Hypercalcemia of malignancy is a metabolic complication of cancer that is potentially life-threatening, with an estimated in-hospital mortality rate of 6.8%.1-4 Hypercalcemia of malignancy affects up to 30% of patients with cancer and occurs most frequently in patients with advanced malignancies, particularly lung cancer, multiple myeloma, and renal-cell carcinoma.2,5 The proposed mechanisms of hypercalcemia of malignancy include increased parathyroid hormone–related peptide, local osteolytic hypercalcemia, excess extrarenal vitamin D activation, and parathyroid hormone secretion.1,6
The clinical symptoms of hypercalcemia of malignancy include lethargy, nausea and vomiting, musculoskeletal pain, and in severe cases, neurocognitive dysfunction, volume depletion, and renal failure.1,7 Because hypercalcemia of malignancy frequently presents with acute kidney injury, it is critical to evaluate each individual patient’s renal function and apply dose reduction strategies as needed to ensure the optimal dosing of pharmacologic therapy.8
Hypercalcemia of malignancy can be treated by targeting the underlying malignancy, but in the acutely hospitalized patient, the initial goal is to lower the serum calcium level by promoting renal excretion and inhibiting further bone resorption.6.9 The management strategies for hypercalcemia of malignancy include fluid resuscitation, intravenous (IV) bisphosphonates, loop diuretics, calcitonin, glucocorticoids, and in bisphosphonate-refractory cases, denosumab.6,9
IV bisphosphonates are typically given immediately after fluid resuscitation, because they produce potent inhibition of osteoclast resorption leading to prompt reduction in serum calcium levels.1 Zoledronic acid and pamidronate are often used in this clinical setting, with zoledronic acid being the preferred agent, because head-to-head comparisons have demonstrated improved efficacy versus pamidronate.7,10,11
However, IV bisphosphonate treatment has been associated with nephrotoxicity, complicating its use in the treatment of hypercalcemia of malignancy, which frequently presents with renal insufficiency.12 Pamidronate-induced nephrotoxicity results from collapsing focal segmental glomerulosclerosis, which occurs often with long-term use (15-48 months) of pamidronate and at higher-than-recommended doses.12 Zoledronic acid is mainly associated with acute tubular necrosis that can progress to renal failure, even at the recommended doses and infusion times.12
Overexposure to IV bisphosphonates in patients with renal insufficiency is associated with a known risk for corresponding hypocalcemia, which can result in significant morbidity, because it is often severe and refractory to calcium repletion.13-15
Zoledronic acid undergoes minimal hepatic metabolism and thus is primarily eliminated via renal excretion, with drug clearance dependent on creatinine clearance (CrCl).16 Pharmacokinetic studies of zoledronic acid in renal insufficiency show an increase in drug exposure with declining renal function.16-18 Of note, although renal dose reduction recommendations are available for zoledronic acid for bone metastases, no such recommendations exist for hypercalcemia of malignancy.16
Prolonging drug infusion time to more than 15 minutes for zoledronic acid, and to more than 2 hours for pamidronate, is suggested to reduce the risk for renal adverse events that have been associated with increased peak levels of bisphosphonates.16,19 Although clinical trials of zoledronic acid for hypercalcemia of malignancy included patients with serum creatinine up to 4.5 mg/dL, patients with moderate-to-severe renal impairment were a minority of the population studied,7,16,18,20 making it difficult to extrapolate safety and efficacy outcomes.
Pamidronate is eliminated exclusively through renal excretion.19 Pharmacokinetic studies demonstrate that patients with CrCl <30 mL/min have more than twice the exposure to pamidronate than patients with CrCl >90 mL/min.20,21 However, there are no dose reduction recommendations for pamidronate for patients with hypercalcemia of malignancy and mild-to-moderate renal impairment, and limited data exist for patients with hypercalcemia of malignancy and severe renal impairment (CrCl <30 mL/min).19
In practice, providers may opt for different dosing strategies for patients with hypercalcemia of malignancy with renal impairment, but the effect of these strategies is not well-characterized in the literature.
Considering the scarcity and limitations of previous studies of IV bisphosphonates for the treatment of hypercalcemia of malignancy in patients with renal dysfunction, it is still unclear whether the presence of baseline renal dysfunction alters the safety and efficacy of bisphosphonates. The objective of this study was to compare the safety and efficacy of zoledronic acid and pamidronate in patients with hypercalcemia of malignancy and with or without baseline renal dysfunction, defined as a cutoff of CrCl ≥60 mL/min.
We chose this cutoff based on the prescribing information for zoledronic acid for patients with multiple myeloma or bone metastases of solid tumors, which recommends a dose reduction with CrCl <60 mL/min, suggesting a clinically relevant decrease in drug clearance and an increase in drug exposure at this CrCl cutoff, based on pharmacokinetic studies.16
Methods
This retrospective cohort study included patients aged ≥18 years who were hospitalized at UC Davis Medical Center between January 1, 2012, and October 1, 2020, and received IV zoledronic acid or IV pamidronate for hypercalcemia of malignancy. Patients were excluded if they had an allergic reaction or sensitivity to bisphosphonates or if they received IV or oral bisphosphonates within the last 90 days for any indication.
The patients were divided into 2 groups based on CrCl. Patients with normal renal function, which was defined as CrCl ≥60 mL/min, were matched in a 1:1 ratio with patients with reduced kidney function, which was defined as CrCl <60 mL/min. The patients were identified using our institutional data analytics software based on documented administration of IV zoledronic acid or IV pamidronate. All the study procedures were approved by our Institutional Review Board.
Hypercalcemia was defined as a corrected serum calcium of ≥10.5 mg/dL, which was calculated using the following equation: corrected serum calcium = serum calcium + 0.8 * (4 − serum albumin) for patients with a serum albumin of <4 g/dL. If no albumin value was available on the day that the calcium was collected, the most recent value was used.
Hypocalcemia was defined as a corrected serum calcium of <8.5 mg/dL. Renal dysfunction was defined using the following cutoffs: grade 1 serum creatinine elevation as an increase in serum creatinine of >1 to 1.5 times baseline or the upper limit of normal (ULN), grade 2 as a serum creatinine increase of >1.5 to 3 times baseline or the ULN, grade 3 as a serum creatinine increase of >3 to 6 times baseline or the ULN, and grade 4 as a serum creatinine elevation of >6 times the ULN. Serum creatinine was estimated using the Cockcroft-Gault equation.22 All definitions for the laboratory abnormalities were based on Common Terminology Criteria for Adverse Events, Version 5.0.23
The primary safety outcome was all-grade serum creatinine elevation by day 7. The secondary safety outcomes were the incidence of grades 1 to 4 serum creatinine elevation; the onset of serum creatinine elevation; hypocalcemia within 30 days; and the lowest corrected serum calcium after the index date (ie, the date of the first bisphosphonate administration) if hypocalcemia occurred. The incidence of osteonecrosis of the jaw up to 1 year after the index date was also collected.
The primary efficacy outcome was complete response, defined as the normalization of corrected serum calcium to ≤10.5 mg/dL by day 10. The secondary efficacy outcomes were the incidence of corrected serum calcium decrease by 1 mg/dL by day 7, the incidence of relapsed or refractory hypercalcemia by day 30, and the number of days of hospitalization. Relapsed or refractory hypercalcemia was defined as the patient requiring an additional dose of zoledronic acid or pamidronate within 30 days.
These outcomes and time points are consistent with those used in clinical trials of bisphosphonates for the treatment of hypercalcemia of malignancy.7 Subgroup analyses were performed based on the severity of the baseline renal function (CrCl <30 mL/min and CrCl ≥30 mL/min) and the specific bisphosphonate received. The efficacy and safety outcomes were also stratified by IV bisphosphonate dose and infusion time.
The baseline serum creatinine and calcium values were defined as the most recent value before the administration of a bisphosphonate. If no calcium value was available on any specified time point, the closest value was used. If the same patient received more than 1 bisphosphonate, the data for each administration were collected if the interval in between the 2 administrations was greater than 6 months.
A sample size of 62 patients per group, for a total of 124 patients, was estimated to provide 80% power to detect a 20% difference in the primary outcome of all-grade serum creatinine elevation. The difference of 20% was estimated from the reported prevalence of renal insufficiency with zoledronic acid in patients with and without renal dysfunction.24
Fisher’s exact test was used to compare the primary safety and efficacy outcomes of all-grade serum creatinine elevation and complete response by day 10 between the patients with and without renal dysfunction, as well as between the zoledronic acid and pamidronate groups.
All the secondary outcomes were analyzed using Fisher’s exact test if they were discrete, and with descriptive statistics if they were continuous. The statistical significance between the comparisons was defined as a P value <.05.
Results
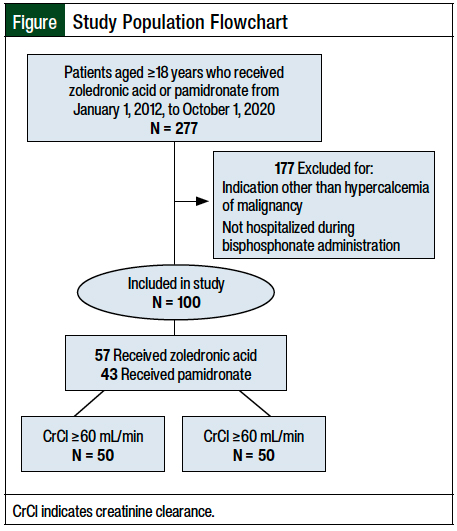
Initially, 277 patients were identified who were aged ≥18 years and received IV zoledronic acid or pamidronate between January 1, 2012, and October 1, 2020 (Figure). The most common reason for study exclusion was not being hospitalized during bisphosphonate administration or receiving bisphosphonates for an indication other than hypercalcemia of malignancy (N = 177).
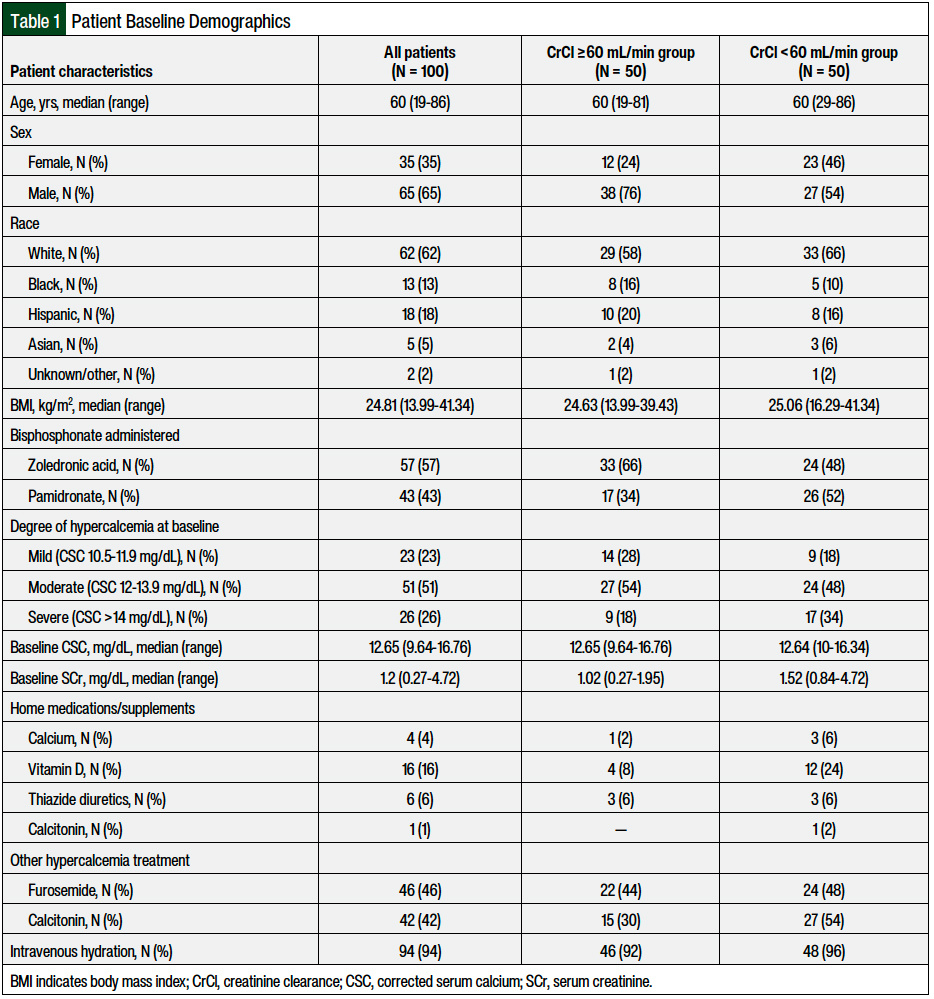
A total of 100 patients met the inclusion criteria and were included in this analysis—50 patients with renal dysfunction and 50 patients without renal dysfunction. The patients’ baseline characteristics are shown in Table 1. The median age was 60 years, and 65% of the patients were men. Among all the patients, 57 patients received zoledronic acid and 43 patients received pamidronate. A total of 33 (66%) and 17 (34%) of the patients with renal dysfunction and 24 (48%) and 26 (52%) of the patients without renal dysfunction received zoledronic acid and pamidronate, respectively. The median baseline corrected serum calcium was 12.65 mg/dL (range, 9.64-16.76 mg/dL), and the median serum creatinine was 1.2 mg/dL (range, 0.27-4.72 mg/dL). The CrCl at baseline was relatively evenly distributed.
A total of 22% of the patients had CrCl ≥90 mL/min, 28% had CrCl of 60 to 90 mL/min, 32% had CrCl of 30 to 60 mL/min, and 18% had CrCl <30 mL/min. Most patients (51%) had moderate hypercalcemia (12-13.9 mg/dL) at baseline. More patients with renal dysfunction (CrCl <60 mL/min) had severe hypercalcemia (>14 mg/dL), received pamidronate, and were taking vitamin D as a home supplement than patients without renal dysfunction (CrCl ≥60 mL/min). No other major differences were observed in the baseline characteristics between the 2 groups (Table 1).
The overall safety and efficacy outcomes are shown in Table 2. No significant differences were observed between the groups for the primary safety outcome of all-grade serum creatinine elevation, which occurred in 18 (36%) of the 50 patients with renal dysfunction and in 14 (28%) of the 50 patients without renal dysfunction (P = .52).
All-grade serum creatinine elevation occurred in 20 (35.1%) of the 57 patients who received zoledronic acid and in 12 (27.9%) of the 43 patients who received pamidronate (P = .52). Statistical power was not met for this primary analysis. There were no significant differences between the cohorts in the primary efficacy end point of complete response by day 10 with respect to the bisphosphonate received or baseline renal function.
In all, 39 (78%) of the 50 patients with renal dysfunction and 33 (66%) of the 50 patients without renal dysfunction (P = .13) achieved a complete response by day 10. A total of 41 (71.9%) of the 57 patients receiving zoledronic acid and 31 (72.1%) of the 43 patients receiving pamidronate achieved a complete response by day 10 (P = .58).
Among the 100 patients in the study, 32 (32%) had all-grade serum creatinine elevations by day 7, of whom 24 (75%) and 8 (25%) patients had grade 1 and grade 2 serum creatinine elevations, respectively. No grade 3 or 4 serum creatinine elevations were identified. There were no significant between-grade differences in serum creatinine elevations in the zoledronic acid versus the pamidronate group.
Of the 57 patients who received zoledronic acid, 16 (28.1%) and 4 (7%) patients had grades 1 and 2 serum creatinine elevations, respectively; of the 43 patients who received pamidronate, 8 (18.6%) and 4 (9.3%) had grades 1 and 2 serum creatinine elevations, respectively (P = .58).
A similar trend was observed in patients with and without renal dysfunction. Of the 50 patients with CrCl <60 mL/min and the 50 patients with CrCl ≥60 mL/min, 10 (20%) and 14 (28%), respectively, had grade 1 serum creatinine elevations, and 4 (8%) and 4 (8%), respectively, had grade 2 serum creatinine elevations (P = .62).
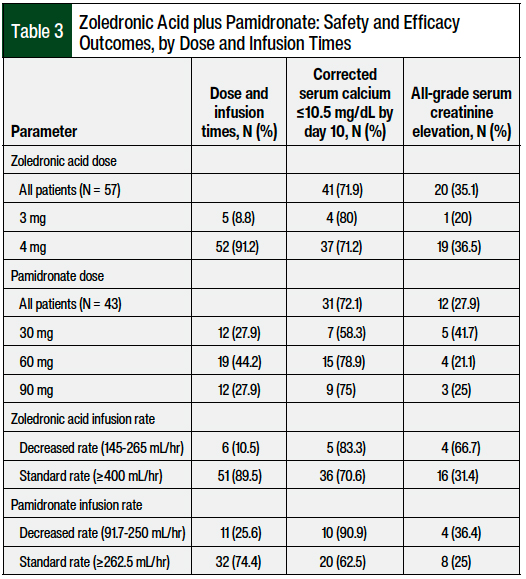
The safety and efficacy outcomes stratified by dose and infusion times are shown in Table 3. The most common dose and infusion time of zoledronic acid was 4 mg over 15 minutes at the standard rate of ≥400 mL/hr, and the most common dose and infusion time of pamidronate was 60 mg over 2 hours at the standard rate of ≥262.5 mL/hr. Of the patients who received zoledronic acid at the standard (4 mg) and reduced (3 mg) doses, 37 (71.2%; N = 52) and 4 (80%; N = 5) patients, respectively, achieved a complete response by day 10. Of the patients who received pamidronate at reduced doses (30 mg), 7 (58.3%; N = 12) achieved a complete response by day 10 compared with 15 (78.9%; N = 19) and 9 (75%; N = 12) patients who received pamidronate at the standard doses of 60 mg and 90 mg, respectively.
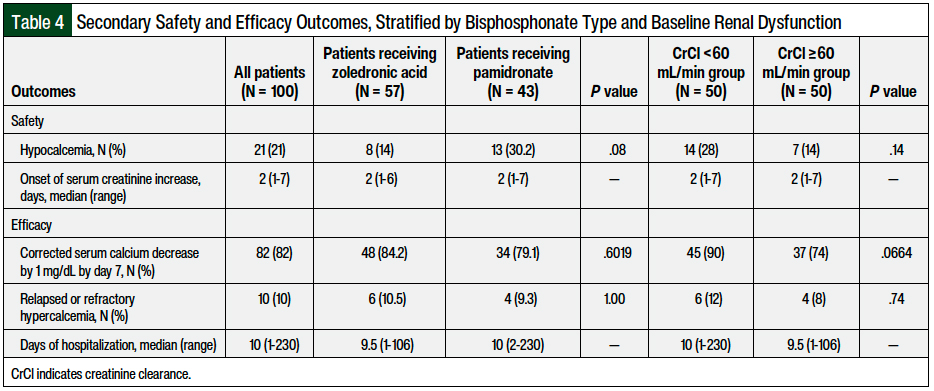
The secondary safety and efficacy outcomes stratified by bisphosphonate type and baseline renal dysfunction are shown in Table 4. Hypocalcemia occurred in 8 (14%) of the 57 patients who received zoledronic acid and in 13 (30.2%) of the 43 patients who received pamidronate (P = .08); a total of 7 (14%) of the 50 patients without renal dysfunction and 14 (28%) of the 50 patients with renal dysfunction (P = .14) had hypocalcemia. The median onset of serum creatinine increase was 2 days (range, 1-7 days).
There were no significant differences between the zoledronic acid and pamidronate groups or between the patients with and without renal dysfunction in the outcomes of corrected serum calcium decrease by 1 mg/dL by day 7 (84.2% with zoledronic acid vs 79.1% with pamidronate; P = .6) or relapsed or refractory hypercalcemia (10.5% with zoledronic acid vs 9.3% with pamidronate; P = 1).
The median duration of hospitalization was 10 days (range, 1-230 days) for all of the patients, which did not differ between the patients with and without renal dysfunction or between the patients receiving zoledronic acid or pamidronate. None of the patients had osteonecrosis of the jaw.
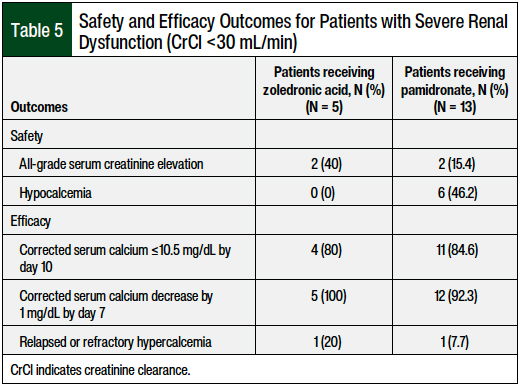
In the subgroup of patients (N = 18) with severe renal dysfunction (CrCl <30 mL/min), the safety and efficacy results were similar overall between the zoledronic acid and pamidronate cohorts, although this sample of 18 patients was notably small (Table 5). Of the 18 patients with CrCl <30 mL/min, 5 (28%) received zoledronic acid and 13 (72%) received pamidronate.
All-grade serum creatinine elevation occurred in 2 (40%) of the 5 patients receiving zoledronic acid and in 2 (15.4%) of the 13 patients receiving pamidronate. Hypocalcemia occurred in none of the 5 patients receiving zoledronic acid and in 6 (46.2%) of the 13 patients receiving pamidronate. There were no differences between the 2 bisphosphonate groups for the primary efficacy outcome of complete response by day 10.
Discussion
To our knowledge, this is the first comparison of the safety and efficacy of IV bisphosphonates in patients with and without renal dysfunction, specifically for the indication of hypercalcemia of malignancy. Our results reveal that acute kidney injury occurs in up to 32% of all patients receiving IV bisphosphonates for hypercalcemia of malignancy, and these findings add to the dearth of literature evaluating the use of IV bisphosphonates in this clinical scenario.
Of note, we found no significant difference in all-grade serum creatinine elevations between the patients receiving IV zoledronic acid or those receiving IV pamidronate for hypercalcemia of malignancy, regardless of baseline renal function. Furthermore, the median days of hospitalization was approximately 10 days in all groups, suggesting a minimal effect of the bisphosphonate received or the baseline renal function on patients’ outcomes.
In patients with acute kidney injury, serum creatinine elevations were mild in severity, with 75% of patients having grade 1 serum creatinine elevations. There were no grade 3 or 4 serum creatinine elevations identified. The use of furosemide was similar in both groups, and therefore is unlikely to be a confounder for these comparisons. Collectively, these results reinforce the clinical notion that IV bisphosphonate-induced nephrotoxicity occurs frequently in patients who are receiving treatment for hypercalcemia, although it is of limited clinical significance, and it may not be impacted by baseline renal function or the choice of bisphosphonate.
Similar to our primary safety analyses, we found no impact of baseline renal function or choice of IV bisphosphonate on the primary efficacy outcome of complete response by day 10. Overall, the complete response rates in our study are within the range of previously reported values of the treatment of hypercalcemia of malignancy with IV bisphosphonates.7,25
However, our results contrast with the seminal article by Major and colleagues, which demonstrated the increased efficacy of zoledronic acid compared with pamidronate for the treatment of hypercalcemia of malignancy.7 This is likely the result of several differences between the protocol used in the study by Major and colleagues and the regular clinical care of patients at our center.
Examples of such differences include the degree of hydration before bisphosphonate administration, the frequency of retreatment with bisphosphonates before day 10, the use of corticosteroids and/or cytotoxic anticancer therapy, and the differences in baseline characteristics that may impact hypercalcemia of malignancy outcomes, such as the percent of patients with bone metastases.7
In clinical practice, the most challenging patients with hypercalcemia of malignancy are those with CrCl <30 mL/min, given the scarcity of safety data to guide the choice of IV bisphosphonate, the appropriateness of dose adjustment, and the rate of infusion. In addition to the potential to worsen the patient’s renal function, the use of IV bisphosphonates for hypercalcemia carries the counterintuitive risk for refractory hypocalcemia, which can occur in patients with low CrCl values by the administration of IV bisphosphonate.14,15
This risk for refractory hypocalcemia is thought to result from a reduction in the renal elimination of bisphosphonates that leads to increased drug exposure. In severe cases, patients with hypocalcemia can be refractory to oral and IV calcium repletion, which may significantly impact morbidity.16,19
Our retrospective review only identified 18 patients with CrCl <30 mL/min. Therefore it is challenging to make any strong conclusions in this subset of patients. In general, in patients with CrCl <30 mL/min (Table 5), pamidronate was more often used than zoledronic acid (13 vs 5 patients, respectively), all-grade serum creatinine elevation occurred at a low incidence (15.4%), hypocalcemia was still a common occurrence (46.2%), and the rates of complete response by day 10 were similar to the rates in patients who received zoledronic acid.
For patients receiving IV pamidronate, the use of a reduced rate of infusion did not affect the efficacy, although it also did not improve safety outcomes. The same was true of dose reductions with pamidronate, with the exception of the 30-mg dose reduction, for which the rate of complete response by day 10 was low (58.3%) compared with the 90-mg (75%) and 60-mg (78.9%) doses. Altogether, our findings suggest that pamidronate doses up to 90 mg, given at a reduced infusion rate, are a safe and effective alternative for patients who present with severe renal dysfunction.
Our findings in patients with CrCl <30 mL/min are similar to the limited published outcomes for this patient population.25,26 Norman and colleagues performed a single-site, retrospective review of IV pamidronate treatment for hypercalcemia, which included 25 patients with preexisting renal dysfunction (defined as CrCl <30 mL/min or serum creatinine >3 mg/dL).26 The results showed that only 2 (8%) of these 25 patients had any grade of acute kidney injury, and this was not influenced by the dose of IV pamidronate used or by the rate of infusion. Furthermore, although complete response rates were not reported, the study showed no impact of the dose of pamidronate on the time to calcium normalization.26
A similar single-site, retrospective analysis of IV bisphosphonate use for the treatment of hypercalcemia by Palmer and colleagues included 41 patients with CrCl <30 mL/min, of whom 33 (81%) received IV pamidronate.25 The results showed no relationship between the dose of pamidronate or the infusion duration and the safety or efficacy outcome measures. Of note, Palmer and colleagues reported a high incidence (10.9%) of grade 3 or 4 serum creatinine elevations in patients receiving IV pamidronate, although this may be confounded by differences in the clinical care of patients with low CrCl values, such as the use of furosemide, or the severity of hypercalcemia.25
The conclusions of these studies align with our findings that pamidronate is a safe alternative to full-dose IV zoledronic acid in patients with CrCl <30 mL/min, and that dose reductions and/or modified infusion rates of IV pamidronate remain of unclear benefit.
Limitations
Our study has several limitations, the first being its retrospective and nonrandomized design, and our patients being limited to a single academic medical center. Therefore, there are likely confounders, such as the preferential use of pamidronate or modified infusion rates for patients with perceived baseline renal insufficiency, which may affect the outcome comparisons.
In addition, concomitant hypercalcemia therapy might have differed between the groups based on the severity of hypercalcemia of malignancy or renal function. In an attempt to control for factors that might influence the outcomes of interest, we obtained information on the home medications that could potentially affect serum calcium, such as diuretics and calcium supplementation, as well as the use of adjunctive hypercalcemia treatments and IV hydration.
Furthermore, statistical power was not met for our primary efficacy or safety outcomes, and therefore caution is warranted in these statistical comparisons. Despite this, a comparison of the raw numbers between these 2 groups of patients with and without renal dysfunction reveals that it is unlikely that additional patient enrollment would result in a significant difference in these outcome measures.
Moreover, missing data points were coded as not meeting the outcome, so it is possible that the incidence of certain outcomes may be underestimated.
Finally, we acknowledge that serum creatinine elevation is only a surrogate for renal injury, and that measuring serum creatinine values before drug administration may not be the most ideal evaluation of renal status. It is possible that the incorporation of other patient data, such as urine output, may provide a more complete picture of renal function. However, these data were not available to us in our retrospective review.
Conclusion
This is the first comparison of the safety and efficacy of IV bisphosphonates in patients with and without renal dysfunction, specifically for the indication of hypercalcemia of malignancy. We found no significant differences in safety or efficacy outcomes with respect to the IV bisphosphonate received or the baseline renal function status in patients with hypercalcemia of malignancy.
Collectively, these results highlight the need for future prospective studies of patients with hypercalcemia of malignancy with CrCl <30 mL/min, with the goal of characterizing the impact of bisphosphonate selection, dose, and rate of infusion on the most clinically relevant outcomes, such as days of hospitalization, symptomatic hypocalcemia, and overall survival.
Author Disclosure Statement
Dr Beechinor has received funding/compensation for consulting and/or research activities unrelated to this study from the National Institute of Health, Eunice Kennedy Shriver National Institute for Child Health and Human Development, Children’s Oncology Group, IQVIA, Cempra Pharmaceutics, Oncology Reimbursement Management, Aptitude Health, The Dedham Group, Trinity Life Sciences, and Pfizer Inc. Dr Hsu has no potential conflict of interests to report.
References
- Goldner W. Cancer-related hypercalcemia. J Oncol Pract. 2016;12:426-432.
- Stewart AF. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373-379.
- Ralston SH, Gallacher SJ, Patel U, et al. Cancer-associated hypercalcemia: morbidity and mortality: clinical experience in 126 treated patients. Ann Intern Med. 1990;112:499-504.
- Wright JD, Tergas AI, Ananth CV, et al. Quality and outcomes of treatment of hypercalcemia of malignancy. Cancer Invest. 2015;33:331-339.
- Jick S, Li L, Gastanaga VM, Liede A. Prevalence of hypercalcemia of malignancy among cancer patients in the UK: analysis of the Clinical Practice Research Datalink database. Cancer Epidemiol. 2015;39:901-907.
- Mirrakhimov AE. Hypercalcemia of malignancy: an update on pathogenesis and management. N Am J Med Sci. 2015;7:483-493.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558-567.
- Rosner MH, Perazella MA. Acute kidney injury in the patient with cancer. Kidney Res Clin Pract. 2019;38:295-308.
- Asonitis N, Angelousi A, Zafeiris C, et al. Diagnosis, pathophysiology and management of hypercalcemia in malignancy: a review of the literature. Horm Metab Res. 2019;51:770-778.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Multiple Myeloma. Version 5.2022. March 9, 2022. www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed May 13, 2022.
- Green JR, Müller K, Jaeggi KA. Preclinical pharmacology of CGP 429446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9:745-751.
- Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74:1385-1393.
- Kim S. A case of severe, irreversible hypocalcemia after one dose of pamidronate administered for hypercalcemia of malignancy. J Oncol Pharm Pract. 2019;25:1787-1793.
- Aldave APN, Jaiswal S. Severe resistant hypocalcemia in multiple myeloma after zoledronic acid administration: a case report. J Med Case Rep. 2014;8:353. doi: 10.1186/1752-1947-8-353.
- Henley D, Kaye J, Walsh J, Cull G. Symptomatic hypocalcaemia and renal impairment associated with bisphosphonate treatment in patients with multiple myeloma. Intern Med J. 2005;35:726-728.
- Zometa (zoledronic acid) injection, for intravenous use [prescribing information]. Novartis Pharmaceuticals Corporation; December 14, 2018. www.accessdata.fda.gov/drugsatfda_docs/label/2018/021223s041lbl.pdf. Accessed May 13, 2022.
- Skerjanec A, Berenson J, Hsu CH, et al. The pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with varying degrees of renal function. J Clin Pharmacol. 2003;43:154-162.
- Cremers S, Drake MT, Ebetino FH, et al. Pharmacology of bisphosphonates. Br J Clin Pharmacol. 2019;85:1052-1062.
- Pamidronate disodium injection/for injection, for intravenous use/powder for solution, for intravenous use [prescribing information]. Bedford Laboratories; December 2014. www.accessdata.fda.gov/drugsatfda_docs/label/2014/021113s017lbl.pdf. Accessed February 12, 2021.
- Chen T, Berenson J, Vescio R, et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42:1228-1236.
- Berenson JR, Rosen L, Vescio R, et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol. 1997;37:285-290.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41.
- US Department of Health & Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. November 27, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed May 13, 2022.
- Zoledronic acid. Lexi-Drugs. Lexicomp. Wolters Kluwer. 2021. http://online.lexi.com/. Accessed May 13, 2022. [Subscription required to access.]
- Palmer S, Tillman F III, Sharma P, et al. Safety of intravenous bisphosphonates for the treatment of hypercalcemia in patients with preexisting renal dysfunction. Ann Pharmacother. 2021;55:303-310.
- Norman SJ, Reeves DJ, Saum LM. Use of pamidronate for hypercalcemia of malignancy in renal dysfunction. J Pharm Pract. 2021;34:553-557.