High-dose methotrexate therapy is primarily used for the treatment of several types of cancer, including central nervous system lymphomas, osteosarcoma, and acute lymphoblastic leukemia. High-dose methotrexate is traditionally defined as doses of ≥1000 mg/m2. Methotrexate exerts its antitumor effect by the inhibition of dihydrofolate reductase, the enzyme responsible for the conversion of folic acid to its reduced form.1 This process ultimately inhibits DNA synthesis by blocking purine synthesis. Several monitoring and management parameters must be considered to promote methotrexate excretion and to minimize the complications associated with the administration of methotrexate, including nephrotoxicity.1
Given the high risk of morbidity and mortality associated with the complications of methotrexate therapy,1 preventive strategies are used when administering high-dose methotrexate treatment regimens. These approaches include adequate hydration with sodium bicarbonate to achieve urine alkalization (which prevents the delayed clearance of methotrexate and kidney injury), routine monitoring of serum methotrexate concentrations, and pharmacokinetically driven leucovorin dosing.2-4
In the inpatient setting at Yale New Haven Hospital, a medication utilization evaluation was conducted between 2012 and 2015 to assess the management of high-dose methotrexate administration at baseline. The findings of that evaluation led to the establishment of a standardized practice guideline in 2017. Before the implementation of this guideline, urinary alkalization with sodium bicarbonate hydration was most often administered to patients at hospital admission, serum methotrexate levels were monitored at the peak, and then once or twice daily thereafter, and the initial leucovorin rescue doses varied among the different treatment regimens.
High-dose methotrexate therapy is primarily used for the treatment of several types of cancer, including central nervous system lymphomas, osteosarcoma, and acute lymphoblastic leukemia. High-dose methotrexate is traditionally defined as doses of ≥1000 mg/m2. Methotrexate exerts its antitumor effect by the inhibition of dihydrofolate reductase, the enzyme responsible for the conversion of folic acid to its reduced form.1 This process ultimately inhibits DNA synthesis by blocking purine synthesis. Several monitoring and management parameters must be considered to promote methotrexate excretion and to minimize the complications associated with the administration of methotrexate, including nephrotoxicity.1
Given the high risk of morbidity and mortality associated with the complications of methotrexate therapy,1 preventive strategies are used when administering high-dose methotrexate treatment regimens. These approaches include adequate hydration with sodium bicarbonate to achieve urine alkalization (which prevents the delayed clearance of methotrexate and kidney injury), routine monitoring of serum methotrexate concentrations, and pharmacokinetically driven leucovorin dosing.2-4
In the inpatient setting at Yale New Haven Hospital, a medication utilization evaluation was conducted between 2012 and 2015 to assess the management of high-dose methotrexate administration at baseline. The findings of that evaluation led to the establishment of a standardized practice guideline in 2017. Before the implementation of this guideline, urinary alkalization with sodium bicarbonate hydration was most often administered to patients at hospital admission, serum methotrexate levels were monitored at the peak, and then once or twice daily thereafter, and the initial leucovorin rescue doses varied among the different treatment regimens.
The doses of methotrexate were then adjusted per the Bleyer nomogram,5,6 and patients were monitored throughout their hospital stay until methotrexate clearance, which was defined as a serum methotrexate level of <1 × 10-7 moles/L. To improve the efficiency of high-dose methotrexate administration and to reduce patients’ length of hospital stay, a high-dose methotrexate management guideline for adults was implemented in 2017 to drive standardized practice measures. This guideline was also incorporated into the treatment plans that contain high-dose methotrexate in our electronic health record (EHR).
The key changes introduced by the guideline included initiating oral sodium bicarbonate 24 hours before hospital admission, starting intravenous (IV) alkalization and laboratory tests in the outpatient setting, and standardizing sodium bicarbonate hydration infusion rates for before and after methotrexate administration. These changes are reflected in 3 publications that are cited as part of the guideline.7-9 In addition, checking serum methotrexate levels was limited to once daily with morning laboratory draws, and leucovorin rescue doses were adjusted as needed, based on the serum methotrexate levels reported in late morning.
Finally, patients were considered for early discharge with oral leucovorin if their methotrexate serum levels were between <1 × 10-7 moles/L and 3 × 10-7 moles/L. These patients were discharged with oral leucovorin dosed at 25 mg every 6 hours, for 48 hours. If the prescription for leucovorin could not be filled at, and secured from, a retail pharmacy, the patient remained in the hospital until his or her methotrexate level was <1 × 10-7 moles/L.
Before the 2017 management guideline for high-dose methotrexate was implemented at our institution, no patients were discharged with oral leucovorin. The purpose of this study was to assess the impact of implementing a management guideline for high-dose methotrexate on the duration of hospital stay and on the incidence of nephrotoxicity among patients with cancer who receive methotrexate therapy.
Methods
This study was conducted in 2 phases at our Smilow Cancer Hospital at Yale New Haven Health. Cohort A included a pre–guideline implementation baseline group of patients who received high-dose methotrexate between February 2012 and September 2015. Cohort B included a post–guideline implementation group of patients who received high-dose methotrexate between January 2017 and February 2018.
The high-dose methotrexate–containing regimens included methotrexate, dexamethasone, ifosfamide, etoposide, and pegaspargase; high-dose methotrexate; rituximab, methotrexate, procarbazine, and vincristine; cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine (Hyper-CVAD) with or without rituximab; cyclophosphamide, doxorubicin, vincristine, and methotrexate; and cisplatin plus doxorubicin and methotrexate.
The EHR was used to assess retrospectively the selected patients. The primary outcomes were length of hospital stay, and the incidence of nephrotoxicity as graded per the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. The secondary outcomes included the time to urine alkalization (ie, urine pH ≥7.5), the frequency of methotrexate levels, methotrexate level at hospital discharge, and the use of leucovorin rescue therapy.
The data collection points included the baseline patient characteristics, oral sodium bicarbonate and/or IV urinary alkalization before hospital admission, the time to methotrexate administration on admission, the frequency of methotrexate level, serum creatinine changes, drug–drug interactions, the methotrexate level at the time of discharge, leucovorin prescription for early discharge, and the length of hospital stay.
Descriptive statistics were used to analyze the data. For the primary outcome of length of hospital stay, statistical significance was calculated using an alpha of 0.05 and the student’s t-test.
Results
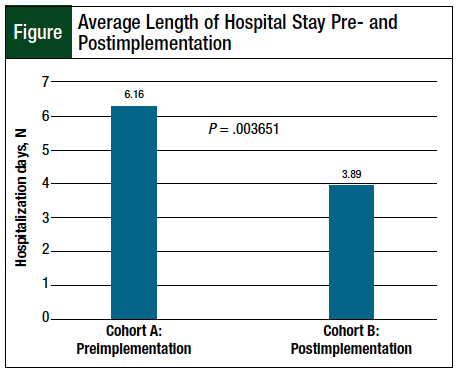
A total of 103 patients were included in the study, with 55 patients in cohort A and 48 patients in cohort B. The baseline characteristics were similar between the 2 groups (Table 1). Of note, cohort A had a higher average methotrexate dose than cohort B (3558 mg/m2 vs 2802 mg/m2, respectively). In addition, cohort A had a lower baseline serum creatinine level than cohort B (0.73 mg/dL vs 0.84 mg/dL, respectively).
The average lengths of hospital stay were 6.16 days (range, 2-30) for cohort A and 3.89 days (range, 1.87-12.85) for cohort B (P = .003651; Figure). The incidence of nephrotoxicity was 1.82% for cohort A and 6.25% for cohort B (P = .249757). All observed nephrotoxicity cases were CTCAE grade 1.
More patients received oral or IV urine alkalization before hospital admission in cohort B (45.8%) than in cohort A (10%). The average time to urine alkalization was 7.63 hours for cohort A and 6.77 hours for cohort B. The time from admission to the administration of methotrexate was 17.66 hours in cohort A and 14.75 hours in cohort B.
The average methotrexate level at discharge was 7.7 × 10-8 moles/L in cohort A compared with 1.2 × 10-7 moles/L in cohort B. A total of 21 (43.8%) patients were discharged early with a methotrexate level of 1 × 10-7 moles/L to 3 × 10-7 moles/L in cohort B.
Discussion
Inpatient admission related to high-dose methotrexate administration is a common practice and is associated with a significant healthcare resource utilization. Limited data are available in the literature that characterize the average length of hospital stay for admissions associated with high-dose methotrexate administration.10,11
May and colleagues reported an average inpatient admission duration of 5.6 days for high-dose methotrexate administration in patients who did not have at least grade 2 renal adverse events.10 The investigators noted that this length of stay increased to 8.9 days when renal-related adverse events were observed.10 Similarly, in a study by Steward and colleagues, the average length of hospital stay was 5 days in the absence of nephrotoxicity compared with 14 days for patients with nephrotoxicity.11
In our study, the average length of inpatient stay among the guideline preimplementation group (cohort A) was 6.16 days, which is consistent with these previously reported data.10,11 Our study shows a significant reduction in the average length of stay for high-dose methotrexate admissions in the postimplementation group (cohort B), with an overall mean of 3.89 days. These results suggest that the implementation of this guideline may improve the efficiency of healthcare resource utilization associated with high-dose methotrexate admissions, by reducing patients’ length of hospital stay.
Kintzel and colleagues showed that the time to acceptable urinary pH was significantly decreased with combined oral and IV alkalization strategies before admission.12 In our study, although more patients received oral or IV urine alkalization before admission in cohort B than in cohort A, there was no difference in the time to urine alkalization compared with cohort A.
Urine alkalization was measured by urine dipsticks with each void, and was documented in our EHR; delays in the documentation of urine pH might have contributed to the observed delayed urine alkalization in both groups in our study.
Although not statistically significant, the increased use of urine alkalization measures in the outpatient setting in our study translated to an improved time from inpatient admission to the start of the high-dose methotrexate infusion for the postimplementation group (cohort B). We suspect that pharmacy delays related to compounding the alkalization fluids or sodium bicarbonate drug shortages at the time might have also contributed to delayed urine alkalization.
We implemented the use of commercially available Lactated Ringer’s solution (contains equivalent to 28 mEq/L of bicarbonate) as alkalization fluid to be initiated immediately on hospital admission, while awaiting the sodium bicarbonate solution to be delivered. Further investigation is needed to develop additional strategies to improve the efficiency of achieving goal urine alkalization before high-dose methotrexate administration.
Nephrotoxicity is a common and often serious complication of high-dose methotrexate therapy. In a retrospective review, Wiczer and colleagues reported an overall incidence of all-grade renal toxicity of 38.6%.13 The majority of these nephrotoxicity events were grade 1, and 10.7% of the events were grade ≥2.13 By comparison, in our study, the rate and severity of nephrotoxicity were low and were comparable before and after the implementation of the methotrexate administration guideline.
In addition, drug interactions, including with beta-lactam antibiotics, can have potentially severe or life-threatening consequences for patients receiving high-dose methotrexate.14 Methotrexate is 90% renally excreted, and the majority of its elimination is mediated by organic anion transport 1 and organic anion transport 3.14 Beta-lactam antibiotics and other drugs that compete for elimination by these pathways may potentially delay methotrexate clearance and increase toxicity.
In our study, 1 patient in the postimplementation group with grade 1 nephrotoxicity received a dose of a beta-lactam antibiotic concurrently with high-dose methotrexate, and this might have contributed to the nephrotoxicity. Our study underscores the importance of identifying and preventing drug interactions.
In our institution, we use a best-practice advisory function in the EHR, which is triggered when the patient has an active order for methotrexate serum level and a provider orders a medication that interacts with methotrexate. The goal of this best-practice advisory is to increase prescribers’ awareness of the drug interactions associated with methotrexate clearance and to prevent nephrotoxicity events related to drug interactions.
Table 2 provides a summary of our high-dose methotrexate administration guideline. One potential challenge to implementing this guideline is patient adherence to filling a prescription for, and taking, an oral sodium bicarbonate medication at home, before hospital admission. Our institution addressed this by including the oral prescription as a take-home medication built directly into the treatment plan. In addition, the ability to initiate hydration in the clinic before admission is limited by infusion chair availability and staffing. Based on the volume of patients, additional resources may need to be used to allow for the initiation of hydration in the clinic.
Overall, among the multiple approaches used in our guideline, the 2 methods with the most significant impact are the optimization of preadmission alkalization, and the option for early discharge, based on a more liberal methotrexate level, with the option for treatment with oral leucovorin to be continued at home if deemed necessary.
Furthermore, several studies have demonstrated that the administration of high-dose methotrexate entirely in the outpatient setting is safe and feasible.15,16
In our study, increased alkalization preadmission translated to a shorter time after hospital admission until methotrexate was administered. In addition, 48.3% of the patients were discharged with a higher methotrexate level than was allowed before the guideline’s implementation. The combination of these 2 strategies contributed to an overall decreased length of hospital stay for patients.
Limitations
Our study has several limitations, including its retrospective design and small sample size. The length-of-stay outcome might have been erroneously affected by factors that are external to the changes made in the guideline, such as an infection or another complication that prolonged a patient’s inpatient stay.
Another potential limitation is that we did not capture data on the time to recovery, mucositis, or the timing of subsequent therapy.
Furthermore, our results may be confounded for patients who are admitted to a hospital for multiday regimens, such as HyperCVAD. In this case, the determining factor of the length of hospital stay may be the completion of cytarabine treatment rather than the clearance of methotrexate. It is also important to note that the lower average methotrexate dose in cohort B might have contributed to the shorter observed length of stay.
Conclusion
The results of this study demonstrate that the implementation of an institutional high-dose methotrexate guideline is safe and can successfully reduce the length of admission for patients receiving high-dose methotrexate. These results also suggest that there is opportunity for further refinement of our high-dose methotrexate guideline, and that outpatient options could be considered for select patients.
Author Disclosure Statement
Dr Kowalski, Dr Mohamed Jaszczur, Dr Nadeau-Nguyen, and Dr Merl have no conflicts of interest to report.
References
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694-703.
- Rouch JA, Burton B, Dabb A, et al. Comparison of enteral and parenteral methods of urine alkalinization in patients receiving high-dose methotrexate. J Oncol Pharm Pract. 2017;23:3-9.
- Pitman SW, Frei E III. Weekly methotrexate-calcium leucovorin rescue: effect of alkalinization on nephrotoxicity; pharmacokinetics in the CNS; and use in CNS non-Hodgkin’s lymphoma. Cancer Treat Rep. 1977;61:695-701.
- Paci A, Veal G, Bardin C, et al. Review of therapeutic drug monitoring of anticancer drugs part 1—cytotoxics. Eur J Cancer. 2014;50:2010-2019.
- Bleyer WA. The clinical pharmacology of methotrexate: new applications of an old drug. Cancer. 1978;41:36-51.
- Bleyer WA. Therapeutic drug monitoring of methotrexate and other antineoplastic drugs. In: Baer DM, Dito WR, eds. Interpretations in Therapeutic Drug Monitoring. Chicago, IL: American Society of Clinical Pathologists; 1981:169-181.
- Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125:1403-1410.
- Alrabiah Z, Luter D, Proctor A, Bates JS. Substitution of sodium acetate for sodium bicarbonate for urine alkalinization in high-dose methotrexate therapy. Am J Health Syst Pharm. 2015;72:1932-1934.
- Shamash J, Earl H, Souhami R. Acetazolamide for alkalinisation of urine in patients receiving high-dose methotrexate. Cancer Chemother Pharmacol. 1991;28:150-151.
- May J, Carson KR, Butler S, et al. High incidence of methotrexate associated renal toxicity in patients with lymphoma: a retrospective analysis. Leuk Lymphoma. 2014;55:1345-1349.
- Steward JS, Bullard HM, O’Rourke TJ, et al. Effect of single agent high-dose methotrexate-related acute kidney injury on length of hospitalization and relative dose intensity in adult patients with central nervous system lymphoma. J Oncol Pharm Pract. 2017;23:496-501.
- Kintzel PE, Campbell AD, Yost KJ, et al. Reduced time for urinary alkalinization before high-dose methotrexate with preadmission oral bicarbonate. J Oncol Pharm Pract. 2012;18:239-244.
- Wiczer T, Dotson E, Tuten A, et al. Evaluation of incidence and risk factors for high-dose methotrexate-induced nephrotoxicity. J Oncol Pharm Pract. 2016;22:430-436.
- Tran HX, Herrington JD. Effect of ceftriaxone and cefepime on high-dose methotrexate clearance. J Oncol Pharm Pract. 2016;22:801-805.
- Zelcer S, Kellick M, Wexler LH, et al. The Memorial Sloan Kettering Cancer Center experience with outpatient administration of high dose methotrexate with leucovorin rescue. Pediatr Blood Cancer. 2008;50:1176-1180.
- Bartholomew JL, Dai H, August KJ, et al. Feasibility of outpatient high-dose methotrexate infusions in pediatric patients with B-lineage acute lymphoblastic leukemia. J Adv Pract Oncol. 2018;9:381-386.
The doses of methotrexate were then adjusted per the Bleyer nomogram,5,6 and patients were monitored throughout their hospital stay until methotrexate clearance, which was defined as a serum methotrexate level of <1 × 10-7 moles/L. To improve the efficiency of high-dose methotrexate administration and to reduce patients’ length of hospital stay, a high-dose methotrexate management guideline for adults was implemented in 2017 to drive standardized practice measures. This guideline was also incorporated into the treatment plans that contain high-dose methotrexate in our electronic health record (EHR).
The key changes introduced by the guideline included initiating oral sodium bicarbonate 24 hours before hospital admission, starting intravenous (IV) alkalization and laboratory tests in the outpatient setting, and standardizing sodium bicarbonate hydration infusion rates for before and after methotrexate administration. These changes are reflected in 3 publications that are cited as part of the guideline.7-9 In addition, checking serum methotrexate levels was limited to once daily with morning laboratory draws, and leucovorin rescue doses were adjusted as needed, based on the serum methotrexate levels reported in late morning.
Finally, patients were considered for early discharge with oral leucovorin if their methotrexate serum levels were between <1 × 10-7 moles/L and 3 × 10-7 moles/L. These patients were discharged with oral leucovorin dosed at 25 mg every 6 hours, for 48 hours. If the prescription for leucovorin could not be filled at, and secured from, a retail pharmacy, the patient remained in the hospital until his or her methotrexate level was <1 × 10-7 moles/L.
Before the 2017 management guideline for high-dose methotrexate was implemented at our institution, no patients were discharged with oral leucovorin. The purpose of this study was to assess the impact of implementing a management guideline for high-dose methotrexate on the duration of hospital stay and on the incidence of nephrotoxicity among patients with cancer who receive methotrexate therapy.
Methods
This study was conducted in 2 phases at our Smilow Cancer Hospital at Yale New Haven Health. Cohort A included a pre–guideline implementation baseline group of patients who received high-dose methotrexate between February 2012 and September 2015. Cohort B included a post–guideline implementation group of patients who received high-dose methotrexate between January 2017 and February 2018.
The high-dose methotrexate–containing regimens included methotrexate, dexamethasone, ifosfamide, etoposide, and pegaspargase; high-dose methotrexate; rituximab, methotrexate, procarbazine, and vincristine; cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine (Hyper-CVAD) with or without rituximab; cyclophosphamide, doxorubicin, vincristine, and methotrexate; and cisplatin plus doxorubicin and methotrexate.
The EHR was used to assess retrospectively the selected patients. The primary outcomes were length of hospital stay, and the incidence of nephrotoxicity as graded per the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. The secondary outcomes included the time to urine alkalization (ie, urine pH ≥7.5), the frequency of methotrexate levels, methotrexate level at hospital discharge, and the use of leucovorin rescue therapy.
The data collection points included the baseline patient characteristics, oral sodium bicarbonate and/or IV urinary alkalization before hospital admission, the time to methotrexate administration on admission, the frequency of methotrexate level, serum creatinine changes, drug–drug interactions, the methotrexate level at the time of discharge, leucovorin prescription for early discharge, and the length of hospital stay.
Descriptive statistics were used to analyze the data. For the primary outcome of length of hospital stay, statistical significance was calculated using an alpha of 0.05 and the student’s t-test.
Results
A total of 103 patients were included in the study, with 55 patients in cohort A and 48 patients in cohort B. The baseline characteristics were similar between the 2 groups (Table 1). Of note, cohort A had a higher average methotrexate dose than cohort B (3558 mg/m2 vs 2802 mg/m2, respectively). In addition, cohort A had a lower baseline serum creatinine level than cohort B (0.73 mg/dL vs 0.84 mg/dL, respectively).
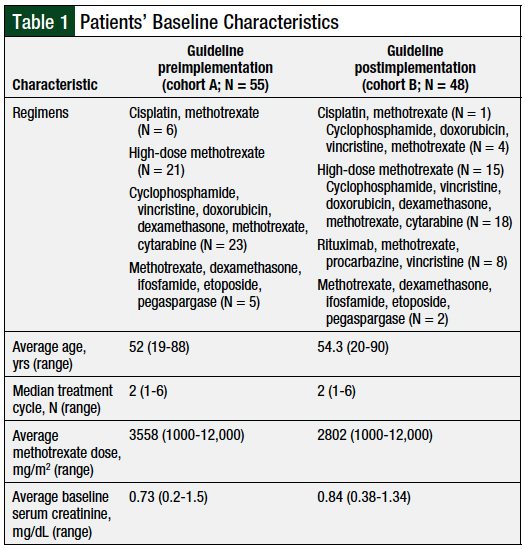
The average lengths of hospital stay were 6.16 days (range, 2-30) for cohort A and 3.89 days (range, 1.87-12.85) for cohort B (P = .003651; Figure). The incidence of nephrotoxicity was 1.82% for cohort A and 6.25% for cohort B (P = .249757). All observed nephrotoxicity cases were CTCAE grade 1.
More patients received oral or IV urine alkalization before hospital admission in cohort B (45.8%) than in cohort A (10%). The average time to urine alkalization was 7.63 hours for cohort A and 6.77 hours for cohort B. The time from admission to the administration of methotrexate was 17.66 hours in cohort A and 14.75 hours in cohort B.
The average methotrexate level at discharge was 7.7 × 10-8 moles/L in cohort A compared with 1.2 × 10-7 moles/L in cohort B. A total of 21 (43.8%) patients were discharged early with a methotrexate level of 1 × 10-7 moles/L to 3 × 10-7 moles/L in cohort B.
Discussion
Inpatient admission related to high-dose methotrexate administration is a common practice and is associated with a significant healthcare resource utilization. Limited data are available in the literature that characterize the average length of hospital stay for admissions associated with high-dose methotrexate administration.10,11
May and colleagues reported an average inpatient admission duration of 5.6 days for high-dose methotrexate administration in patients who did not have at least grade 2 renal adverse events.10 The investigators noted that this length of stay increased to 8.9 days when renal-related adverse events were observed.10 Similarly, in a study by Steward and colleagues, the average length of hospital stay was 5 days in the absence of nephrotoxicity compared with 14 days for patients with nephrotoxicity.11
In our study, the average length of inpatient stay among the guideline preimplementation group (cohort A) was 6.16 days, which is consistent with these previously reported data.10,11 Our study shows a significant reduction in the average length of stay for high-dose methotrexate admissions in the postimplementation group (cohort B), with an overall mean of 3.89 days. These results suggest that the implementation of this guideline may improve the efficiency of healthcare resource utilization associated with high-dose methotrexate admissions, by reducing patients’ length of hospital stay.
Kintzel and colleagues showed that the time to acceptable urinary pH was significantly decreased with combined oral and IV alkalization strategies before admission.12 In our study, although more patients received oral or IV urine alkalization before admission in cohort B than in cohort A, there was no difference in the time to urine alkalization compared with cohort A.
Urine alkalization was measured by urine dipsticks with each void, and was documented in our EHR; delays in the documentation of urine pH might have contributed to the observed delayed urine alkalization in both groups in our study.
Although not statistically significant, the increased use of urine alkalization measures in the outpatient setting in our study translated to an improved time from inpatient admission to the start of the high-dose methotrexate infusion for the postimplementation group (cohort B). We suspect that pharmacy delays related to compounding the alkalization fluids or sodium bicarbonate drug shortages at the time might have also contributed to delayed urine alkalization.
We implemented the use of commercially available Lactated Ringer’s solution (contains equivalent to 28 mEq/L of bicarbonate) as alkalization fluid to be initiated immediately on hospital admission, while awaiting the sodium bicarbonate solution to be delivered. Further investigation is needed to develop additional strategies to improve the efficiency of achieving goal urine alkalization before high-dose methotrexate administration.
Nephrotoxicity is a common and often serious complication of high-dose methotrexate therapy. In a retrospective review, Wiczer and colleagues reported an overall incidence of all-grade renal toxicity of 38.6%.13 The majority of these nephrotoxicity events were grade 1, and 10.7% of the events were grade ≥2.13 By comparison, in our study, the rate and severity of nephrotoxicity were low and were comparable before and after the implementation of the methotrexate administration guideline.
In addition, drug interactions, including with beta-lactam antibiotics, can have potentially severe or life-threatening consequences for patients receiving high-dose methotrexate.14 Methotrexate is 90% renally excreted, and the majority of its elimination is mediated by organic anion transport 1 and organic anion transport 3.14 Beta-lactam antibiotics and other drugs that compete for elimination by these pathways may potentially delay methotrexate clearance and increase toxicity.
In our study, 1 patient in the postimplementation group with grade 1 nephrotoxicity received a dose of a beta-lactam antibiotic concurrently with high-dose methotrexate, and this might have contributed to the nephrotoxicity. Our study underscores the importance of identifying and preventing drug interactions.
In our institution, we use a best-practice advisory function in the EHR, which is triggered when the patient has an active order for methotrexate serum level and a provider orders a medication that interacts with methotrexate. The goal of this best-practice advisory is to increase prescribers’ awareness of the drug interactions associated with methotrexate clearance and to prevent nephrotoxicity events related to drug interactions.
Table 2 provides a summary of our high-dose methotrexate administration guideline. One potential challenge to implementing this guideline is patient adherence to filling a prescription for, and taking, an oral sodium bicarbonate medication at home, before hospital admission. Our institution addressed this by including the oral prescription as a take-home medication built directly into the treatment plan. In addition, the ability to initiate hydration in the clinic before admission is limited by infusion chair availability and staffing. Based on the volume of patients, additional resources may need to be used to allow for the initiation of hydration in the clinic.
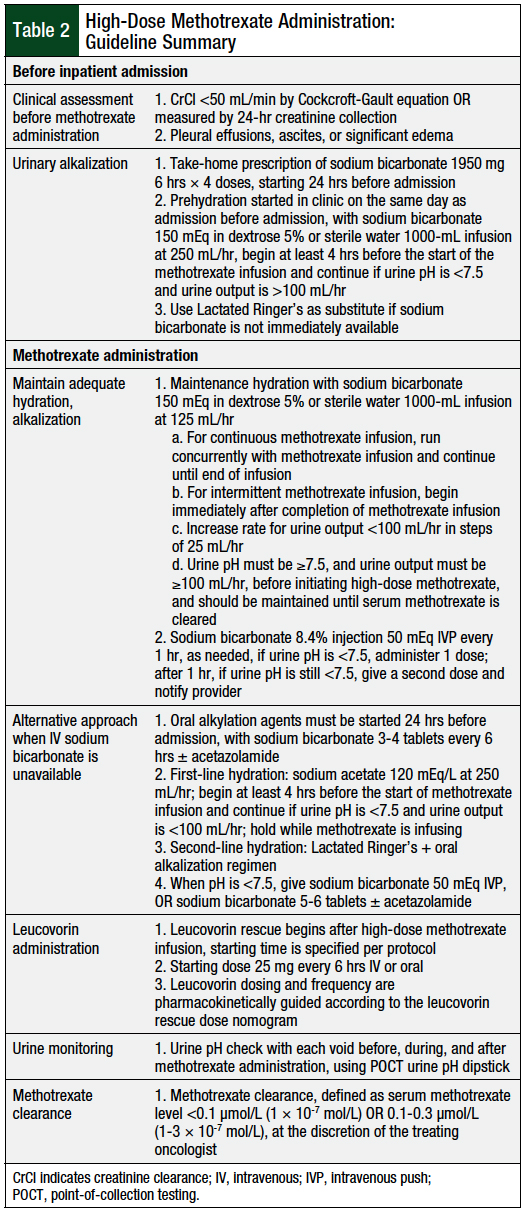
Overall, among the multiple approaches used in our guideline, the 2 methods with the most significant impact are the optimization of preadmission alkalization, and the option for early discharge, based on a more liberal methotrexate level, with the option for treatment with oral leucovorin to be continued at home if deemed necessary.
Furthermore, several studies have demonstrated that the administration of high-dose methotrexate entirely in the outpatient setting is safe and feasible.15,16
In our study, increased alkalization preadmission translated to a shorter time after hospital admission until methotrexate was administered. In addition, 48.3% of the patients were discharged with a higher methotrexate level than was allowed before the guideline’s implementation. The combination of these 2 strategies contributed to an overall decreased length of hospital stay for patients.
Limitations
Our study has several limitations, including its retrospective design and small sample size. The length-of-stay outcome might have been erroneously affected by factors that are external to the changes made in the guideline, such as an infection or another complication that prolonged a patient’s inpatient stay.
Another potential limitation is that we did not capture data on the time to recovery, mucositis, or the timing of subsequent therapy.
Furthermore, our results may be confounded for patients who are admitted to a hospital for multiday regimens, such as HyperCVAD. In this case, the determining factor of the length of hospital stay may be the completion of cytarabine treatment rather than the clearance of methotrexate. It is also important to note that the lower average methotrexate dose in cohort B might have contributed to the shorter observed length of stay.
Conclusion
The results of this study demonstrate that the implementation of an institutional high-dose methotrexate guideline is safe and can successfully reduce the length of admission for patients receiving high-dose methotrexate. These results also suggest that there is opportunity for further refinement of our high-dose methotrexate guideline, and that outpatient options could be considered for select patients.
Author Disclosure Statement
Dr Kowalski, Dr Mohamed Jaszczur, Dr Nadeau-Nguyen, and Dr Merl have no conflicts of interest to report.
References
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694-703.
- Rouch JA, Burton B, Dabb A, et al. Comparison of enteral and parenteral methods of urine alkalinization in patients receiving high-dose methotrexate. J Oncol Pharm Pract. 2017;23:3-9.
- Pitman SW, Frei E III. Weekly methotrexate-calcium leucovorin rescue: effect of alkalinization on nephrotoxicity; pharmacokinetics in the CNS; and use in CNS non-Hodgkin’s lymphoma. Cancer Treat Rep. 1977;61:695-701.
- Paci A, Veal G, Bardin C, et al. Review of therapeutic drug monitoring of anticancer drugs part 1—cytotoxics. Eur J Cancer. 2014;50:2010-2019.
- Bleyer WA. The clinical pharmacology of methotrexate: new applications of an old drug. Cancer. 1978;41:36-51.
- Bleyer WA. Therapeutic drug monitoring of methotrexate and other antineoplastic drugs. In: Baer DM, Dito WR, eds. Interpretations in Therapeutic Drug Monitoring. Chicago, IL: American Society of Clinical Pathologists; 1981:169-181.
- Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125:1403-1410.
- Alrabiah Z, Luter D, Proctor A, Bates JS. Substitution of sodium acetate for sodium bicarbonate for urine alkalinization in high-dose methotrexate therapy. Am J Health Syst Pharm. 2015;72:1932-1934.
- Shamash J, Earl H, Souhami R. Acetazolamide for alkalinisation of urine in patients receiving high-dose methotrexate. Cancer Chemother Pharmacol. 1991;28:150-151.
- May J, Carson KR, Butler S, et al. High incidence of methotrexate associated renal toxicity in patients with lymphoma: a retrospective analysis. Leuk Lymphoma. 2014;55:1345-1349.
- Steward JS, Bullard HM, O’Rourke TJ, et al. Effect of single agent high-dose methotrexate-related acute kidney injury on length of hospitalization and relative dose intensity in adult patients with central nervous system lymphoma. J Oncol Pharm Pract. 2017;23:496-501.
- Kintzel PE, Campbell AD, Yost KJ, et al. Reduced time for urinary alkalinization before high-dose methotrexate with preadmission oral bicarbonate. J Oncol Pharm Pract. 2012;18:239-244.
- Wiczer T, Dotson E, Tuten A, et al. Evaluation of incidence and risk factors for high-dose methotrexate-induced nephrotoxicity. J Oncol Pharm Pract. 2016;22:430-436.
- Tran HX, Herrington JD. Effect of ceftriaxone and cefepime on high-dose methotrexate clearance. J Oncol Pharm Pract. 2016;22:801-805.
- Zelcer S, Kellick M, Wexler LH, et al. The Memorial Sloan Kettering Cancer Center experience with outpatient administration of high dose methotrexate with leucovorin rescue. Pediatr Blood Cancer. 2008;50:1176-1180.
- Bartholomew JL, Dai H, August KJ, et al. Feasibility of outpatient high-dose methotrexate infusions in pediatric patients with B-lineage acute lymphoblastic leukemia. J Adv Pract Oncol. 2018;9:381-386.

