Vascular endothelial growth factor (VEGF) is a signaling protein and potent angiogenic factor that is upregulated in many solid tumors. In addition to promoting angiogenesis to increase nutrient delivery to tumor cells, VEGF also protects cells from apoptosis.1 Therapies that inhibit the VEGF pathway decrease the proliferation of vascular endothelial cells and promote apoptosis, thereby inhibiting the growth of tumor cells.1 Multiple FDA-approved therapeutic agents are available that target the VEGF signaling pathway.
Bevacizumab is a recombinant, humanized monoclonal antibody that binds VEGF ligand extracellularly, thereby preventing the binding of VEGF to receptors on the endothelial cell surface.2 Similarly, ziv-aflibercept serves as a decoy receptor or VEGF trap to bind VEGF ligand and prevent interaction with VEGF receptors.2 Ramucirumab, another recombinant monoclonal antibody, binds the VEGF receptor 2, blocking the binding of VEGF ligand.2 Intracellularly, tyrosine kinase inhibitors (TKIs) target the VEGF pathway by binding the tyrosine kinase domain of the VEGF receptors (VEGFR1, VEGFR2, VEGFR3), thereby blocking intracellular signaling.3 Orally administered small-molecule TKIs that primarily target this specific pathway include axitinib, lenvatinib, pazopanib, regorafenib, sorafenib, and sunitinib, among others.3
VEGF pathway inhibitor therapies are associated with therapeutic advantages in multiple malignancies, but they also possess an array of adverse events. Proteinuria, which is characterized by the presence of excess protein in the urine, is an adverse event that is frequently reported with VEGF pathway inhibitor therapies.4 Potential mechanisms for VEGF inhibitor–induced proteinuria include treatment-associated hypertension and alterations in glomerular endothelial cells.5 Recommendations for the management of VEGF inhibitor–induced proteinuria have included maintaining blood pressure at <130/80 mm Hg and withholding therapy for severe cases of proteinuria (ie, those with proteinuria in the nephrotic range).5
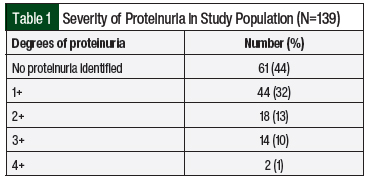
Common Terminology Criteria for Adverse Events version 5.0 defines grade 1 proteinuria as ≥1+ proteinuria (30 mg of protein/dL of urine) or 24-hour urinary protein greater than or equal to the upper limit of normal; grade 2 is defined as 2+ and 3+ proteinuria or urinary protein 1.0 to 3.4 g every 24 hours; and grade 3 is defined as 4+ proteinuria or urine protein ≥3.5 g every 24 hours.6,7 Proteinuria occurs in up to 38% of patients receiving bevacizumab for colon cancer and in up to 64% of patients with renal cell carcinoma.8,9 In a meta-analysis of patients receiving TKIs that target VEGFR, the incidence of proteinuria was 18.7%, with 2.4% of patients having grade ≥3 proteinuria.10 Independent predictors of proteinuria from an analysis of pooled clinical trial data in a population of patients receiving TKIs that target VEGFR for renal cell carcinoma included preexisting proteinuria, Asian ethnicity, diabetes, elevated baseline systolic blood pressure, and previous nephrectomy.4
VEGF inhibitor adverse events, such as proteinuria, may represent biomarkers that indicate an increased likelihood of benefit from therapy. In a pooled analysis of proteinuria in patients who received pazopanib or sunitinib for renal cell carcinoma, a significant association between the severity of proteinuria and overall survival was seen.4 Because proteinuria may be associated with survival and a risk for the alteration of the treatment course, including the withholding of and discontinuation of therapy, it is important to understand the overall impact of this adverse event on therapy.
Herein, we describe our experience with proteinuria in patients receiving VEGF inhibitor therapy in a real-world community setting, and we review the pertinent literature related to VEGF inhibition–induced proteinuria.
Methods
This retrospective analysis included patients receiving treatment with a VEGF inhibitor therapy as outpatients at our community oncology clinic in Indiana from January 1, 2017, to January 1, 2019. Patients were considered for inclusion if they received at least 1 dose of bevacizumab or an anti-VEGFR TKI (axitinib, lenvatinib, pazopanib, regorafenib, sorafenib, sunitinib) during this time frame and if they were aged ≥18 years. Patients with baseline proteinuria without worsening while receiving VEGF inhibitor therapy were excluded from the study. We did not include ramucirumab and ziv-aflibercept for analysis because of the infrequency of use at the location. The local institutional review board approved this analysis.
We assessed the patients to describe the effect of new or worsening proteinuria on the course of therapy by comparing the incidence of withholding therapy and the incidence of treatment discontinuation resulting from a documented adverse event. The additional points that were reviewed to describe the occurrence of proteinuria and its consequences included the time to the first documentation of new or worsened proteinuria, the time to the most severe proteinuria (defined as time from start of therapy to most severe protein level in the urine), the serum creatinine level at end of therapy compared with baseline, and the incidence of an elevation in blood pressure during treatment (defined as a >20–mm Hg increase in systolic blood pressure or a >20–mm Hg increase in diastolic blood pressure from baseline). Last, the data from patients with ≥2+ proteinuria (clinically significant proteinuria) and 3+ or 4+ proteinuria (severe proteinuria) were analyzed and compared with the data from the patients with no or less severe proteinuria.
Data Collection
The data extracted from the electronic medical record included the following baseline characteristics: sex; cancer diagnosis; presence of metastases; medical history of hypertension, diabetes mellitus, chronic kidney disease, or cardiovascular disease; receipt of previous VEGF inhibitor therapy; use of concurrent nonsteroidal anti-inflammatory drugs (NSAIDs), angiotensin-converting enzyme inhibitors (ACEIs), or angiotensin receptor blockers (ARBs) during treatment with VEGF inhibitor therapy; baseline serum creatinine; baseline urine protein; and duration of the VEGF inhibitor therapy. The outcome variables that were extracted included urine protein throughout treatment duration, treatment interruption or withholding for any reason, treatment discontinuation resulting from an adverse event (based on the prescriber’s documentation of the reason for treatment discontinuation), serum creatinine at time of therapy discontinuation, blood pressure throughout the treatment duration, the time to the first documentation of proteinuria, and the time to the most severe proteinuria.
Data were collected from the start of VEGF inhibitor therapy until the discontinuation of treatment with the VEGF inhibitor. Proteinuria was defined as the occurrence of worsening proteinuria in the patients with baseline proteinuria and progression to a higher level of proteinuria or new proteinuria and classified as 1+ proteinuria for a urine protein of 30 mg/dL to 99 mg/dL, 2+ proteinuria for 100 mg/dL to 299 mg/dL, 3+ proteinuria for 300 mg/dL to 999 mg/dL, and 4+ proteinuria for ≥1000 mg/dL.7 The standard-of-care method for urine protein measurement at the clinic was via a complete laboratory urinalysis at the start of each new treatment cycle (ie, every 3-4 weeks). The clinic standard of care was to measure blood pressure at each clinic visit.
Statistical Analysis
Categorical variables were compared with the chi-square test or Fisher exact test, as appropriate. Continuous variables were compared with an independent samples t test or a Mann–Whitney U test, as appropriate. A 2-sided alpha level of 5% was used for all analyses. Statistical analyses were completed using IBM SPSS Statistics 25 (IBM Corp; Armonk, NY) software.
Results
A total of 225 patients were identified for inclusion in the study; however, 16 patients were excluded from the study with baseline proteinuria without further worsening of urine protein levels, and 70 patients were excluded from the study for not receiving at least 1 dose of VEGF inhibitor therapy or for being aged <18 years. A total of 139 patients were included for analysis, of whom 107 received bevacizumab and 32 received a TKI. Of the TKIs included, the patients received sunitinib (n=8), pazopanib (n=7), regorafenib (n=7), sorafenib (n=5), lenvatinib (n=3), or axitinib (n=2). Overall, the patient population had a mean age of 64 years, 55% were men, and the most common malignancy was colorectal cancer. Documented metastases were present in 88% of the population, and the majority of patients (67%) were receiving VEGF inhibition in the first-line treatment setting.
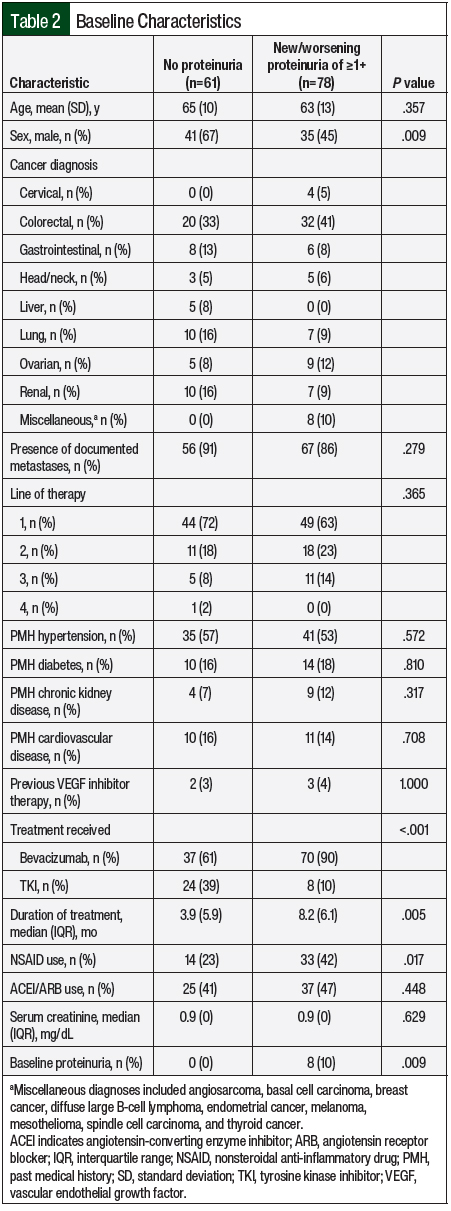
The median duration of therapy was 5.6 months. Of the included patients, 61 (44%) did not have proteinuria whereas 78 (56%) had new or worsening proteinuria of ≥1+ (Table 1). The median time to the first presentation of proteinuria was 2 months, and the median time to the most severe proteinuria was 2.9 months. Patients with proteinuria were more likely to be women, receive bevacizumab, have baseline proteinuria, and receive therapy longer (Table 2). In addition, NSAID use was more common in the patients with proteinuria than in those without proteinuria (42% vs 23%, respectively; P=.017; Table 2). All other baseline characteristics, including age, relevant comorbidities, cancer diagnosis, the presence of metastatic disease, line of therapy, and serum creatinine, were similar between groups (Table 2).
Outcomes
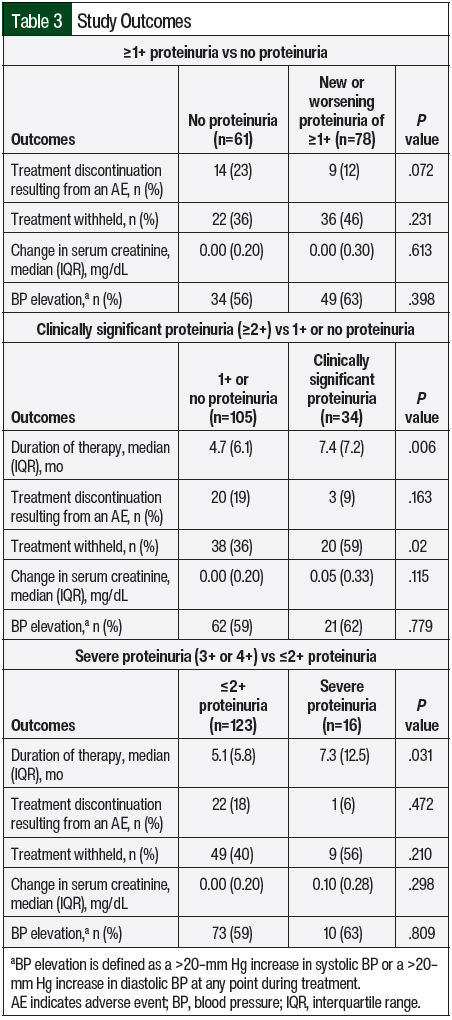
The patients with new or worsening proteinuria were not significantly more likely than the patients without proteinuria to discontinue therapy because of an adverse event (12% vs 23%, respectively; P=.072), nor were they more likely to withhold therapy (46% vs 36%; P=.231; Table 3). The median duration of therapy was significantly longer in the patients with new or worsening proteinuria compared with those without new or worsening proteinuria (8.2 months vs 3.9 months, respectively; P=.005). Elevations in blood pressure were common in both groups (63% in those with proteinuria vs 56% in those without proteinuria), and there was no effect on serum creatinine in either group (Table 3).
A total of 34 patients (24%) had clinically significant proteinuria (≥2+ proteinuria). Of these patients, 29 received bevacizumab and 5 received a TKI. The overall incidence of clinically significant proteinuria in the patients receiving bevacizumab was 27% compared with 16% of those receiving a TKI. Some patients with clinically significant proteinuria had baseline proteinuria, whereas patients with less than clinically significant proteinuria did not (23% vs 0%, respectively; P<.001). NSAID use was similar between the groups (41% of those with clinically significant proteinuria vs 31% of those with 1+ or no proteinuria; P=.296), as was ACEI and ARB use (59% vs 40%, respectively; P=.055). Despite withholding therapy more frequently in the patients with clinically significant proteinuria compared with those with 1+ or no proteinuria (59% vs 36%, respectively; P=.02), the median duration of therapy was significantly longer in the former group (7.4 months vs 4.7 months, respectively; P=.006). No differences were seen in the incidence of blood pressure elevation or changes in serum creatinine between the groups (Table 3).
A total of 16 (12%) patients had severe proteinuria (3+ or 4+ proteinuria); 15 patients received bevacizumab and 1 received a TKI. The median time to the most severe proteinuria among the patients with severe proteinuria was 120 days. The duration of therapy was significantly longer in the patients with severe proteinuria compared with those with ≤2+ proteinuria (7.3 months vs 5.1 months, respectively; P=.031; Table 3). Patients with severe proteinuria were more likely than those with ≤2+ proteinuria to have baseline proteinuria (31% vs 2%, respectively; P<.001). All of the other outcomes that were assessed were similar, including the incidence of elevations in blood pressure and change in serum creatinine (Table 3).
Discussion
Proteinuria is a common occurrence in patients receiving VEGF pathway inhibitor therapy. New or worsening proteinuria occurred in 56% of the patients in our analysis, with 12% having severe (3+ or 4+) proteinuria. In a meta-analysis of studies including 42,510 patients receiving bevacizumab or a control for a variety of malignancies (including glioblastoma, lymphoma, melanoma, mesothelioma, leiomyosarcoma, and colorectal, lung, renal, pancreatic, ovarian, gastric, prostate, and cervical cancers), ≥1+ proteinuria occurred in 18% (2.4% had severe proteinuria).11 However, in a study of patients with renal cell carcinoma, the incidence of ≥1+ bevacizumab-induced proteinuria was 64%.8
In a meta-analysis of 6882 patients with a variety of malignancies, including soft-tissue sarcoma, glioblastoma, mesothelioma, and hepatocellular, lung, ovarian, nasopharyngeal, breast, renal, thyroid, pancreatic, and colorectal cancers, the incidence of TKI-induced proteinuria was 18.7% (grade ≥3, 2.4%).10
An analysis of 2 studies that included a total of 1392 patients receiving pazopanib or sunitinib for the treatment of renal cell carcinoma showed a proteinuria rate of 15% (grade ≥3, 3.7% ).4 The relatively low incidence of severe proteinuria demonstrates that for most patients with proteinuria, the occurrence remains at a low grade; however, the rate of low-grade proteinuria in clinical practice may be higher than that observed in clinical trials.
The population in our analysis had a diverse set of malignancies, with colorectal cancer being the most common, yet the incidence of proteinuria more closely aligned with the previously reported population receiving bevacizumab for renal cell carcinoma, which is described above.8 Likewise, the rate of more severe proteinuria may be higher in clinical practice than that observed in clinical trials.
Despite the common occurrence of proteinuria, patients are able to remain on VEGF inhibitor therapy. In fact, the patients with proteinuria in our analysis had a longer duration of therapy that was consistent across all 3 levels of proteinuria assessed, even among those patients with severe proteinuria.
The median duration of therapy was 4.3 months longer in those with ≥1+ proteinuria, 2.7 months longer in those with clinically significant proteinuria (compared with those with 1+ or no proteinuria), and 2.2 months longer in those with severe proteinuria (compared with those with ≤2+ proteinuria). The increased duration observed could be a result of an increased risk for proteinuria over time or may be a result of an increased likelihood of benefit.
In the previously published analysis of patients with renal cell carcinoma receiving pazopanib or sunitinib described above, overall survival was associated with the severity of proteinuria (hazard ratio [HR], 0.86; P=.015), with severe proteinuria demonstrating the strongest association (HR, 0.53; 95% confidence interval, 0.30-0.92).4 It is possible that the increased duration of therapy observed in our population with proteinuria resulted from patients deriving more benefit from therapy; however, given the inclusion of multiple VEGF-inhibiting agents and diagnoses, it is not possible to confirm this correlation.
In an analysis of 40 patients receiving lenvatinib 24 mg daily for the treatment of differentiated thyroid cancer in Japan, a decrease in glomerular filtration rate (GFR) was noted over time with significant decreases compared to baseline demonstrated throughout therapy.12 A total of 13 (32.5%) patients had a decrease in GFR >15 mL/min/1.73 m2 for ≥6 months and a final GFR decrease of >20 mL/min/1.73 m2. The risk factors for declines in GFR were time and the presence of 4+ proteinuria. Despite the common occurrence of proteinuria in our patients, nephropathy was not observed when comparing the baseline and end-of-therapy serum creatinine levels.
The patients in our analysis differed from the patients in the study of differentiated thyroid cancer,12 with a much shorter duration of therapy in our population (median 29.5 months vs 5.6 months, respectively), which may have resulted in less impact on kidney function. Elevations in blood pressure were similar among the groups assessed in our analysis (56% in those without proteinuria, 63% in those with ≥1+ proteinuria, 62% in those with clinically significant proteinuria, and 63% in those with severe proteinuria).
Similar proportions of patients requiring the withholding of therapy were found in the analysis of ≥1+ proteinuria and severe proteinuria; however, those with clinically significant proteinuria had a significant increase in the rate of withholding therapy. No significant difference existed between the groups in the proportion of patients discontinuing therapy as a result of an adverse event, despite being less common in those with ≥1+ proteinuria compared with those without proteinuria (12% vs 23%, respectively). These data are reassuring in that patients may be able to continue tolerating therapy without having untoward consequences despite having proteinuria.
The current practice of monitoring for proteinuria includes frequent urine protein assessments. Despite the frequent monitoring of proteinuria (at the beginning of each treatment cycle), no difference was seen in the withholding of VEGF inhibitor therapy between the patients with any level of proteinuria and those without proteinuria. In a study of 55 patients receiving 338 treatments with bevacizumab, the reduction of urine protein monitoring frequency to every other treatment did not have any impact on proteinuria-related adverse events.13 Based on these data, the institution completing the analysis of reduced urine protein monitoring further reduced their standard monitoring to occur with every third dose of bevacizumab. This strategy of reduced monitoring has not been evaluated with VEGF inhibitors other than bevacizumab; however, reductions in monitoring have the potential to decrease the use of resources and increase patient satisfaction through decreased testing.
Previously demonstrated risk factors for proteinuria among patients receiving pazopanib or sunitinib for renal cell carcinoma included Asian ethnicity, diabetes, baseline systolic blood pressure, preexisting proteinuria, and previous nephrectomy.4 In another study of Japanese patients receiving axitinib for renal cell carcinoma, proteinuria at baseline correlated with axitinib-induced proteinuria more than the other baseline markers of renal function or hypertension.14
In our population, female sex, baseline proteinuria, and the use of NSAIDs were more common in the patients with new or worsening proteinuria, as was a longer duration of therapy. Proteinuria was also more frequently observed in those receiving bevacizumab compared with those receiving TKIs; 65% of those receiving bevacizumab had proteinuria, whereas 25% of patients receiving a TKI had proteinuria. Interestingly, the previously demonstrated risk factor of a history of hypertension was not more common among the group with proteinuria. Other factors that can increase the risk for renal dysfunction, such as diabetes, chronic kidney disease, cardiovascular disease, and elevated baseline serum creatinine, were also not more common among the group with proteinuria. NSAIDs have effects on renal homeostasis15 and were more frequently received by those with proteinuria in our study. To our knowledge, this is the first analysis describing a potential relationship between NSAID use and proteinuria in patients receiving VEGF pathway–inhibiting therapies. Moreover, female sex was not a risk factor for proteinuria in a previous study of pazopanib and sunitinib in patients with renal cell carcinoma; however, in that study, 28% of the patients were women,4 whereas in our population women accounted for 45% of the total population.
The management of patients with proteinuria while receiving VEGF inhibitor therapy is not well established; however, the use of ACEI or ARB antihypertensive therapy is frequently recommended.5,16 In our patients, concomitant ACEI or ARB use during therapy was common (45% of the population) and was not protective. In fact, the use of ACEIs or ARBs was numerically higher in the patients with clinically significant proteinuria than without (59% vs 40%). In a preclinical study of sunitinib treatment in rats, ACE inhibition prevented angiogenesis inhibition–induced proteinuria.16 However, in mouse models of VEGF inhibition in hepatocellular carcinoma, ACE inhibition was not effective in delaying or reducing the occurrence of proteinuria and demonstrated a stimulatory effect on the cancer cells via the increased production of erythropoietin.17
It is possible that with lower rates of the use of ACEI or ARB therapies, proteinuria could have been higher in our population. It is also possible that the common use of ACEI or ARB therapy in our population limited proteinuria to lower severity, because severe proteinuria was uncommon. In patients with diabetes, ACEI or ARB therapy has demonstrated a decrease in the progression of proteinuria in those with hypertension and microalbuminuria; however, ACEI or ARB therapy has not demonstrated an impact on renal function in patients without hypertension.18 The incidence of elevations in blood pressure in our population was 60%. Therefore, the use of ACEIs or ARBs may have limited progression of proteinuria, similar to that demonstrated in patients with diabetes.
Limitations
Our study has limitations. The limitations of our analysis include the retrospective nature of the data, the number of VEGF-inhibiting agents and diagnoses, and the reliance on documentation within the medical record for NSAID and ACEI or ARB use through patient medication lists, adverse events, and reasons for discontinuation. The small number of patients receiving a TKI was also a limitation. Despite these limitations, our data were in line with the available literature.
The exclusion of patients with baseline proteinuria without worsening is also a limitation; however, if included, there would be no plausible way to determine if continued or stable proteinuria resulted from VEGF inhibition or from an unassociated underlying renal pathology.
Another study limitation was the relatively small number of patients with clinically significant or severe proteinuria. This may result from the natural progression of VEGF inhibitor–induced proteinuria with the majority of patients having low-grade proteinuria without clinically significant renal adverse events or negative impacts on the patients’ VEGF inhibitor treatment course.
Conclusion
New or worsening proteinuria that results from VEGF pathway inhibitor therapy does not lead to an increase in treatment discontinuation because of adverse events or to an increase in the risk for additional adverse events. Nephropathy is rare; however, the risk for nephropathy may increase in patients receiving prolonged courses of VEGF pathway inhibition. Despite this potential risk, our results help support the suggestion that decreasing the frequency of urine protein monitoring for bevacizumab to every other treatment or every third treatment may not increase proteinuria-related adverse events. More research is needed to quantify the potential negative impact of concomitant NSAID use on the risk for new or worsening proteinuria while receiving VEGF pathway inhibitor therapy.
Author Disclosure Statement
Dr Murdock, Dr Duco, and Dr Reeves have no conflicts of interest to report.
References
- Duffy AM, Bouchier-Hayes DJ, Harmey JH. Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: autocrine signalling by VEGF. Madame Curie Bioscience Database. Landes Bioscience; 2000-2013. www.ncbi.nlm.nih.gov/books/NBK6482/. Accessed June 2022.
- Oguntade AS, Al-Amodi F, Alrumayh A, et al. Anti-angiogenesis in cancer therapeutics: the magic bullet. J Egypt Natl Canc Inst. 2021;33:15.
- Gotink KJ, Verheul HMW. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010;13:1-14.
- Sorich MJ, Rowland A, Kichenadasse G, et al. Risk factors of proteinuria in renal cell carcinoma patients treated with VEGF inhibitors: a secondary analysis of pooled clinical trial data. Br J Cancer. 2016;114:1313-1317.
- Izzedine H, Massard C, Spano JP, et al. VEGF signalling inhibition-induced proteinuria: mechanisms, significance and management. Eur J Cancer. 2010;46:439-448.
- Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. November 27, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed May 2022.
- Carroll MF, Temte JL. Proteinuria in adults: a diagnostic approach. Am Fam Physician. 2000;62:1333-1340.
- Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti–vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427-434.
- Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697-3705.
- Zhang ZF, Wang T, Liu LH, Guo HQ. Risks of proteinuria associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a systematic review and meta-analysis. PLoS One. 2014;9:e90135.
- Zhao T, Wang X, Xu T, et al. Bevacizumab significantly increases the risks of hypertension and proteinuria in cancer patients: a systematic review and comprehensive meta-analysis. Oncotarget. 2017;8:51492-51506.
- Masaki C, Sugino K, Kobayashi S, et al. Impact of lenvatinib on renal function: long-term analysis of differentiated thyroid cancer patients. BMC Cancer. 2021;21:894.
- Schiffer M, Zukovic L, Hall S, Merl MY. Assessment of extended urine protein monitoring frequency in patients receiving bevacizumab. J Oncol Pharm Pract. 2021;27:902-906.
- Tomita Y, Uemura H, Fujimoto H, et al. Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell carcinoma. Eur J Cancer. 2011;47:2592-2602.
- Hörl WH. Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals (Basel). 2010;3:2291-2321.
- Lankhorst S, Kappers MHW, van Esch JHM, et al. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: preclinical study. Hypertension. 2014;64:1282-1289.
- Zhang S, Cao M, Hou Z, et al. Angiotensin-converting enzyme inhibitors have adverse effects in anti-angiogenesis therapy for hepatocellular carcinoma. Cancer Lett. 2021;501:147-161.
- American Diabetes Association. Section 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(suppl 1):S135-S151.