Immune checkpoint inhibitors are widely used for the treatment of various types of cancer, and approximately 45% of US patients with cancer are eligible to receive treatment with these drugs.1,2 Currently, 9 immune checkpoint inhibitors are approved by the US Food and Drug Administration (FDA), including ipilimumab, which inhibits cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4); nivolumab, pembrolizumab, dostarlimab, and cemiplimab, which inhibit programmed cell death 1 (PD-1) activity; atezolizumab, avelumab, and durvalumab, which inhibit PD ligand 1 (PD-L1); and the first FDA-approved LAG-3 inhibitor, relatlimab (which was approved in March 2022).
Immune checkpoint inhibitors have changed the landscape of cancer treatments as a result of their ability to improve overall survival; however, they can also cause immune-related adverse events (AEs) in up to 60% to 85% of patients, which can lead to treatment interruption and discontinuation.3 The rate of AEs increases to 95% when nivolumab and ipilimumab are given in combination.3 Thus, it is important to identify potential AEs and understand the management of these toxicities.
One common immune-related AE caused by checkpoint inhibitor therapy is endocrine dysregulation, which has been reported in 1% to 50% of patients with cancer receiving checkpoint inhibitors.4 The spectrum of endocrinopathies includes hypothyroidism, hypopituitarism, hyperthyroidism, adrenal insufficiency, and hyperglycemia.5
The reported incidence of hypothyroidism related to the use of checkpoint inhibitors is between 1.5% and 22%; the incidence varies based on the choice of checkpoint inhibitor, the dose, and the frequency.1,6,7 The incidence of hypothyroidism is highest when 2 checkpoint inhibitors are given in combination, followed by a PD-1 inhibitor, then a PD-L1 inhibitor, and then anti–CTLA-4 immunotherapy.8,9
The clinical presentation of hypothyroidism can include (but is not limited to) weight gain, cold intolerance, xerosis, hair loss, fatigue, and depression.7,10,11 The mechanism of checkpoint inhibitor–induced endocrinopathies is still uncertain but is likely multifactorial, including autoimmune mechanisms, because the functional changes are associated with the appearance of thyroid antibodies.4 Another proposed mechanism of endocrinopathies is immune tolerance, which is defined as “a state of unresponsiveness of the immune system toward antigens or tissues able to elicit an immune response.”12 Hence, immune-related AEs such as endocrinopathies may be related to an activated immune system that has reacted against tumor antigens and healthy tissue antigens.1,10-12
As a result of the frequency of thyroid-related AEs, clinicians are advised to monitor thyroid function with thyroid-stimulating hormone (TSH) and free thyroxine tests at baseline and then every 4 to 6 weeks, because early recognition may warrant therapeutic management.11,13
The time to onset of hypothyroidism after the initiation of checkpoint inhibitors ranges from 3 weeks to 10 months.5 In general, hypothyroidism is treated with thyroid hormone replacement therapy, whereas patients with subclinical hypothyroidism are monitored closely for clinical symptoms.5,13 Checkpoint inhibitors may be continued in patients with hypothyroidism, but it is essential to manage patients with overt hypothyroidism, because untreated hypothyroidism can increase the risks for morbidity and mortality.1,14
Endocrine AEs can lead to permanent organ dysfunction, which may require indefinite steroid replacement.1,11 Although checkpoint inhibitor–induced hypothyroidism is not reversible, early recognition and management of immune-related AEs are essential to improve patients’ quality of life and adherence to immunotherapy.15
Several clinical studies have demonstrated that thyroid dysfunction that is induced by checkpoint inhibitor treatment may be linked to survival outcomes.8,16-18 However, no independent study has evaluated the use of all checkpoint inhibitors in any type of cancer to confirm this correlation.
The primary objective of our study was to evaluate the incidence and management of new-onset hypothyroidism in adults who received checkpoint inhibitor therapy at our academic institution for any FDA-approved indication. The secondary objectives were to assess the number of endocrinology referrals and the percentage of patients with thyroid function test normalization after the initiation of hypothyroidism treatment, and to evaluate the outcomes of new-onset hypothyroidism on switching to another checkpoint inhibitor, nonresponse to treatment, disease progression, and overall survival. In addition, we compared the outcomes of patients who had any immune-related AEs with patients who did not have such AEs in reaction to switching to another checkpoint inhibitor, treatment nonresponse, disease progression, and overall survival.
Methods
This retrospective chart review was conducted at the Medical University of South Carolina-Hollings Cancer Center. We first identified all adults who received checkpoint inhibitors at our institution between January 1, 2019, and December 31, 2019.
Patients were excluded if they had primary hypothyroidism or had a history of hypothyroidism at baseline. Specifically, patients were excluded from this study if they (1) were aged <18 years; (2) had immune-related thyroid dysregulation other than hypothyroidism (which usually requires referral to the endocrinology department); (3) had hypothyroidism at baseline; (4) had missing data; (5) were participating in a clinical trial; (6) were receiving checkpoint inhibitors for off-label indications; (7) had thyroid carcinoma; or (8) were receiving checkpoint inhibitor therapy in combination with a tyrosine kinase inhibitor (TKI), because TKIs can cause hypothyroidism.7,19
The key data we collected included (1) the type of checkpoint inhibitor therapy, (2) serum TSH and free thyroxine levels at baseline and during treatment with checkpoint inhibitor therapy, (3) the frequency of thyroid function tests performed, (4) the numbers of referrals to the endocrinology department, (5) presence of symptoms related to hypothyroidism, (6) presence of subclinical or overt hypothyroidism, (7) treatment of checkpoint inhibitor interruption, (8) disease progression, (9) overall survival, (10) switching to another checkpoint inhibitor, (11) having nonthyroid immune-related AEs, (12) initial dose of levothyroxine, and (13) thyroid function test normalization after starting hypothyroidism management. All data were deidentified, and the study was approved by the Institutional Review Board and Ethics Committee of the Medical University of South Carolina.
Thyroid function test normalization was defined as TSH and free thyroxine within the euthyroid range (ie, 0.45 to 4.49 mIU/L for TSH; 0.7 to 1.9 mcg/dL for free thyroxine). Subclinical hypothyroidism was scored if the TSH level was between 4 and 10 mIU/L with normal free thyroxine levels, or if the TSH level was ≥10 mIU/L with normal free thyroxine levels, and the patient was asymptomatic. Overt hypothyroidism was scored if the TSH level was ≥10 mIU/L, with a decreased free thyroxine level, or if the patient was symptomatic.
Patients were considered to have checkpoint inhibitor–induced hypothyroidism if they had overt hypothyroidism after checkpoint inhibitor initiation, or if they were symptomatic. Any patient who had checkpoint inhibitor–induced hypothyroidism continued to receive checkpoint inhibitor therapy while hypothyroidism was also managed.
Treatment nonresponse was defined as the time from starting checkpoint inhibitor therapy to treatment discontinuation because of a lack of efficacy, intolerability, or patient choice. Progression-free survival was defined as the time from starting therapy to disease progression. Overall survival was defined as the time from starting therapy to death from any cause. In addition, nonthyroid immune-related AEs were defined as AEs involving organs other than the thyroid gland and that required intravenous or oral glucocorticoid therapy for resolution.
Continuous data are presented in this article as medians (and interquartile range); categorical data are presented as counts (and percentages). We used Mann–Whitney U tests for comparisons of the continuous outcomes, and chi-square or Fisher’s exact tests to compare categorical outcomes. The times to event outcomes were evaluated via Kaplan-Meier analysis and log-rank tests. To account for the chance of error in multiple hypothesis testing, we applied a Bonferroni correction and defined statistical significance as P <.004.
All laboratory reference intervals were determined per Medical University of South Carolina Laboratory Services. All our analyses were conducted using IBM SPSS Statistics version 25 (IBM Corporation; Armonk, NY).
Results
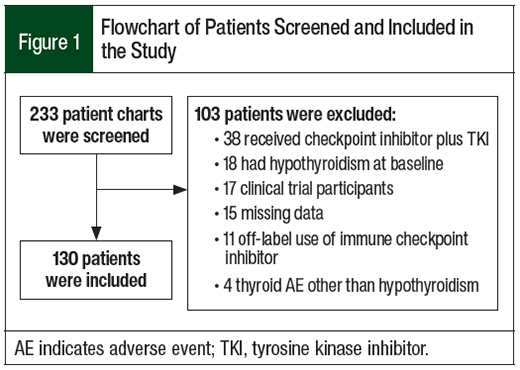
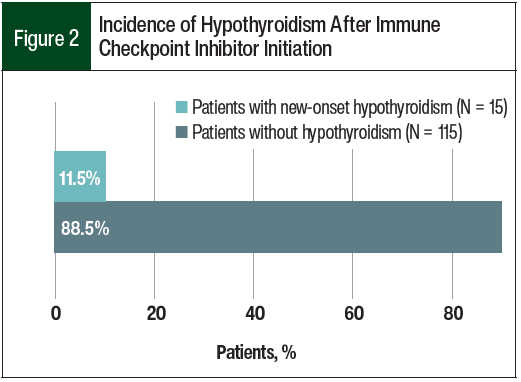
Of the 233 patients who were identified for this study, 130 patients were included in the analysis (Figure 1). In all, 15 (11.5%) patients had checkpoint inhibitor–induced hypothyroidism (Figure 2).
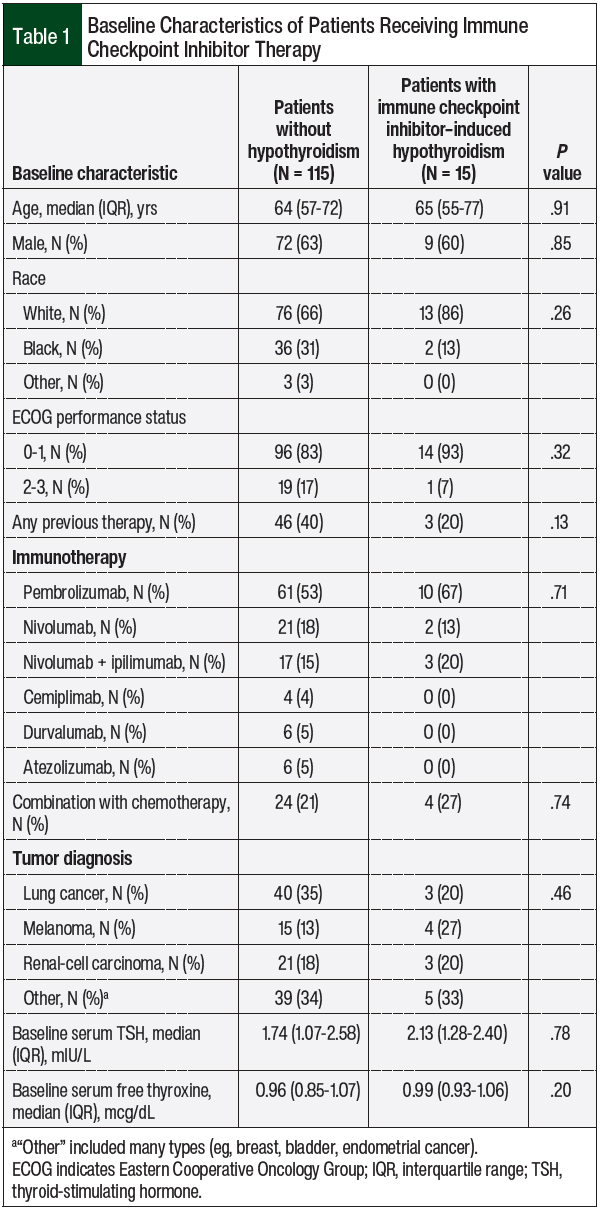
Table 1 shows the baseline demographic characteristics of the study patients and their use of checkpoint inhibitors. Overall, pembrolizumab was the most often used checkpoint inhibitor, followed by nivolumab monotherapy or in combination with ipilimumab. The most prevalent cancer diagnoses were lung cancer and melanoma, followed by renal-cell carcinoma. At baseline, all patients had normal median TSH and free thyroxine levels.
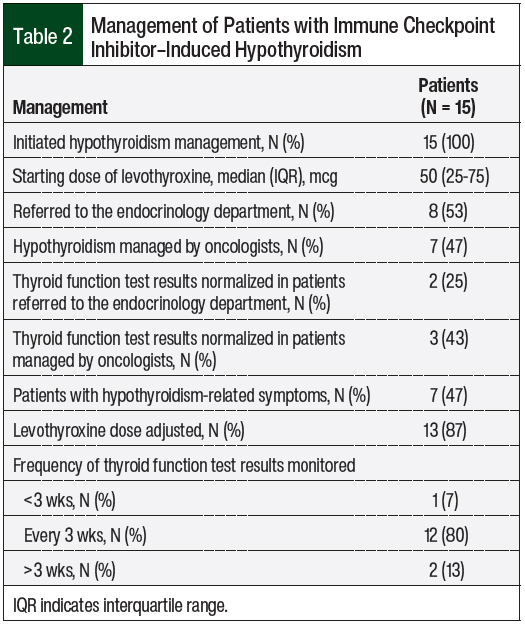
All the patients who had checkpoint inhibitor–induced hypothyroidism were managed with levothyroxine therapy, with a median starting dose of 50 mcg, and 47% of those patients were symptomatic (Table 2). A total of 13 (87%) patients required levothyroxine dose adjustment, and 8 (53%) patients were referred to the endocrinology department. Thyroid function normalization was higher in patients who were managed by their oncologist (43%) than in patients who were referred to the endocrinology department (25%).
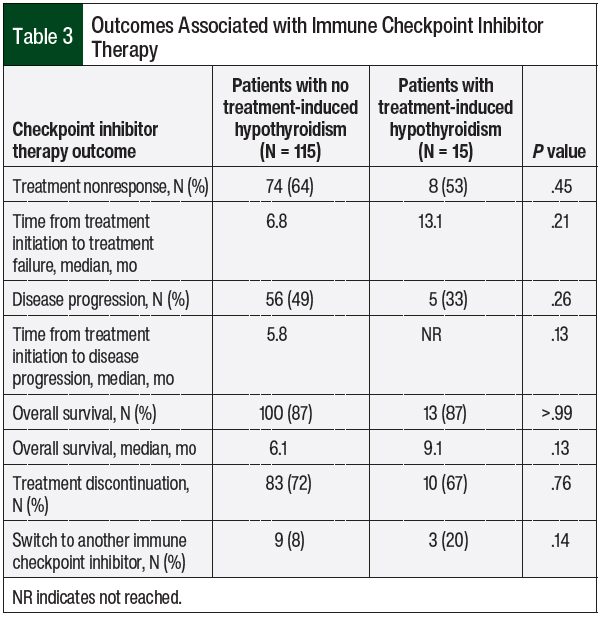
No significant difference was found in nonresponse to checkpoint inhibitor treatment between patients who had hypothyroidism and patients who did not have thyroid-related AEs (53% vs 64%, respectively; P = .45; Table 3). The time to treatment nonresponse was also not significantly different between the 2 groups. A total of 56 (49%) patients who did not have checkpoint inhibitor–induced hypothyroidism had disease progression while receiving checkpoint inhibitors compared with only 5 (33%) patients who had thyroid AEs (P = .26).
No significant difference was found in overall survival or in the rate of checkpoint inhibitor discontinuation between the 2 groups; however, although not significant, the median overall survival in the patients who had checkpoint inhibitor–induced hypothyroidism was longer than in the patients who did not have hypothyroidism (9.1 months vs 6.1 months, respectively; P = .13). More patients with hypothyroidism switched to another checkpoint inhibitor compared with patients without thyroid AEs, but that finding was not statistically significant (20% vs 8%, respectively; P = .14; Table 3).
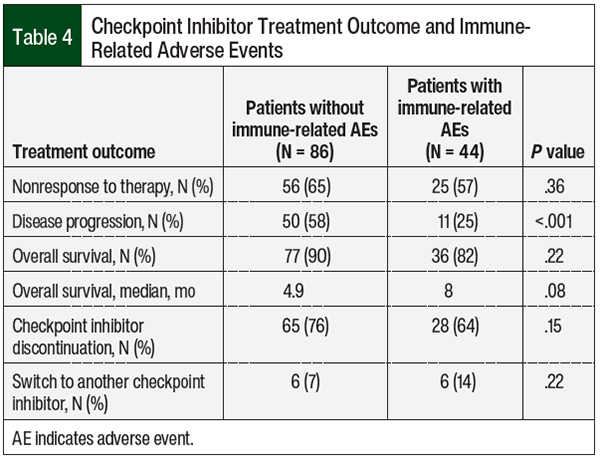
The treatment outcomes were assessed in the patients who had any immune-related AEs (Table 4). Disease progression was less common in patients with any immune-related AEs than in the patients without immune-related AEs (25% vs 58%, respectively; P <.001). No differences were seen in treatment nonresponse or in overall survival between the 2 groups; however, the median overall survival in the patients with any immune-related AEs was numerically greater by 3 months than in patients without any immune-related AEs, although this was not significant (8 months vs 4.9 months, respectively; P = .08; Table 4).
Discussion
In our retrospective chart review, the incidence of checkpoint inhibitor–induced hypothyroidism was within the range reported in previous studies.1,6,8,19-21 In our study, however, 11.5% of patients had checkpoint inhibitor–induced hypothyroidism, whereas in previous studies the frequency of hypothyroidism varied from 5% to 22%.1,6,8,19-21 This wide variation, as well as the increased frequency of hypothyroidism in more recent reports22,23 compared with earlier reports,1,6,8 indicate an improvement in screening.
Moreover, the incidence of hypothyroidism is higher when a combination of 2 checkpoint inhibitors is used compared with the use of monotherapy based on some published data.19,20 In our study, 20% of the patients who were receiving checkpoint inhibitor combination therapy had checkpoint inhibitor–induced hypothyroidism compared with 10% to 22% of the patients who were receiving checkpoint inhibitor combination therapy in previous studies.19,20
In our study, 53% of the patients who had hypothyroidism were referred to the endocrinology department. Thyroid function test results returned to normal more quickly in patients managed by oncologists than in those managed by endocrinologists, which validates the recommendation of current treatment guidelines that hypothyroidism can be initially managed by the treating oncologist and does not require endocrinology referral.11,13 The average monitoring with thyroid function test was every 3 weeks, which is more frequent than current treatment guidelines recommend.13,24
In addition, referrals to endocrinology can delay the management of hypothyroidism, potentially resulting in the worsening of the patient’s thyroid function and leading to more delays in checkpoint inhibitor therapy than when patients are managed by an oncologist at the time of diagnosis.
In our study, the patients who had checkpoint inhibitor–induced hypothyroidism had a higher median serum TSH level at baseline compared with those who did not have hypothyroidism, although the difference was not significant (2.13 mIU/L vs 1.74 mIU/L, respectively; P = .78; Table 1).
Some studies have indicated that a higher TSH level at baseline may predict an increased risk for hypothyroidism after treatment initiation with a checkpoint inhibitor.25,26 Two studies have indicated that baseline TSH levels of >1.67 mIU/L and of >2.19 mIU/L are risk factors for immune-related thyroid AEs.26,27 In our study, patients who had hypothyroidism after checkpoint inhibitor initiation had a median baseline TSH level of 2.13 mIU/L before therapy.
Although the use of checkpoint inhibitors can lead to a variety of AEs, some preclinical studies and several clinical studies have shown that thyroid dysfunction may be associated with a better response to checkpoint inhibitor therapy and better overall survival compared with patients without thyroid dysfunction.7,15-17,28
A retrospective study by Kotwal and colleagues showed that patients who had immune-related thyroid AEs while receiving PD-L1 inhibitors had longer overall survival and lower mortality than patients without immune-related thyroid AEs (P = .027).17 A 2020 study by Basak and colleagues demonstrated that patients with non–small-cell lung carcinoma, renal-cell carcinoma, or melanoma who had overt thyroid dysfunction while receiving checkpoint inhibitor therapy had higher overall survival (P = .02) and progression-free survival (P = .05) than patients who did not have thyroid dysfunction while receiving checkpoint inhibitor therapy.18 And a recent study by Baek and colleagues showed that patients with new overt or subclinical hypothyroidism had a significantly reduced hazard ratio for mortality of 0.324 compared with patients without thyroid dysfunction (P = .002).29
In our study, however, we found no correlation between checkpoint inhibitor treatment outcomes and the emergence of hypothyroidism, which most likely is the result of our nonpowered secondary end points (Table 3) used to demonstrate any beneficial or harmful effects. By contrast, our study showed a significant reduction in disease progression in the patients who had any immune-related AEs compared with the patients without immune-related AEs (25% vs 58%, respectively; P <.001), yet there were no differences in the treatment nonresponse or overall survival, which is likely a result of the small sample size of our study.
A recent retrospective cohort study by Shankar and colleagues showed that thyroiditis, pneumonitis, and dermatitis are the most common multisystem immune-related AE patterns that are associated with improved treatment outcomes.30 Moreover, Shankar and colleagues showed that patients with multisystem immune-related AEs had better survival outcomes than patients with a single immune-related AE.30
The cohort study by Shankar and colleagues may help explain why we did not find a correlation between hypothyroidism and improved treatment outcomes in our study, which excluded patients with other thyroid-related immune-related AEs and did not evaluate for single versus multisystem immune-related AEs, which were beyond the scope of our study.
Currently, there are no confirmed risk factors to help predict which patients are at an increased risk for hypothyroidism; thus, further studies are needed.
Limitations
Our study had several limitations. In addition to the small sample size and possible missing information in medical records, the retrospective nature of this study posed a risk for patient selection bias, although this risk was minimized by randomly assigning unique numbers to each patient before data collection.
Moreover, our study was likely underpowered as a result of the small sample size and our exclusion of patients with other thyroid-related immune-related AEs, which might have led to nonsignificant treatment outcomes as a consequence of type II error.
We also excluded patients who had a history of hypothyroidism at baseline, because of the inability to interpret changes in thyroid function as being related solely to the underlying disease, as a result of treatment with immune-related AEs, or a combination of both.
Conclusion
Our findings suggest that medical oncologists can appropriately manage new-onset hypothyroidism in patients receiving checkpoint inhibitor therapy without a referral to the endocrinology department. In addition, referrals can delay the management of hypothyroidism and may lead to worsening of the patient’s thyroid function and delays in checkpoint inhibitor therapy.
Early detection of checkpoint inhibitor–induced hypothyroidism is key to preventing further complications of hypothyroidism and lowering the number of referrals to the endocrinology department. Studies are needed to identify and assess the risk factors for thyroid-related immune-related AEs.
Author Disclosure Statement
Dr Sion is an employee of Merck and is on the Advisory Board of Exelixis and Merck; Dr Alsuhebany, Dr Graham, and Dr Weeda have no conflicts of interest to report.
References
- Cukier P, Santini FC, Scaranti M, Hoff AO. Endocrine side effects of cancer immunotherapy. Endocr Relat Cancer. 2017;24:T331-T347.
- Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2:e192535. doi: 10.1001/jamanetworkopen.2019.2535.
- Haanen J, Obeid M, Spain L, et al; for the ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1217-1238.
- Chalan P, Di Dalmazi G, Pani F, et al. Thyroid dysfunctions secondary to cancer immunotherapy. J Endocrinol Invest. 2018;41:625-638.
- Girotra M, Hansen A, Farooki A, et al; for the Investigational Drug Steering Committee (IDSC) Immunotherapy Task Force collaboration. The current understanding of the endocrine effects from immune checkpoint inhibitors and recommendations for management. JNCI Cancer Spectr. 2018;2:pky021. doi: 10.1093/jncics/pky021.
- Illouz F, Drui D, Caron P, Do Cao C. Expert opinion on thyroid complications in immunotherapy. Ann Endocrinol (Paris). 2018;79:555-561.
- Jannin A, Penel N, Ladsous M, et al. Tyrosine kinase inhibitors and immune checkpoint inhibitors-induced thyroid disorders. Crit Rev Oncol Hematol. 2019;141:23-35.
- Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4:173-182.
- Zhan L, Feng HF, Liu HQ, et al. Immune checkpoint inhibitors-related thyroid dysfunction: epidemiology, clinical presentation, possible pathogenesis, and management. Front Endocrinol (Lausanne). 2021;12:649863. doi: 10.3389/fendo.2021.649863.
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28:583-589.
- Del Rivero J, Cordes LM, Klubo‐Gwiezdzinska J, et al. Endocrine‐related adverse events related to immune checkpoint inhibitors: proposed algorithms for management. Oncologist. 2020;25:290-300.
- Esfahani K, Meti N, Miller WH Jr, Hudson M. Adverse events associated with immune checkpoint inhibitor treatment for cancer. CMAJ. 2019;191:E40-E46.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Management of Immunotherapy-Related Toxicities. Version 1.2022. February 28, 2022. www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed October 10, 2022.
- Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9:e002435. doi: 10.1136/jitc-2021-002435.
- Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073-4126. Erratum in: J Clin Oncol. 2022;40:315.
- Yamauchi I, Yasoda A, Matsumoto S, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One. 2019;14:e0216954. doi: 10.1371/journal.pone.0216954.
- Kotwal A, Kottschade L, Ryder M. PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid. 2020;30:177-184.
- Basak EA, van der Meer JWM, Hurkmans DP, et al. Overt thyroid dysfunction and anti-thyroid antibodies predict response to anti-PD-1 immunotherapy in cancer patients. Thyroid. 2020;30:966-973.
- Ferrari SM, Fallahi P, Galetta F, et al. Thyroid disorders induced by checkpoint inhibitors. Rev Endocr Metab Disord. 2018;19:325-333.
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093-2104.
- Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789-1801.
- Muir CA, Clifton-Bligh RJ, Long GV, et al. Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab. 2021;106:e3704-e3713.
- Lu D, Yao J, Yuan G, et al. Immune checkpoint inhibitor-related new-onset thyroid dysfunction: a retrospective analysis using the US FDA Adverse Event Reporting System. Oncologist. 2022;27:e126-e132.
- Higham CE, Olsson-Brown A, Carroll P, et al. Society for Endocrinology Endocrine Emergency Guidance: acute management of the endocrine complications of checkpoint inhibitor therapy. Endocr Connect. 2018;7:G1-G7.
- Delivanis DA, Gustafson MP, Bornschlegl S, et al. Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab. 2017;102:2770-2780.
- Luongo C, Morra R, Gambale C, et al. Higher baseline TSH levels predict early hypothyroidism during cancer immunotherapy. J Endocrinol Invest. 2021;44:1927-1933.
- Brilli L, Danielli R, Campanile M, et al. Baseline serum TSH levels predict the absence of thyroid dysfunction in cancer patients treated with immunotherapy. J Endocrinol Invest. 2021;44:1719-1726.
- Thuillier P, Joly C, Alavi Z, et al. Thyroid dysfunction induced by immune checkpoint inhibitors is associated with a better progression-free survival and overall survival in non-small cell lung cancer: an original cohort study. Cancer Immunol Immunother. 2021;70:2023-2033.
- Baek HS, Jeong C, Shin K, et al. Association between the type of thyroid dysfunction induced by immune checkpoint inhibitors and prognosis in cancer patients. BMC Endocr Disord. 2022;22:89. doi: 10.1186/s12902-022-01004-8.
- Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952-1956.