Pain is one of the most feared symptoms of cancer, with uncontrolled pain being the most frequently reported symptom in hospitalized patients with cancer.1,2 As cancer stage progresses, the pain intensity and incidence increase, with cancer pain reported in 62% to 86% of patients with advanced disease.1 Poor pain control negatively affects patients’ quality of life, their family interactions, as well as their psychological and functional health.1,2 In addition, appropriate symptom management, including pain control, has been associated with improved survival in patients with cancer.3 In hospitalized patients with cancer, poor pain control constitutes a major problem that has been associated with increased length of hospital stay, medical complications, and increased healthcare resource utilization, as well as decreased patient satisfaction.2 Additional efforts to improve patient satisfaction related to cancer pain control during hospitalization have increasingly been implemented.
Patient satisfaction with pain control is a key element in the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.4 The HCAHPS survey is the first national and standardized survey created by the Centers for Medicare & Medicaid Services (CMS) that measures patients’ perspectives on their hospital care using different key elements, such as communication with nurses and physicians, the responsiveness of hospital staff, the cleanliness and quietness of the hospital environment, and pain management. HCAHPS performance scores are publicly reported, allowing for comparison of hospitals’ performances and affecting their reimbursement by CMS.4
Undertreatment of cancer pain is frequently reported despite the availability of effective therapies and pain management guidelines, and implementation of pain management guidelines in clinical practice remains problematic. A review that assessed compliance with the Joint Commission standards for pain management in patients with cancer showed that pain intensity and pain reassessment were documented in 53% and 44%, respectively, of inpatients’ charts.5
The National Comprehensive Cancer Network (NCCN) guidelines for adult cancer pain lists the principles of appropriate cancer pain control as comprehensive assessment, management, and ongoing care of pain, emphasizing the importance of multidisciplinary collaboration in cancer pain management.6 Appropriate pain assessment should include evaluation of pain intensity and pain characteristics, as well as the patient’s pain management goal, which helps to individualize management. In addition, a pain reassessment postintervention is crucial to guide therapy and help reach pain relief faster.7
Pharmacologic treatment, particularly opioid therapy, is the mainstay of cancer pain management. In hospitalized patients with cancer, parenteral opioids are often needed because of the rapid onset of analgesia associated with opioid therapy, patient’s inability to swallow medications, and/or impairment of enteral absorption.6
The standard of care for pain assessment at our institution is in compliance with the NCCN guidelines for adult cancer pain, which includes assessment and documentation of pain intensity, pain characteristics, and the patient’s goal for pain management based on the level of pain control the patient desires to achieve. In addition, pain reassessment should be documented within 1 hour of administering any opioid dose, according to NCCN’s and our institution’s guidelines.
Because of the comprehensive approach to cancer pain management and the prospective improvement of pain practices that can be implemented, we conducted a review to determine the level of compliance with the NCCN guidelines in pain assessment practices at our institution. Our goal was to evaluate the pain assessment in patients with solid tumors who were receiving parenteral opioids as documented in their charts.
Methods
This study was conducted at a 900-bed, private, nonprofit teaching institution at Texas Medical Center, Houston.
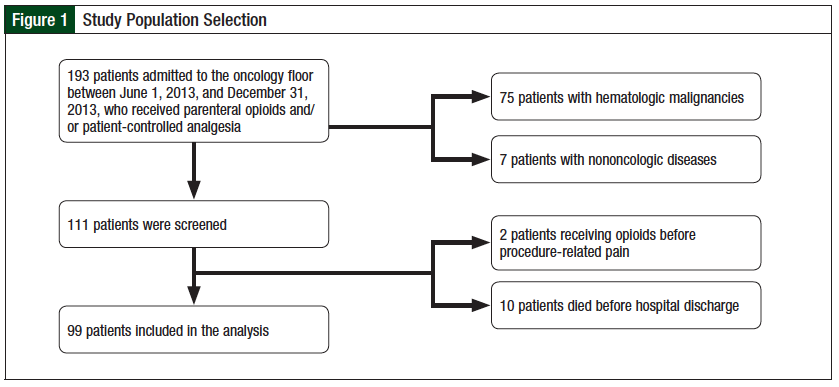
We conducted a retrospective electronic chart review of all patients admitted to the oncology floor between June 1, 2013, and December 31, 2013, who received parenteral opioids. Patients who had hematologic malignancies or nononcologic diseases, received opioid therapy in anticipation of procedure-related pain, or died before discharge were excluded from the study. We obtained approval for the study from the hospital Institutional Review Board, and informed consent was waived.
The primary outcome measure was alignment with the NCCN guidelines for cancer pain assessment practices. Documentation of pain intensity, pain characteristics, pain management goal, and pain reassessment in a patient’s chart was measured as part of the pain assessment practices.
Pain intensity was considered “assessed” if a pain score, or any noncommunicative assessment of pain, was documented at the patient’s admission to the hospital. Pain intensity at our institution is based on a 0 to 10 scale, and/or categorical associated scale (ie, “none” for a score of 0, “mild” for a score of 1 to 3, “moderate” for a score of 4 to 6, and “severe” for a score of 7 to 10) as reported by the patient, and the Faces Pain Scale for patients who are unable to communicate.
Pain characteristics, such as pain location, features, exacerbating factors, and/or presence during rest or during activity, were counted as “assessed” if they were documented at pain onset. The patient’s goal was considered evaluated if it was documented in the chart anytime during the patient’s hospital stay.
According to the NCCN pain management guidelines, pain reassessment should be performed 15 minutes after parenteral opioid administration6; pain management practice at our organization requires reassessment of any oral or parenteral opioid dose 1 hour after administration. For the purpose of this analysis, chart documentation of any pain score or noncommunicative pain reassessment within 1 hour after parenteral opioid dose was considered an appropriate pain reassessment practice.
Secondary outcome measures included the percentage of patients who met their pain management goal at discharge. In addition, we assessed the presence of breakthrough analgesics and a bowel regimen in the discharge medication list for patients discharged with a scheduled opioid regimen.
Descriptive statistics were used to analyze the primary and secondary outcome measures.
Results
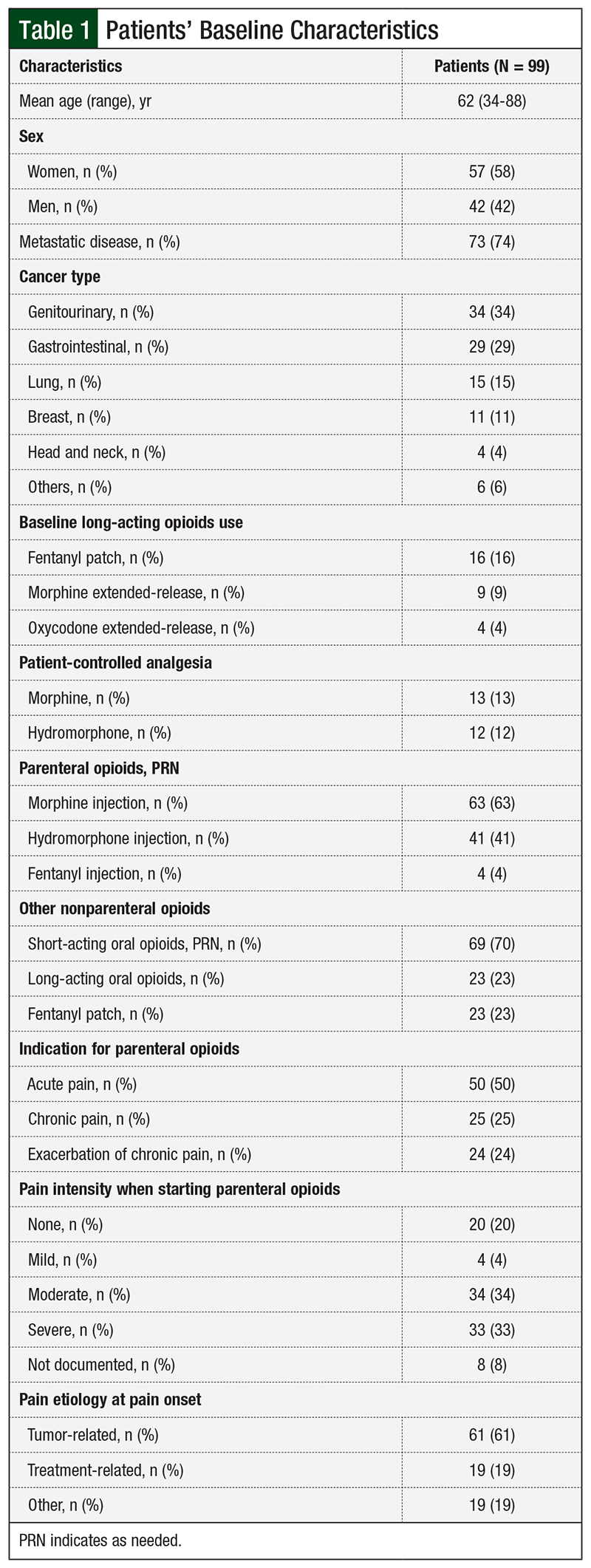
A total of 99 patients with solid tumors who received parenteral opioids at our institution were included in the analysis (Figure 1). Baseline characteristics included age, sex, cancer type, presence of metastatic disease, type of parenteral opioid used, type of nonparenteral opioids used in addition to the parenteral regimen, indication for parenteral opioids, pain intensity when parenteral opioids were started, and most probable pain etiology at onset.
Overall, 74% of patients had metastatic disease, with genitourinary tumors being the most common type. The most common opioids used, as needed, were morphine injections. Acute pain was the most common indication for parenteral opioid use, which was documented for 50% of patients. In addition, 67% of patients had moderate or severe pain intensity when parenteral opioid therapy was initiated. Moreover, the etiology of pain at onset of pain was tumor-related in 61% of patients (Table 1).
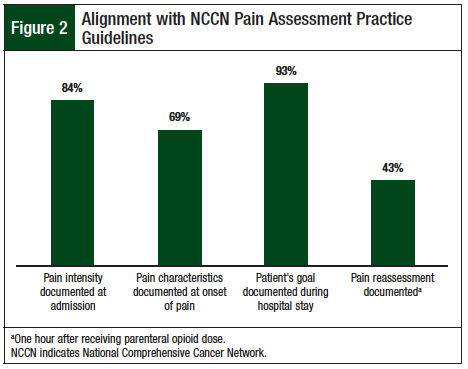
Evaluation of compliance with pain assessment practices according to the NCCN guidelines showed 84% compliance with pain intensity documentation at hospital admission. Pain characteristics were reported in 69% of patients at pain onset, and pain management goals were documented for 93% of patients during their hospital stay. In addition, we stratified compliance with pain assessment practices based on indication of parenteral opioids (ie, acute pain, chronic pain, or exacerbation of chronic pain) and pain intensity (ie, none/mild, moderate, or severe) when parenteral opioids were started.
Compliance with pain assessment was consistent between the different indications of parenteral opioids, with similar rates of compliance reported for acute pain, chronic pain, and exacerbation of chronic pain. However, compliance with assessment practices was higher in patients with moderate and severe pain intensity compared with those with no pain or mild pain intensity (Table 2).
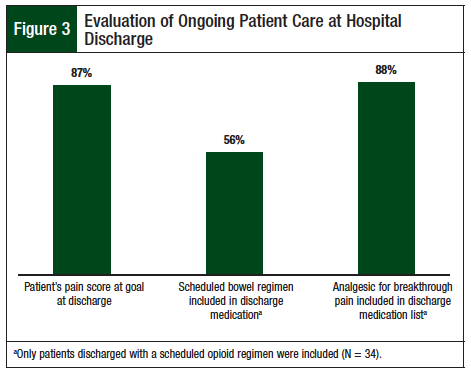
The primary end point, which was compliance with the NCCN pain reassessment documentation guidelines within 1 hour of parenteral opioid dose, was achieved 43% of the time (Figure 2). Analysis of the secondary outcomes showed that 87% of patients met their pain management goal at discharge. The secondary end point was ongoing patient care at discharge; 56% of patients discharged with a scheduled opioid dose had a scheduled bowel regimen in their discharge medication list, and 88% had a breakthrough analgesic in addition to their scheduled opioid (Figure 3).
Moreover, a review of the discharge opioid regimen for hospitalized patients who were receiving a long-acting opioid (N = 29) showed that 11 (38%) had an increase in their long-acting opioid dose or were switched to another type of long-acting opioid (Table 3). Of those 11 patients, 3 were started with parenteral opioids for acute pain and 5 for an exacerbation of chronic pain. Furthermore, 11 of the patients who were started with parenteral opioids, either for acute pain (n = 6) or for an exacerbation of chronic pain (n = 5) did not have a dose increase in their long-acting opioid at discharge (Table 3).
Furthermore, because of the effect of adjuvant therapies on pain control, we further reported their appropriate use based on pain nature. In our analysis, we identified 17 patients with bone metastasis as the main contributor to pain; 16 of those patients received ≥1 adjuvant treatments (not shown). We also determined that 8 patients had pain mainly related to bowel obstruction, and all of them received adjuvant treatment in addition to their opioid regimens. In addition, neuropathic pain was identified as the main pain etiology in 4 patients who received gabapentin as adjuvant treatment.
These results show that adjuvant therapies have been appropriately used at our institution when indicated.
Discussion
Our analysis revealed that compliance with the NCCN pain assessment guidelines remains problematic. Pain reassessment documentation has been identified as a major barrier to appropriate alignment with pain evaluation practices at our institution. The pain reassessment rate was 44%, which is comparable to the compliance rate (44%) reported in the Joint Commission report.5 A study of 42 registered nurses from the medical and surgical oncology units of a US cancer hospital reported documentation of pain reassessment in 61% of patients preintervention,7 which is similar to our preintervention rate.
Inappropriate pain reassessment may delay pain relief; opioid dose titration based on reassessment scores allows for faster resolution of pain, which permits an earlier transition to oral opioids, when appropriate. In our analysis, 93% of patients received parenteral opioids until the day of discharge. Delay in transitioning to an oral opioid regimen can lead to inappropriate dose conversion, and to prolonged hospital stay. In fact, the NCCN guidelines recommend switching to an oral regimen once opioid requirements have been stable for 24 hours and an appropriate level of comfort and function has been achieved.6,8
The results of our study show that a bowel regimen was prescribed in 56% of patients discharged with regularly scheduled opioids (Figure 3). Opioid-related constipation has been reported as an economic burden in patients with cancer, with approximately 33% of patients with cancer who receive opioids experiencing constipation, which can lead to increased medical complications, increased hospitalizations, and increased length of hospital stay.9-11
As was shown in Table 3, several patients who had been receiving parenteral opioids did not have their long-acting opioid dose increased at discharge, which further highlights the need for a better discharge strategy and the involvement of a palliative care team to identify patients who can benefit from a dose increase of their opioid regimen at discharge.
Inadequate knowledge of appropriate cancer pain management, incomplete pain assessment, underutilization of pain management tools, and misconceptions about opioids have been reported as possible reasons for inadequate pain assessment.5,12 In addition, nonflexible work environment, lack of leadership support, and lack of nurse decision-making abilities have been described as institutional barriers to pain management.7
Several efforts are currently being implemented at our institution as a joint effort between nursing and pharmacy leadership in an attempt to improve pain assessment and management strategies. These efforts include nurses’ education regarding the importance of pain assessment, pain reassessment, and documentation of both in the patient’s electronic chart. This has been one of the main education points in the orientations for new nurses, but audits are being performed by nursing management to assess nurses’ performance in that area periodically. In addition, pain reassessment documentation is continuously being reinforced during nursing rounds by nursing leadership and clinical pharmacists.
A multidisciplinary team that includes nursing leadership, nurse practitioners, pharmacists, oncologists, and hematologists is currently working on including pain medications (eg, “as needed” and scheduled opioids) and bowel regimens in the oncology admission order set that will also include a nursing order for documentation of pain assessment at the patient’s admission and every 4 hours after, as well as a reassessment pain score after any opioid dose is given to the patient.
An additional strategy that is currently being implemented includes a clinical pharmacist consult to help support opioid dosing, titration, and side-effects management, which will further help patients reach their goal for pain control. Finally, a palliative care consult is currently being developed as an automatic referral for eligible patients admitted to the oncology floor, which will allow a specialized physician in pain management to follow and manage the pain regimen and adjuvant therapies for patients with cancer.
Limitations
The results of our analysis should be interpreted with several limitations in mind. First, we relied on the presence of documentation in patients’ charts to report our compliance with pain assessment practices; however, the absence of documentation does not always imply lack of appropriate assessment.
Second, cancer-related pain can be multifactorial, with treatment, procedures, and complications contributing to the pain nature and severity, in addition to the disease itself. In fact, the NCCN guidelines emphasize the importance of adjuvant therapies for additional relief of pain associated with neuropathy (ie, anticonvulsants, antidepressants, corticosteroids, local or topical anesthetics), bone pain (ie, radiation, surgery, bisphosphonates, denosumab, corticosteroids), and bowel obstruction (ie, surgery, chemotherapy, radiation, bowel rest, nasogastric suction, corticosteroids, octreotide).6
Finally, pain reassessment was considered appropriate if documented within 1 hour of parenteral opioid administration; however, appropriate reassessment should be done 15 minutes after parenteral opioid administration per NCCN guidelines, because of their rapid onset. More frequent reassessment, especially for severe, intractable pain, allows for faster pain relief because of quicker dose titration and more frequent monitoring of side effects.
Conclusion
Cancer pain management should be considered a priority in daily practice. Nursing education regarding the importance of pain assessment and reassessment documentation is continuously being reinforced at our institution. In addition, appropriate interventions are being implemented to help improve our compliance rates with pain assessment. Future efforts should focus on multidisciplinary and organizational collaboration to improve pain management practices in addition to comprehensive planning after discharge in accordance with national guidelines.
Acknowledgments
We are grateful to Beverly Hughes, RN, MS, AOCNS, CHPN, Houston Methodist Hospital, TX; Hongmei Wang, PharmD candidate, and My-Hue Manh, PharmD candidate, Texas Southern University, Houston; Lang Nguyen, PharmD candidate, University of Houston College of Pharmacy, TX; and Nisrine El-Hadi, PharmD candidate, Lebanese American University, Beirut, Lebanon.
Author Disclosure Statement
Dr El Rahi, Dr Zaghloul, and Dr Murillo have no conflicts of interest to report.
References
1. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449.
2. Goldberg GR, Morrison RS. Pain management in hospitalized cancer patients: a systematic review. J Clin Oncol. 2007;25:1792-1801.
3. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733-742.
4. Centers for Medicare & Medicaid Services. HCAHPS fact sheet. March 2008. www.hcahpsonline.org/files/FINAL-HCAHPS%20Fact%20Sheet_3-26-08.pdf. Accessed March 22, 2014.
5. Cohen MZ, Easley MK, Ellis C, et al. Cancer pain management and the JCAHO’s pain standards: an institutional challenge. J Pain Symptom Manage. 2003;25:519-527.
6. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Adult Cancer Pain. Version 2.2017. Published May 10, 2017. www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Accessed June 29, 2017.
7. Idell CS, Grant M, Kirk C. Alignment of pain reassessment practices and National Comprehensive Cancer Network guidelines. Oncol Nurs Forum. 2007;34:661-671.
8. Mercandante S, Villari P, Ferrera P, et al. Rapid titration with intravenous morphine for severe cancer pain and immediate oral conversion. Cancer. 2002;95:203-208.
9. Panchal SJ, Müller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract. 2007;61: 1181-1187.
10. Iyer S, Davis KL, Candrilli S. Opioid use patterns and healthcare resource utilization in patients prescribed opioid therapy with and without constipation. Manag Care. 2010;19:44-51.
11. Candrilli SD, Davis KL, Iyer S. Impact of constipation on opioid use patterns, healthcare resource utilization, and costs in cancer patients on opioid therapy. J Pain Palliat Care Pharmacother. 2009;23:231-241.
12. Xue Y, Schulman-Green D, Czaplinski C, et al. Pain attitudes and knowledge among RNs, pharmacists, and physicians on an inpatient oncology service. Clin J Oncol Nurs. 2007;11:687-695.