Single-dose vials include a formulation that lacks antimicrobial preservatives to prevent microbial contamination. The Centers for Disease Control and Prevention have recently clarified inquiries related to the use of single-dose vials, indicating that they should be used only once for a single patient’s procedure or injection.1 United States Pharmacopeia (USP) General Chapter 797 (USP <797>) has established the guidance of sterility expiration of single-dose vials once they have been punctured.2
According to USP <797>, single-dose vials that have been opened or perforated with a needle can be used for up to 6 hours when handled in an air quality equal to or better than ISO Class 5.2
Vial conservation techniques take into consideration the medication’s beyond-use date, which is defined as “the date or time after which a [compounded sterile preparation or compounded nonsterile preparation] shall not be stored or transported,” and is calculated from the date or time of compounding. The current standards establish a beyond-use date based on the risk for microbial contamination in single-use and multi-use vials, which considers the conditions present during preparation and the time frame in which the medication is required to be used.2,3
Healthcare facilities in Europe have reported retaining partially used medication vials to reduce drug waste and conserve medications for future compounding needs.4,5 USP <797> presents a beyond-use date model that is referenced by international guidelines to determine the beyond-use date length of sterile hazardous and nonhazardous medicines.2 In USP <797>, the stability and sterility of sterile preparations and multidose vials are governed by the potential risk for contamination associated with the conditions present during the compounding process.2
Although currently postponed, draft USP <797> revisions were postponed in September 2021 that extended the beyond-use date of single-dose vials to up to 12 hours, provided that the vial is handled in an ISO Class 5 environment.6 The impossibility of reclosing the container once opened, and the absence of preservatives, imply an increased risk for microbiologic contamination, which is reflected in the short periods of recommended use for these drugs. In the current version of USP <797> that was implemented in 2008, and in the draft revisions proposed in 2021, the short sterility allowed in manufactured sterile, single-use preparations may lead to the discarding of medication and losses for pharmacy services that result in higher costs for preparation and potential medication shortage when the drug supply is constrained.7,8
The Canadian National Association of Pharmacy Regulatory Authorities (NAPRA) Model Standards for Pharmacy Compounding of Hazardous Sterile Preparations states that single-use vials have a 6-hour beyond-use date if punctured in a containment primary engineering control environment that maintains ISO Class 5 air quality, whereas multiple-use vials have a 28-day beyond-use date.9 The discarding of partial vials has raised concerns from healthcare professionals, because it would increase hazardous drug wastage and cost, and potentially exacerbate drug shortages.7,8
To address the Canadian NAPRA beyond-use date requirements, Cancer Care Ontario established a Beyond-Use Date Mitigation Strategy Working Group to develop clinical practices consistent with the NAPRA standards that target the reduction of drug waste.10 A focus of the working group was on evidence supporting the use of closed-system drug-transfer devices (CSTDs) to access vials and prevent the contamination of the vial and microbial contamination.10
The US National Institute for Occupational Safety and Health defines a CSTD as “a drug transfer device that mechanically prohibits the transfer of environmental contaminants into the system and the escape of the hazardous drug or vapor concentrations outside the system.”11 When used to access a vial, CSTDs enable fluid transfer between the drug vial and the final container without entry of airborne contaminants or other contaminants, while also preventing the manipulated medication from entering the environment.12-14 CSTD systems allow for the maintenance of vial sterility despite having been opened or perforated.12-14
The use of a CSTD system to access single-use vials could address the contamination concerns that drive the short (ie, 6 hours) beyond-use date period given by the USP <797> and the Canadian NAPRA.2,9
The goal of this study was to determine if the use of a CSTD in the local compounding environment can prevent microbial contamination of single-use vials and maintain sterility after vial access and repeated use for up to 9 days.
Methods
Tests were performed by a single technician who followed standard work procedures for hazardous drugs in the cytotoxic drug preparation unit of the pharmacy department of the Hospital Universitari Arnau de Vilanova in Lleida, Spain. The protocol tasks took place in a Class II, type B2 biological safety cabinet (ie, ISO-4 environment) installed in a Class B environment (ie, ISO-5).15,16
ChemoLock CSTD barrier vial adapters and syringe adapters were used for vial access and fluid transfer, respectively. The ChemoLock barrier system is a needle-free CSTD with a double membrane system that prevents the transfer of environmental contaminants into the system and prevents the escape of drug or vapor outside of the system. The CSTD system vial adapter access devices and syringe adapters are passively closed until the fluid path is opened with a connection.
In accordance with the USP <71> guidance for sterility tests,17 potential contamination from gram-positive and gram-negative microorganisms, anaerobic microorganisms, yeast, and mold was assessed using the 2 types of growth media of tryptic soy broth and fluid thioglycolate medium. All the media vials had manufacturer’s certificates for sterility and stimulation of microbial growth. Two temperature ranges (20°C-25°C and 30°C-35°C) were used for incubation to ensure optimum growth conditions for different microorganisms.
The method to test the sterility of single-use vials when accessed repeatedly was performed following guidance established by the European Pharmacopoeia and the USP <71>.17,18 These guidelines identify direct inoculation of the culture media with the drug to be examined, along with membrane filtration, as a suitable sterility test.17,18 For the study, the used vial itself was considered the drug to be examined, and thus no additional inoculation was needed.
The European Pharmacopoeia and the USP <71> guidelines also state the minimum number of items to be tested in relation to the size of the batch.17,18 For batches with more than 100 containers, but with no more than 500 containers, a minimum of 10 items with each medium is required.17,18
The definition of a batch is complex in continuous aseptic preparations of patient individual mixtures. The number of drugs prepared in the same working session was determined to be used as an appropriate reference batch size. The output of a typical pharmacy-based cytotoxic preparation unit is 75 to 125 preparations daily. Using the daily output as reference, a typical batch size would be approximately 100 vials, but no more than 500 vials. Using USP <71> and European Pharmacopoeia 5.0 as guidance, which indicate a minimum of 10 vials to be tested for this batch range,17,18 ten 100-mL vials of each growth media were tested.
The test vials were accessed using ChemoLock vial spikes under institutional standard procedures and were kept within the biological safety cabinet for the remainder of the sampling period at room temperature. One-mL aliquots were withdrawn and discarded from each vial at 0, 2 (48 hours), 4 (96 hours), 6 (144 hours), 7 (168 hours), and 9 days (216 hours).
Before each sample, vials were visually examined for evidence of contamination. At the end of this 9-day period, the vials were transferred to the microbiologic laboratory for incubation. The technician’s identity, the date and time of vial use, the sample removal identifier, and the record of transfer to the microbiologic laboratory were recorded in a laboratory notebook.
Five media vials of tryptic soy broth and fluid thioglycolate medium from each lot were segregated to act as negative and positive controls: 2 for negative controls and 3 for positive controls. The negative control vials were placed under the hood during the compounding process but were not manipulated. For the positive controls, we used the suitable strains of the test microorganisms according to the European Pharmacopoeia 5.0.18
Two positive fluid thioglycolate medium vials were inoculated with <102 colony-forming units of one of 2 American Type Culture Collection (ATCC) Stock strains—Pseudomonas aeruginosa or Staphylococcus aureus. The third fluid thioglycolate medium control vial was inoculated with a Clostridium perfringens strain from the microbiologic laboratory archive. Each of the 3 positive 100-mL control tryptic soy broth vials were inoculated with ≥102 colony-forming units of an ATCC Stock strain—Bacillus subtilis, Aspergillus brasiliensis, or Candida albicans—and were then incubated with the test samples.
All control vials were stored under the hood in identical conditions to the test vials and were transferred to the microbiologic laboratory for incubation at the end of the vial sampling period.
The test vials and controls were delivered to the hospital microbiology laboratory under temperature control after the completion of the 9-day cycle. Before incubation, the microbiology technicians ensured that the media solution was in contact with all the vial surfaces. The sample vials and the positive and negative controls were then incubated at 20°C to 25°C for 7 days, followed by incubation at 30°C to 35°C for an additional 7 days. The turbidity of the vials was visually inspected for growth every 2 days, and the data were recorded contemporaneously. Any positive growth was noted at the time of observation.
Results
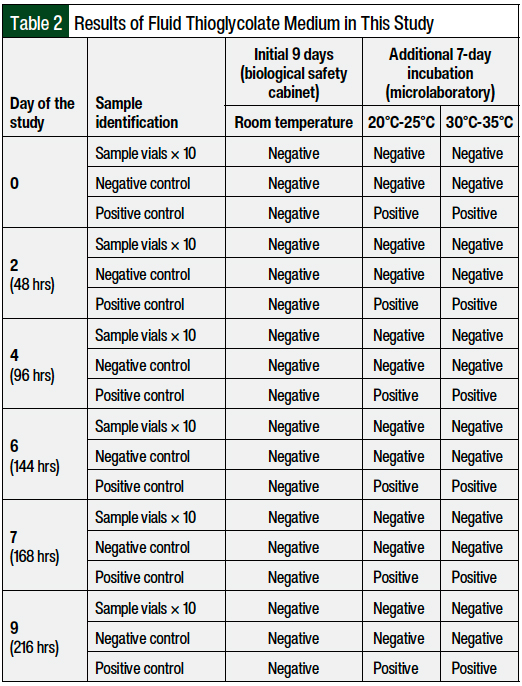
No deviations from the protocol were made, and no contamination was detected during the study period. A daily assessment of the vials for the 9-day period did not identify any turbidity or abnormality that would indicate contamination. After the additional 7-day incubation periods at 20°C to 25°C and 30°C to 35°C, the study vials and negative controls remained free of contamination, whereas all positive controls had microbial growth. Positive control growth, which was verified by turbidity, was evident in all positive controls at 48 hours.
The full turbidity test results, by growth medium, are shown in Table 1 and Table 2. All sample and negative control vials remained uniform, clear, and in light-amber solutions.
The ability of growth promotion by the media used was guaranteed by the manufacturer in the batch certificates. In addition, the reliability of the diluted tryptic soy broth products and the process was demonstrated by the positive controls. As shown in Tables 1 and 2, all the selected strains used in low numbers (102 colony-forming units in 100 mL of growth media) caused turbidity in all the positive controls after 48 hours of incubation, except those inoculated with A brasiliensis, where turbidity was observed at 144 hours of incubation.
Discussion
Vials containing nutrient media remained sterile over a 9-day period when using the ChemoLock barrier CSTD system. A low level of inoculation was used (100 colony-forming units per 100 mL of media fill), as stated by the European Pharmacopeia for sterility tests.18 The experimental procedure in this study was consistent with the USP <797> guidelines, which state that the validation of an aseptic procedure should be performed by simulation with nutrient media, also called media fills, to identify any contaminants.2
Our findings are consistent with previous studies, with or without the incorporation of CSTDs, that demonstrate the use of media-fill techniques to validate the compounding practices.19-21 Krämer and colleagues used the media-fill technique without a CSTD to confirm that individual ready-to-use injection solutions could be prepared in a sterile manner with a robotic system and manually in cytotoxic workbenches in the same cleanroom environment.19
Garrigue and colleagues compared 2 CSTD systems for the maintenance of vial sterility after a single vial access using the media-fill test and showed that both systems were successful.20
Ho and colleagues confirmed the effectiveness of a CSTD system in the prevention of microbiologic contamination of a growth media bag after repeated injection of an antineoplastic agent.21 In their study, aliquots of the test agent were transferred to tryptic soy broth culture medium in intravenous bags over a 2-week period using a CSTD system.21 No microbial growth was observed throughout a 14-day period, which supports the conclusion that the CSTD system maintained the sterility of the unpreserved injectable solutions and may be used to extend the beyond-use date of single-use vials to 7 days or longer.21
These 3 studies simulated the preparation of sterile solutions, but they did not address repeated growth media vial use with a CSTD,19-21 which may best represent the maintenance of single-use vial sterility after an initial vial puncture. Our results provide further evidence supporting the potential for the extension of beyond-use date for single-use vials, when a CSTD system is used in compounding.
The use of CSTDs for vial access and fluid transfer may help to address deficiencies in sterile compounding practices. Canadian practice surveys have led to recommendations advocating for critical assessment and improvement in practice.22 The contamination rates of injectable doses prepared in pharmacy-controlled environments may be unacceptably high, as concluded in a review study by Austin and Elia.23
Beyond-use date limits are established to address these and other contamination considerations with medication handling under varying conditions. The published evidence evaluating the impact of CSTDs on compounding vial sterility highlights the importance of validating additional compounding practices that reduce contamination and that may justify increasing the beyond-use date limits for single-dose vials beyond 6 hours.
The Cancer Care Ontario Beyond-Use Date Mitigation Strategy Working Group provides recommendations to address medication wastage by using a CSTD in compounding single-use vials while complying with the Canadian NAPRA standards.10 The working group recommended that a CSTD may be used to extend the beyond-use date of single-dose vials up to 7 days when supported by the results of facility-level sterility testing using the specific device and when done in accordance with the NAPRA Model Standards (using nutrient or culture media as outlined in the NAPRA Model Standards).9,10
The results of this study meet the recommendations for the beyond-use date extension from the Ontario working group and suggest that beyond-use date sterility may be studied for up to 9 days after the initial vial access.10 Additional studies are required to support a beyond-use date extension of up to 9 days in CSTD-facilitated vial conservation practices. The outcomes of vial conservation include financial benefits and the preservation of medication when a drug supply is constrained.24
Limitations
This study has several limitations. Determining contamination by visual inspection may be inferior to inoculating and incubating plates of growth media. We chose visual examination over plating, however, because we desired to maintain a completely closed system to eliminate any possibility of an additional source of contamination, which may confound our results.
Bacterial endotoxin testing was not performed in our study. This test did not apply in this study, because our aim was to show that CSTD use prevents bacterial contamination in single-use vials that are otherwise sterile and free of endotoxins. Because no additional liquid was introduced in the tested vials when accessed, there was no risk for endotoxin contamination of the tested vials.
Process simulation tests using growth media are valuable for evaluating aseptic procedures and the microbiologic stability of sterile preparations.25 However, previously published results evaluating the test method raise a concern about such tests’ sensitivity to determine proper aseptic technique in compounding sterile preparations.26
In our study, the test vials were kept within the biological safety cabinet for the entire sampling period at room temperature. If an extension of the beyond-use date is accepted by the facility, keeping the vials in the biological safety cabinet would not be advisable. In new uses, used vials should be stored in a refrigerator outside the biological safety cabinet; however, not all injectable solutions can be refrigerated. Moreover, in the context of our study, refrigeration would have introduced a confounder in the interpretation of the results, because cold temperature negatively affects the viability of the microorganisms that were eventually introduced in the test vials during the procedures. For this reason, our test method of incubating the test vials at room temperature was deemed to represent a more challenging test method, by being more conducive to microbial growth.
Drug stability may limit the application of extending the use of single-dose vials and other medication vials. The drug stability characteristics were not evaluated in this study and should be considered when determining the extension of the beyond-use date. The drug’s stability, as communicated by a drug manufacturer’s prescribing information, along with other published resources,27 should be referenced when establishing a beyond-use date of a medication.
Conclusion
Based on our findings, the sterility of the media fills after 9 days of repeated access suggests that the use of the ChemoLock barrier CSTD system may prevent microbial contamination of single-use vials for up to 9 days. These results address procedural breakdowns in the current practice leading to potential contamination and provide evidence for extending the single-use vial beyond-use date recommendations of USP <797> to more than 6 hours when used with the CSTD.
Prolonged sterility of single-dose vials may result in improved medication supply in times of drug shortage, increased efficiency, and could prevent waste of high-cost medications. This CSTD compounding protocol may be applicable to many medications, including cytotoxic drugs that are intended for administration or further dilution.
Disclaimer
The information presented in this study is not meant to replace the drug manufacturers’ prescribing information or current guidance for single-use vials.1,2 The extension of the beyond-use date may be specific to certain facilities and should not be applied without consideration for drug- or prescribing-specific characteristics and facility-specific sterility confirmation.
Acknowledgments
We are grateful to the Hospital Pharmacy and Microbiology technicians of the Hospital Universitari Arnau de Vilanova for their help in performing this study.
Funding Source
Funding for this study was provided by ICU Medical.
Author Disclosure Statement
Dr Schoenenberger-Arnaiz is a Consultant to Biogen, AstraZeneca, and Bayer; Mr Battle is an employee of ICU Medical, which funded this study; Dr Nevot-Blanc, Dr Garcia-Gonzalez, Dr Martinez-Sogues, Ms Moroba-Estor, and Dr Rumi-Carrera have no conflicts of interest to report.
References
- Centers for Disease Control and Prevention. Questions about single-dose/single-use vials. June 20, 2019. www.cdc.gov/injectionsafety/providers/provider_faqs_singlevials.html. Accessed June 27, 2022.
- USP <797> pharmaceutical compounding—sterile preparations. Design. 2007;5(Class 100):1-61.
- United States Pharmacopeial Convention. USP General Chapter <800> Hazardous Drugs–Handling in Healthcare Settings. 2017. Reprinted from USP 40—NF 35, Second Supplement (2017). www.usp.org/sites/default/files/usp/document/our-work/healthcare-quality-safety/general-chapter-800.pdf. Accessed June 15, 2022.
- Ripoll Gallardo A, Meneghetti G, Ragazzoni L, et al. Multiple withdrawals from single-use vials: a study on sterility. Int J Pharm. 2015;485:160-163.
- Örnek K, Karahan ZC, Ergin A, et al. Bevacizumab sterility in multiple doses from a single-use vial. Ann Pharmacother. 2008;42:1425-1428.
- US Pharmacopeia. Proposed revisions to <797> pharmaceutical compounding—sterile preparations. September 1, 2021. https://go.usp.org/l/323321/2021-08-31/5kmjww/323321/16304645801icecobH/797_PHARMACEUTICAL_COMPOUNDING_STERILE_PREPARATIONS_POST_Revised.pdf. Accessed June 15, 2022.
- Rowe EC, Savage SW, Rutala WA, et al. Economic and microbiologic evaluation of single-dose vial extension for hazardous drugs. J Oncol Pract. 2012;8:e45-e49.
- Edwards MS, Solimando DA Jr, Grollman FR, et al. Cost savings realized by use of the PhaSeal closed-system transfer device for preparation of antineoplastic agents. J Oncol Pharm Pract. 2013;19:338-347.
- National Association of Pharmacy Regulatory Authorities. Model Standards for Pharmacy Compounding of Hazardous Sterile Preparations. Revised November 2016. www.napra.ca/sites/default/files/2017-09/Mdl_Stnds_Pharmacy_Compounding_Hazardous_Sterile_Preparations_Nov2016_Revised_b.pdf. Accessed June 15, 2022.
- Cancer Care Ontario. Cancer Care Ontario Beyond-Use Date Recommendations Report. March 2018. www.cancercareontario.ca/sites/ccocancercare/files/guidelines/full/BUD%20Recommendations%20Report%20final_0.pdf. Accessed June 15, 2022.
- National Institute for Occupational Safety and Health. NIOSH Alert: preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. September 2004. www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf?id=10.26616/NIOSHPUB2004165. Accessed February 2, 2022.
- De Prijck K, D’Haese E, Vandenbroucke J, et al. Microbiological challenge of four protective devices for the reconstitution of cytotoxic agents. Lett Appl Microbiol. 2008;47:543-548.
- McMichael DM, Jefferson DM, Carey ET, et al. Utility of the PhaSeal closed system drug transfer device. Am J Pharm Benefits. 2011;3:9-16.
- Carey ET, Forrey RA, Haughs D, et al. Second look at utilization of a closed-system transfer device (PhaSeal). Am J Pharm Benefits. 2011;3:311-318.
- NSF International; American National Standards Institute. NSF International Standard/American National Standard: NSF/ANSI 49–2008: Biosafety Cabinetry: Design, Construction, Performance, and Field Certification. Revised October 2008. www.halco-products.com/links/resources/brochures/NSF_49-08e-rep-watermarked.pdf. Accessed February 2, 2022.
- International Organization for Standardization. ISO 14644-1:2015: Cleanrooms and associated controlled environments—Part 1: classification of air cleanliness by particle concentration. 2nd ed. December 15, 2015. www.iso.org/standard/53394.html. Accessed February 2, 2022. [Requires payment to access.]
- United States Pharmacopeial Convention. USP <71> Sterility Tests. December 1, 2012. www.triphasepharmasolutions.com/Private/USP%2071%20STERILITY%20TESTS.pdf. Accessed July 1, 2022.
- Council of Europe. 2. Methods of analysis: Section 2.6.1. Sterility. In: European Pharmacopoeia 5.0. Strasbourg, France: Council of Europe; 2005:145-149.
- Krämer I, Federici M, Kaiser V, Thiesen J. Media-fill simulation tests in manual and robotic aseptic preparation of injection solutions in syringes. J Oncol Pharm Pract. 2016;22:195-204.
- Garrigue P, Montana M, Ventre C, et al. Safe cytotoxic drug preparation using closed-system transfer device: technical and practical evaluation of a new device (Vialshield/Texium) comparatively to a reference one (Phaseal). Int J Pharm Compd. 2016;20:148-154.
- Ho KV, Edwards MS, Solimando DA Jr, Johnson AD. Determination of extended sterility for single-use vials using the PhaSeal closed-system transfer device. J Hematol Oncol Pharm. 2016;6(2):46-50.
- Warner T, Nishi C, Checkowski R, Hall KW. Survey of sterile admixture practices in Canadian hospital pharmacies: Part 1. Methods and results. Can J Hosp Pharm. 2009;62:100-111.
- Austin PD, Elia M. A systematic review and meta-analysis of the risk of microbial contamination of aseptically prepared doses in different environments. J Pharm Pharm Sci. 2009;12:233-242.
- Gilbar PJ, Chambers CR, Vandenbrouche J, et al. How can the use of closed system transfer devices to facilitate sharing of drug vials be optimised to achieve maximum cost savings? J Oncol Pharm Pract. 2019;25:205-209.
- Pharmaceutical Inspection Co-operation Scheme. Recommendation on the validation of aseptic processes. PI 007-6. January 1, 2011. https://picscheme.org/docview/3446. Accessed March 15, 2021.
- Moody CA, Eckel SF, Amerine LB. Evaluating the sensitivity of a media-fill challenge test under various situations as a reliable method for recommended aseptic technique competency assessment. J Pharm Technol. 2016;32:47-53.
- Engel J, Lazar N. Guidelines for the establishment of appropriate beyond use dating of sterile compounded admixtures. Hosp Pharm. 2016;51:654-655.