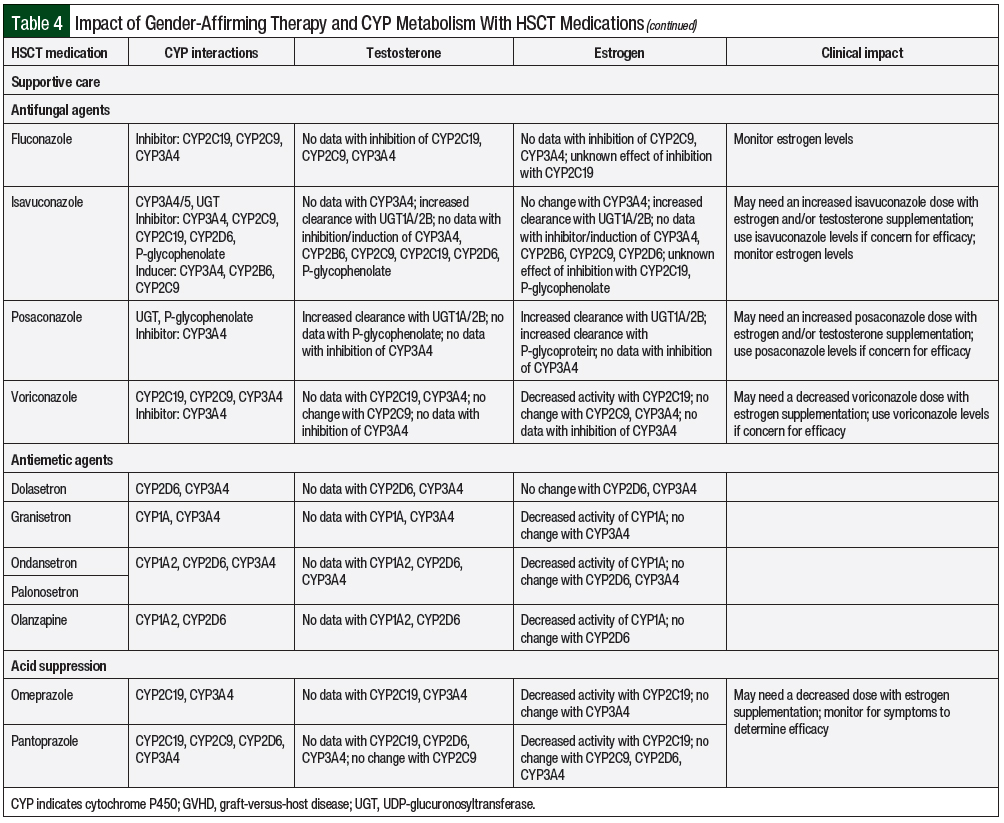
The liver cytochrome P450 (CYP) metabolic system is the primary site for drug metabolism. In the cisgender population, sex-related CYP metabolism has demonstrated differences based on the levels of hormones present.9 This has also been demonstrated with pregnancy-related altered CYP metabolism coinciding with hormone fluctuations.9 In transgender adults, there is a lack of data on the influence of hormonal therapy on CYP metabolism, with most recommendations extrapolated from cisgender patient data. Transgender adults undergoing HSCT could have changes in drug metabolism during hormone therapy; however, the clinical significance of these changes is unknown. Potential drug interactions from CYP metabolism for chemotherapy regimens and supportive care for undergoing HSCT are summarized in Table 4.
CYP1A2
Sex gender differences in CYP1A2 reveal that cisgender women have lower activity of this enzyme than cisgender men.9 This is also observed in cisgender women with elevated levels of estrogen in pregnancy, estrogen replacement therapy, and oral contraceptive therapy where CYP1A2 activity is inhibited.9
The effect of testosterone therapy on CYP1A2 activity is not known. Lifestyle habits, such as cigarette smoking, can induce CYP1A2 activity regardless of hormonal supplementation. Supportive care medications used in HSCT, including olanzapine and ondansetron for nausea and vomiting and mirtazapine for appetite stimulation and mood enhancement, are minor substrates of the CYP1A2 pathway. Although the likelihood for CYP1A2 to impact these medications is low, monitoring for adverse events should be recommended to assess therapeutic efficacy.
CYP2B6
Chemotherapy metabolized through CYP2B6 consists of cyclophosphamide, ifosfamide, and thiotepa. Cyclophosphamide and thiotepa are often used in conditioning regimens for autologous and allogeneic HSCT. A decrease in the concentration of these agents could result in a decrease of efficacy, and an increase could result in additive adverse events of myelosuppression, hemorrhagic cystitis, and mucositis. However, sex-related differences in CYP2B6 between cisgender women and cisgender men have not demonstrated any differences in drug metabolism.9 With the addition of estrogen and testosterone cisgender hormonal therapy, there is no independent effect on CYP2B6. Based on the above observations in cisgender adults, medications that use the CYP2B6 metabolism pathway would not need a dose adjustment in transgender adults who are receiving gender-affirming hormone therapy.
CYP2C9 and CYP2C19
The CYP2C gene complex is composed of 4 enzymes, with CYP2C9 and CYP2C19 involved in the majority of drug metabolism.23 CYP2C9 metabolizes the chemotherapy agent idarubicin, which is not frequently used as part of HSCT conditioning regimens. Supportive care therapy potentially metabolized through CYP2C9 during HSCT consists of nonsteroidal anti-inflammatory drugs, which are rarely used because of the increased risk for bleeding from thrombocytopenia and sulfonylureas (eg, glimepiride, glipizide). Prophylactic medications used during HSCT, such as phenytoin for the prevention of busulfan-induced seizures and sulfamethoxazole for the prevention of Pneumocystis jirovecii pneumonia (PJP), are metabolized by CYP2C9. The sex-related differences between cisgender men and cisgender women are similar; therefore, it is unlikely that dose adjustments would be needed for transgender adults.9
Chemotherapy consisting of cyclophosphamide, ifosfamide, and thalidomide involves the CYP2C19 pathway for drug metabolism.9 Additional supportive care medications with CYP2C19 metabolism include antidepressants (eg, citalopram, escitalopram, sertraline, diazepam, olanzapine), proton pump inhibitors (eg, omeprazole, pantoprazole), and phenytoin. Antimicrobial agents used during HSCT that are substrates of CYP2C19 include intravenous pentamidine for PJP prophylaxis and voriconazole for antifungal prophylaxis. Data regarding the differences in CYP2C19 metabolism between cisgender men and cisgender women are conflicting because of the addition of CYP2C19 having individual pharmacokinetic variability that is affected by ethnicity and medications.9 There are limited data demonstrating cisgender women receiving oral contraception therapy to decrease CYP2C19 metabolism compared with cisgender women who are not using oral contraception.9 Currently, no data exist on testosterone influence on CYP2C19 metabolism. Based on this information of estrogen decreasing CYP2C19 metabolism, future studies should be considered to determine the impact of sex hormones on drug metabolism. Currently, there is no clinical recommendation on medication dose adjustments for CYP2C19 in the setting of gender-affirming therapy.
CYP2D6
CYP2D6 is used in the metabolism of approximately 25% of medications.24 The most common medications metabolized by CYP2D6 include antidepressants (eg, amitriptyline, citalopram, duloxetine, fluoxetine, nortriptyline, paroxetine, venlafaxine) and antihypertension medications (eg, carvedilol, metoprolol, propranolol). In addition, codeine and trazodone are prodrugs that require conversion to their active metabolites via the CYP2D6 pathway. Additional supportive care for HSCT consists of 5-HT3 (5-hydroxytryptamine type 3) inhibitors for nausea or vomiting prophylaxis. CYP2D6 is similar to CYP2C19 metabolism in that the metabolism between cisgender men and cisgender women has revealed conflicting data resulting from additional individual pharmacokinetic variability that is not regulated by sex.9 Gender-affirming hormonal therapy has not demonstrated an effect on CYP2D6 drug metabolism.9
CYP3A
CYP3A is the most predominate enzyme in drug metabolism, involving more than 50% of medications.25 Many chemotherapy agents, such as busulfan, cyclophosphamide, etoposide, and thiotepa, which are used for conditioning regimens for HSCT, are metabolized by CYP3A. The majority of supportive care medications used during HSCT also rely on the CYP3A pathway for metabolism, including, but not limited to, medications for antimicrobial prophylaxis (eg, azoles, dapsone), hypertension (eg, amlodipine), hyperlipidemia (eg, atorvastatin, simvastatin), HIV treatment, immunosuppression agents for graft-versus-host disease (GVHD) prophylaxis (eg, cyclosporine, sirolimus, tacrolimus), nausea and vomiting prophylaxis (eg, aprepitant, granisetron), and seizure prophylaxis (eg, carbamazepine, phenobarbital, phenytoin). Maintenance therapy after HSCT also consists of medications that use the CYP3A4 pathway, including tyrosine kinase inhibitors, FLT3 inhibitors, and venetoclax.
CYP3A metabolism has a higher clearance of substrates in cisgender women than in cisgender males. These sex-related differences likely result from P-glycoprotein (P-gp) activity and not CYP3A activity.26 Sex hormones (eg, estrogen replacement therapy, oral contraceptives) do not significantly alter the metabolism of CYP3A4 substrates in cisgender females.27 Currently, the impact of testosterone replacement on CYP3A drug metabolism is unknown. Although dose adjustments for CYP3A medications with gender-affirming therapy are not needed, the identification of CYP3A inhibitors and inducers should be noted when assessing medication therapy as part of routine care. In addition, the use of busulfan pharmacokinetics for individualized dosing should be incorporated along with the monitoring of drug concentration levels when applicable (eg, for azoles, cyclosporine, sirolimus, tacrolimus, carbamazepine, phenobarbital, phenytoin).
Phase II Metabolism
UGT1/2
Phase II metabolism consists of conjugative enzymes such as UDP-glucuronosyltransferase (UGT) along with influx and efflux cell membrane transporters.28 UGT glucuronidation reactions are responsible for approximately 35% of drugs metabolized by phase II enzymes.28 The UGT family consists of UGT1 and UGT2, with 3 subfamilies. These UGT enzymes are expressed in the liver and extrahepatic tissues.28 Medications that are substrates of UGT1A include estrogen, benzodiazepines (eg, lorazepam, clozapine), HIV medications, and pain medications (eg, acetaminophen, codeine, ibuprofen, morphine). Chemotherapy agents catalyzed by UGT that are used for conditioning regimens for HSCT include etoposide. Another common UGT1 and UGT2 substrate is mycophenolate, which is frequently used for GVHD prophylaxis.29
In the total population, the weight-adjusted oral clearance of select UDP-UGT substrates is higher in cisgender men than in cisgender women.9 With the addition of sex hormones from pregnancy, estrogen replacement therapy, and oral contraceptives, the clearance of UGT substrates can increase.9 Similarly, testosterone replacement also accelerates the clearance of UGT substrates.9 Although the clearance may increase when patients receive gender-affirming hormonal therapy, dose adjustments are not recommended given the lack of clinical studies that identify the known etiology of the interaction from the many UGT polymorphisms or the hormonal therapy that affect the metabolism of UGT substrates.29
Drug Transport Proteins
P-glycoprotein
P-gp is expressed in the intestines, liver, and kidneys as an efflux membrane transporter. This transporter membrane is involved in the absorption, distribution, and excretion of drugs.9 A chemotherapy agent that uses the transport membrane is etoposide. HSCT supportive care medications include dexamethasone and cyclosporine. Sex-related differences in the plasma exposure of P-gp in cisgender adults have not been noted.9 P-gp activity increases during pregnancy, but the effect of hormonal therapy is unclear.9 P-gp intracellular activity is coupled with CYP3A metabolism, which can contribute to the different observed clearance of medications.26,30 Additional studies on P-gp activity in transgender adults are needed to understand the impact of gender-affirming hormones on drug metabolism.
Unique Considerations for Patients Undergoing HSCT
As with any patient undergoing stem-cell transplant, there are several important considerations that should be addressed before HSCT in a transgender patient. During the pretransplant consultation, a thorough medical history, including medication reconciliation, should be performed. Identifying potential drug interactions, as well as assessing renal function and body composition in conjunction with long-term gender-affirming therapy, is essential to create a safe medication management plan.
The adverse events with estrogen and testosterone can overlap or be additive in combination with chemotherapy and immunosuppressive adverse events. In addition, overlapping metabolic adverse events of renal and liver dysfunctions can result from immunosuppression, chemotherapy agents, and other supportive care medications. Pharmacodynamic interactions between hormonal therapy, chemotherapy, and immunosuppression medications are unknown because of the lack of data available. It is important to identify, disclose, and discuss the potential adverse events and the risks of therapy with patients before undergoing a transplant.
Chemotherapy and Conditioning Regimens
Chemotherapy used in HSCT preparative regimens often comes with undesirable side effects. The use of chemotherapy agents, such as cyclophosphamide, methotrexate, and etoposide, as well as total body irradiation in conditioning regimens is likely to cause alopecia. Among cisgender females, 47% of patients undergoing chemotherapy describe hair loss as traumatic, and it may even be a barrier to the pursuit or continuation of treatment.31 In addition, any topical formulation of estrogens or testosterone would be contraindicated during treatment with thiotepa because of the risk for increased skin adverse events.
Medications for Prophylaxis and Treatment of GVHD
Medications that are frequently used for prophylaxis against GVHD include calcineurin inhibitors (eg, tacrolimus and cyclosporine), sirolimus, mycophenolate, and methotrexate. Estrogen and testosterone are extensively metabolized by the liver. Several case reports have examined the hepatotoxicity of using cyclosporine in combination with testosterone.32-34 In these reports, cyclosporine concentrations increased 100% to 200% in combination with the testosterone derivative danazol. Of note, danazol is not recommended for use in hormone replacement for transgender persons, and current therapy has not demonstrated these elevations in cyclosporine concentrations.
Testosterone specifically can result in elevated transaminases, coronary artery disease, and hypertension after testosterone initiation.10-12,35 Calcineurin inhibitors and sirolimus can also result in cardiovascular disease, hypertension, and hypertriglyceridemia.36 Depending on the timeline of testosterone initiation and immunosuppression, there is a potential for compounding adverse events. However, liver function, cholesterol, triglycerides, and blood pressure should be monitored if the patient is receiving gender-affirming hormonal therapy.
Hypertrichosis is also a common side effect of cyclosporine used to prevent GVHD. This onset of hair growth may occur on the face, back, and arms, and is generally observed within 6 months of starting therapy.37 The reversal of hypertrichosis is only achievable with the cessation of cyclosporine, but it may take months to see the full results depending on the cycle of the hair follicle.38
Glucocorticoids are the primary treatment for GVHD after transplant. Because of the nature of GVHD, long-term exposure to steroids, including prolonged tapers, may be necessary for the treatment of this complication. Systemic steroid treatment is associated with undesirable side effects, such as cushingoid changes, as well as neuropsychiatric complications. The use of glucocorticoid in combination with gender-affirming hormone therapy, especially testosterone, could result in additive effects and body composition changes. Newer agents used in steroid-refractory GVHD, such as ruxolitinib and ibrutinib, may also have overlapping adverse events. For example, ibrutinib can increase blood pressure, which could have a confounding effect when used concomitantly with testosterone.39
Supportive Care Medications in HSCT
Several medications used in supportive care for HSCT have drug interactions. For example, careful consideration of antiepileptic drugs (AEDs) for seizure prophylaxis during busulfan therapy should be taken. Enzyme-inducing AEDs, such as phenobarbital, phenytoin, and carbamazepine, may decrease serum concentrations of CYP substrates. Studies of the concomitant use of ethinyl estradiol and the CYP3A4 inducers rifampin, phenytoin, and carbamazepine have shown decreased areas under the curve (AUCs) of ethinyl estradiol between 42% and 66%.40-42 Enzyme-inducing AEDs may also affect the serum concentration of busulfan. To avoid this interaction, alternative AEDs with little-to-no effect on hormone or busulfan levels can be used, such as levetiracetam.
Fungal prophylaxis with azole antifungals are often started shortly after HSCT and are known for having numerous drug interactions via the CYP pathway. When used in combination with oral ethinyl estradiol, ketoconazole increased the AUC by 140%, and voriconazole increased the AUC by 160%.43,44 Routine monitoring of antifungal medications, such as isavuconazole, posaconazole, and voriconazole, as well as increased monitoring of testosterone and estrogen levels, should be used when adding or discontinuing an azole antifungal.
Late Adverse Events in Patients Undergoing HSCT
Patients undergoing HSCT have an increased risk for late adverse events and should be routinely screened for the long-term complications of transplant.45 Endocrinopathies occur often after transplant, including hypothyroidism, hypoandrogenism, and hypogonadism. Gonadal dysfunction is common, with 92% of cisgender males and 99% of cisgender females having elevated gonadotropin levels.46 Increased levels of follicle-stimulating hormone and luteinizing hormone occur in cisgender females and males after transplant. In a study examining testosterone and estrogen levels after transplant, testosterone was low in 81% of cisgender men and progesterone was below the detection limit in 100% of cisgender females.47 Currently, there are no studies that examine gonadal function after transplant in the transgender population, but hormonal levels should be closely monitored.
Conclusion
Clinical pharmacists play a pivotal role in medication management before and after HSCT, patient education, and in the long-term care of patients undergoing HSCT. Extensive evaluation must be done for any patient undergoing HSCT, and patients identifying as transgender are no exception to this standard. Optimizing therapeutic drug monitoring, minimizing overlapping adverse events of conditioning and hormone therapy, and identifying potential drug interactions are opportunities for pharmacists to engage in patient care. Because no data exist on specific dosing modifications for transgender patients, pharmacists should use their clinical knowledge and expertise to identify safety concerns and discuss them with the multidisciplinary team and patient. However, given this unmet need for transgender representation in clinical trials, larger studies that include transgender populations are needed in HSCT as well as in oncology.
Author Disclosure Statement
Dr Barthelmess, Dr Perreault, Dr Sabus, and Dr Topal have no conflicts of interest to report.
References
- Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet. 2016;388:390-400.
- Herman JL, Flores AR, Brown TNT, et al. Age of individuals who identify as transgender in the United States. The Williams Institute, UCLA School of Law; January 2017. https://williamsinstitute.law.ucla.edu/pulications/age-trans-individuals-us. Accessed August 16, 2022.
- Webb AJ, McManus D, Rouse GE, et al. Implications for medication dosing for transgender patients: a review of the literature and recommendations for pharmacists. Am J Health Syst Pharm. 2020;77:427-433.
- Safer JD, Tangpricha V. Care of transgender persons. N Engl J Med. 2019;381:2451-2460.
- Bishop BM. Pharmacotherapy considerations in the management of transgender patients: a brief review. Pharmacotherapy. 2015;35:1130-1139.
- Arcelus J, Bouman WP, Van Den Noortgate W, et al. Systematic review and meta-analysis of prevalence studies in transsexualism. Eur Psychiatry. 2015;30:807-815.
- Kronk CA, Everhart AR, Ashley F, et al. Transgender data collection in the electronic health record: Current concepts and issues. J Am Med Inform Assoc. 2022;29:271-284.
- Martinez EO, Rubin M, Miller T, Cortina CS. Transgender and non-binary persons in contemporary oncology randomized clinical trials. Ann Surg Oncol. 2022;29:7958-7960.
- Cirrincione LR, Huang KJ. Sex and gender differences in clinical pharmacology: implications for transgender medicine. Clin Pharmacol Ther. 2021;110:897-908.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102:3869-3903. Errata in: J Clin Endocrinol Metab. 2018;103:699; J Clin Endocrinol Metab. 2018;103:2758-2759.
- Coleman E, Radix AE, Bouman WP, et al. Standards of Care for the Health of Transgender and Gender Diverse People, Version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S258.
- Deutsch MB, ed. Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People. 2nd ed. UCSF Gender Affirming Health Program, Department of Family and Community Medicine, University of California San Francisco; June 17, 2016. https://transcare.ucsf.edu/guidelines. Accessed August 16, 2022.
- Meriggiola MC, Gava G. Endocrine care of transpeople part II. A review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015;83:607-615.
- Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5:877-884.
- Klaver M, Dekker MJHJ, de Mutsert R, et al. Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: a meta-analysis. Andrologia. 2017;49:e12660.
- Bubalo J, Carpenter PA, Majhail N, et al. Conditioning Chemotherapy Dose Adjustment in Obese Patients: A Review and Position Statement by the American Society for Blood and Marrow Transplantation Practice Guideline Committee. Biol Blood Marrow Transplant. 2014;20:600-616.
- Anderson GD. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health (Larchmt). 2005;14:19-29.
- Humble RM, Imborek KL, Nisly N, et al. Common hormone therapies used to care for transgender patients influence laboratory results. J Appl Lab Med. 2019;3:799-814.
- Robinson JD, Lupkiewicz SM, Palenik L, et al. Determination of ideal body weight for drug dosage calculations. Am J Hosp Pharm. 1983;40:1016-1019.
- Carasco CF, Fletcher P, Maconochie I. Review of commonly used age-based weight estimates for paediatric drug dosing in relation to the pharmacokinetic properties of resuscitation drugs. Br J Clin Pharmacol. 2016;81:849-856.
- Giltay EJ, Hoogeveen EK, Elbers JMH, et al. Effects of sex steroids on plasma total homocysteine levels: a study in transsexual males and females. J Clin Endocrinol Metab. 1998;83:550-553.
- Olson J, Schrager SM, Clark LF, et al. Subcutaneous testosterone: an effective delivery mechanism for masculinizing young transgender men. LGBT Health. 2014;1:165-167.
- Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679-1691.
- Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5:6-13.
- Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211-2221.
- Cummins CL, Wu CY, Benet LZ. Sex-related differences in the clearance of cytochrome P450 3A4 substrates may be caused by P-glycoprotein. Clin Pharmacol Ther. 2002;72:474-489.
- Gorski JC, Wang Z, Haehner-Daniels BD, et al. The effect of hormone replacement therapy on CYP3A activity. Clin Pharmacol Ther. 2000;68:412-417.
- Kiang TKL, Ensom MHH, Chang TKH. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther. 2005;106:97-132.
- Stingl JC, Bartels H, Viviani R, et al. Relevance of UDP-glucuronosyltransferase polymorphisms for drug dosing: a quantitative systematic review. Pharmacol Ther. 2014;141:92-116.
- Meibohm B, Beierle I, Derendorf H. How important are gender differences in pharmacokinetics? Clin Pharmacokinet. 2002;41:329-342.
- McGarvey EL, Baum LD, Pinkerton RC, Rogers LM. Psychological sequelae and alopecia among women with cancer. Cancer Pract. 2001;9:283-289.
- Ross WB, Roberts D, Griffin PJA, Salaman JR. Cyclosporin interaction with danazol and norethisterone. Lancet. 1986;1:330.
- Blatt J, Howrie D, Orlando S, Burckart G. Interaction between cyclosporine and danazol in a pediatric patient. J Pediatr Hematol Oncol. 1996;18:95.
- Koneru B, Hartner C, Iwatsuki S, Starzl TE. Effect of danazol on cyclosporine pharmacokinetics. Transplantation. 1988;45:1001.
- Mueller A, Haeberle L, Zollver H, et al. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals. J Sex Med. 2010;7:3190-3198.
- Farouk SS, Rein JL. The many faces of calcineurin inhibitor toxicity—what the FK? Adv Chronic Kidney Dis. 2020;27:56-66.
- Fernandes Souza K, Barbosa de Camargo Andrade PF, de Freire Cassia F, Ribeiro de Castro MC. Cyclosporine-induced childhood generalized hypertrichosis. An Bras Dermatol. 2020;95:402-403.
- Trüeb RM. Causes and management of hypertrichosis. Am J Clin Dermatol. 2002;3:617-627.
- Lee DH, Hawk F, Seok K, et al. Association between ibrutinib treatment and hypertension. Heart. 2022;108:445-450.
- Wiesinger H, Klein S, Rottmann A, et al. The effects of weak and strong CYP3A induction by rifampicin on the pharmacokinetics of five progestins and ethinylestradiol compared to midazolam. Clin Pharmacol Ther. 2020;108:798-807.
- Barditch-Crovo P, Braun Trapnell C, Ette E, et al. The effects of rifampin and rifabutin on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive. Clin Pharmacol Ther. 1999;65:428-438.
- LeBel M, Masson E, Guilbert E, et al. Effects of rifabutin and rifampicin on the pharmacokinetics of ethinylestradiol and norethindrone. J Clin Pharmacol. 1998;38:1042-1050.
- Wiesinger H, Berse M, Klein S, et al. Pharmacokinetic interaction between the CYP3A4 inhibitor ketoconazole and the hormone drospirenone in combination with ethinylestradiol or estradiol. Br J Clin Pharmacol. 2015;80:1399-1410.
- Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
- Majhail NS, Rizzo JD, Lee SJ, et al; for the Center for International Blood and Marrow Transplant Research (CIBMTR), American Society for Blood and Marrow Transplantation (ASBMT), European Group for Blood and Marrow Transplantation (EBMT), Asia-Pacific Blood and Marrow Transplantation Group (APBMT), Bone Marrow Transplant Society of Australia and New Zealand (BMTSANZ), East Mediterranean Blood and Marrow Transplantation Group (EMBMT), and Sociedade Brasileira de Transplante de Medula Ossea (SBTMO). Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:348-371.
- Mertens AC, Ramsay NKC, Kouris S, Neglia JP. Patterns of gonadal dysfunction following bone marrow transplantation. Bone Marrow Transplant. 1998;22:345-350.
- Claessens JJM, Beerendonk CCM, Schattenberg AVMB. Quality of life, reproduction and sexuality after stem cell transplantation with partially T-cell-depleted grafts and after conditioning with a regimen including total body irradiation. Bone Marrow Transplant. 2006;37:831-836.