Non-Hodgkin lymphoma (NHL) represents a heterogeneous group of predominantly mature malignancies originating from B lymphocytes, T lymphocytes, or natural killer cells.1 The incidence of NHL has increased since the 1970s, paralleling a major decrease in deaths from other causes for patients in their sixth and seventh decades of life. The increase is also attributed to the development of AIDS-related NHL in patients infected with HIV. Diffuse large B-cell lymphoma (DLBCL) is the most common B-cell NHL, representing 30% to 35% of cases, and affecting patients of all ages.
Treatment options for patients with aggressive NHL are driven by pathologic subtype and biologic events that contribute to the clinical behavior of the disease. Emerging technology, including gene-expression profiling, RNA interference screening, and DNA sequencing, has led to the identification of several new signaling pathways and targets for drug development.2 The World Health Organization (WHO) classifies DLBCL into 4 major categories, and recent developments using gene-expression profiling have led to subdivision of the largest category—DLBCL not otherwise specified—into 3 molecular subtypes based on cell of origin, including germinal center B-cell–like (GCB), activated B-cell (ABC), and primary mediastinal B-cell lymphoma (PMBL).2,3 Although these 3 molecular subtypes are not yet recognized by the WHO, new treatment strategies based on these subtypes are being evaluated in ongoing clinical trials. Clinically, stratification based on cell of origin has prognostic value, because the overall survival (OS) rates are better for patients with GCB-DLBCL than for those with ABC-DLBCL. It is well-recognized that DLBCL is clinically and biologically diverse, yet treatment choices for most patients continue to depend on clinical features, including age and disease stage according to the validated International Prognostic Index (IPI) score, but the biologic mechanisms that may contribute to treatment failure are not taken into account.3
Before the 1970s, treatment using single-agent chemotherapy produced dismal responses; complete response (CR) was achieved in less than 5% of patients. The combination regimen of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) was studied during the 1970s, and quickly became the standard of care. Rituximab, an anti-CD20 monoclonal antibody, transformed the treatment of B-cell malignancies, resulting in durable responses and improved event-free survival (EFS) and OS rates. The addition of rituximab to CHOP (R-CHOP) is currently considered the standard of care for most patients with aggressive B-cell NHL. However, even with the addition of rituximab, subsets of patients still experience disease progression or relapse secondary to disease progression.
Infusion with the dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin (DA-EPOCH) regimen—which is designed to increase intracellular concentrations of cytotoxic chemotherapy, decrease drug resistance, and maximize fractional cell kill—was originally studied as a salvage regimen.4,5 Because DLBCL is a heterogeneous group of diseases, molecular subtyping is quickly transforming care, allowing for more patient-specific directed care. Emerging data suggest that certain subsets of patients with NHL may benefit from infusional DA-EPOCH plus rituximab (DA-EPOCH-R), including those with AIDS or with PMBL, and patients with double- or triple-hit B-cell NHL.4
The objective of this article is to discuss the current role of the R-CHOP and DA-EPOCH-R regimens in the treatment of patients with NHL. The benefits and risks of these 2 regimens are examined specifically for elderly patients and for patients diagnosed with AIDS-related NHL. In addition, special considerations in the care of subsets of patients with NHL are identified.
The CHOP Regimen
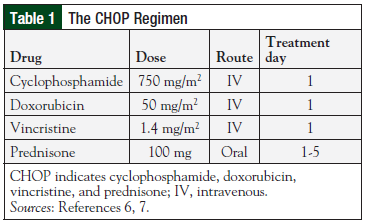
The CHOP regimen (Table 1) was established as the standard of care for patients with intermediate- or high-grade NHL after publication of results of the Southwest Oncology Group (SWOG)/Eastern Cooperative Oncology Group (ECOG) phase 3 clinical trial in which CHOP was compared with more intensive, advanced-generation regimens, including methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone (m-BACOD); prednisone, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, and methotrexate (ProMACE-CytaBOM); and methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin (MACOP-B) in approximately 900 treatment-naïve patients.6,7
No significant differences were noted between the 4 treatment regimens with respect to CR rates or disease-free survival at 3 years. However, there were significantly fewer (P = .001) fatal reactions and grade 4 toxicities in patients treated with CHOP or with ProMACE-CytaBOM than with m-BACOD or MACOP-B.6 Because the OS and disease-free survival rates with CHOP were similar to the other regimens, but with fewer serious toxicities, CHOP remained the recommended regimen for advanced-stage, intermediate-grade, and high-grade lymphoma.
The addition of rituximab to chemotherapy regimens such as CHOP has improved EFS and OS. In SWOG 0014, a phase 2 clinical trial of patients with limited-stage disease and ≥1 adverse factors as defined by the modified IPI, 3 cycles of R-CHOP were administered followed by involved field radiation. The 4-year progression-free survival (PFS) rates were 88% (95% confidence interval [CI], 80%-96%) versus 78% (95% CI, 68%-88%) for controls, and the OS rates were 92% (95% CI, 85%-95%) versus 88% (95% CI, 81%-96%) for the controls—better than CHOP historical controls.7
The MabThera International Trial was a phase 3 comparison trial of 6 cycles of CHOP-like chemotherapy versus 6 cycles of R-CHOP. All patients enrolled had 0 to 1 IPI risk factors, and the majority (75%) of patients had limited-stage disease. The investigators reported a significant benefit with the addition of rituximab; the 6-year OS rate was 90% (vs 80%; P = .0004), and the PFS was 80% (vs 63.9%; P <.0001).8
The Groupe d&rsqo;Études des Lymphomes de l&rsqo;Adulte (GELA) LNH98-5 trial demonstrated that the addition of rituximab to CHOP, administered every 21 days, improved PFS and OS for patients aged ≥60 years with advanced disease. A long-term follow-up analysis of this trial demonstrated that the PFS was significantly better for R-CHOP (36.5%; 95% CI, 37.9%-43.3%) than CHOP (20%; 95% CI, 14.6%-26.2%), as was the OS at 10 years: 43.5% (95% CI, 36.4%-5.4%) versus 27.6% (95% CI, 21.4%-34.3%), respectively.9
These findings are similar to the ECOG/Cancer and Leukemia Group B (CALGB) 9703 trial, in which the PFS (52% vs 39%; P = .03) and OS at 3 years (67% vs 58%; P = .05) were significantly improved for patients aged ≥60 years who received rituximab.10 In that trial, maintenance rituximab did not provide additional benefit for patients who achieved a CR with R-CHOP induction chemotherapy.10
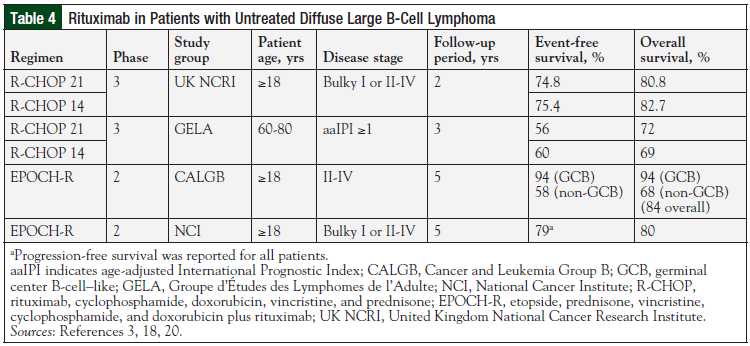
Before the standard use of rituximab, the German High-Grade NHL Study Group reported that 6 cycles of CHOP administered every 14 days (CHOP-14) was superior to 6 cycles of CHOP administered every 21 days (CHOP-21).11 In a large, phase 3 clinical trial performed by the United Kingdom National Cancer Research Institute, more than 1000 patients were randomly assigned to receive R-CHOP every 14 or 21 days.12 At 2 years, the EFS (75.4% vs 74.8%) and OS (82.7% vs 80.8%) did not differ significantly. GELA reported similar outcomes from their phase 3 trial; EFS of 56% versus 60%, and OS of 69% versus 72% for 14- and 21-day intervals, respectively, did not differ significantly after 3 years of follow-up.13 Collectively, these studies suggest that the addition of rituximab does not improve the outcome of dose-dense CHOP. Therefore, R-CHOP-21 remains a standard treatment regimen for patients with newly diagnosed DLBCL.
Although the R-CHOP regimen is an effective regimen, it is associated with noteworthy toxicities. Grade 3 or 4 toxicities can include neutropenia, febrile neutropenia, and infection.12,13 Neurologic toxicities, including vincristine-induced peripheral neuropathy, are common. Cardiac toxicity has been associated with the doxorubicin component of R-CHOP.14 In a retrospective analysis of patients with aggressive NHL who received treatment with R-CHOP and were monitored for 10 years, the 5-year cumulative risk for cardiac events (left ventricular ejection fraction [LVEF] of <50%, or a decline of ≥20%) was 29%, suggesting that providers should weigh the risks and benefits of R-CHOP for patients with a significant cardiac history.
Even though NHL is diagnosed most frequently in older adults (aged ≥65 years), it is common for elderly people to be excluded from randomized trials. Data reported for younger, healthier patients may not be applicable to elderly patients with NHL. An observational, population-based study was conducted among patients aged ≥75 years who received treatment for newly diagnosed DLBCL.15 Of the 70 patients who received R-CHOP, CR was achieved in 30 of 39 patients who completed all treatment cycles. OS at 2 years was 70% for patients who received all cycles of chemotherapy. However, significant toxicities occurred, including treatment-related mortality. Eight of the 10 reported deaths were attributed to sepsis.15 In patients aged >75 years, R-CHOP is reportedly effective if patients are able to complete a full course of therapy; however, it is associated with severe treatment-related toxicities.
Although the R-CHOP regimen has demonstrated durable responses, improving on this regimen continues to be an area of investigation. Administering the CHOP regimen every 14 days combined with prolonged administration of rituximab has produced encouraging results. Among patients with IPI scores of 3 to 5, the 3-year EFS (67% vs 54%) and OS (80% vs 67%) were better than that for patients receiving standard R-CHOP-14 (respectively), with an acceptable toxicity profile.16 The addition of targeted agents, such as lenalidomide, ibrutinib, or bortezomib, to the R-CHOP regimen has the potential to improve outcomes even further.17
Individually, each of these agents has demonstrated activity in DLBCL in preclinical or in phase 1 trials. Adding these targeted therapies to traditional chemotherapy may have an additive, or even synergistic, effect. As the understanding of tumor biology improves, targeted therapies combined with R-CHOP may provide the opportunity to individualize therapy to optimize outcomes.
The DA-EPOCH Regimen
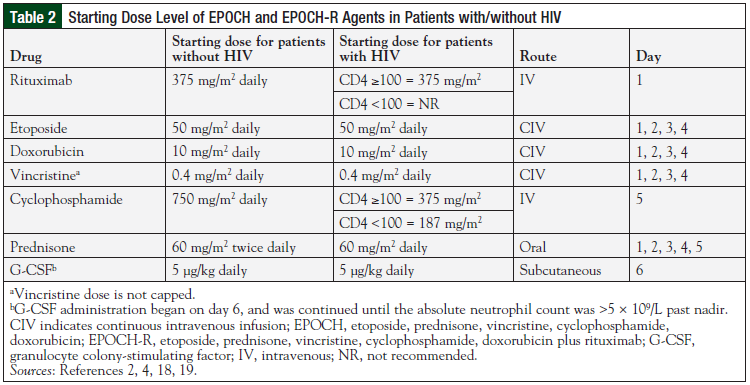
Treatment failure is multifactorial and dependent on tumor biology, tumor volume, pharmacokinetics, and pharmacogenomics. The DA-EPOCH regimen, developed at the National Cancer Institute (NCI), was based on the hypothesis that optimization of drug selection, drug schedule, and pharmacokinetics would produce better outcomes than the CHOP regimen in patients with aggressive NHL.18 The 96-hour continuous infusion schedule was developed from in vitro modeling data of drug resistance and pharmacodynamics. DA-EPOCH is administered every 21 days and allows for topoisomerase II targeting by doxorubicin, and pharmacodynamic dosing of etoposide and vincristine (Table 2).2,4,18,19
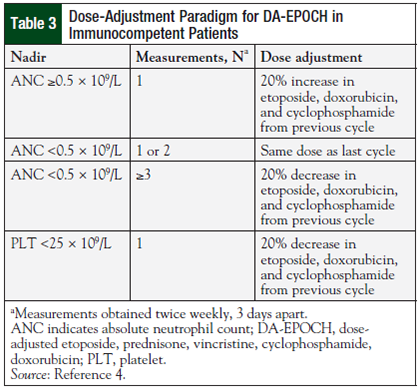
Based on the hypothesis that variations in drug clearance among patients would affect the low steady-state concentrations achieved during continuous infusion, the dose adjustment protocol was developed. Dose adjustments to doxorubicin, etoposide, and cyclophosphamide are made based on the absolute neutrophil count nadir in the previous cycle (Table 3).4
The first phase 2 multi-institutional study of DA-EPOCH included 50 untreated patients with DLBCL; 24% were aged ≥60 years, and 44% had high intermediate-risk or high-risk IPI scores.4 Among the 49 patients assessed, the overall response rate was 100%; 45 (92%) achieved CRs, and 4 (8%) had partial responses. At the median follow-up of 62 months, the PFS was 70%, and the OS was 73%. There was no significant association between any validated IPI risk factor and the survival end point. When patients with low-risk and high-risk IPI scores were compared, there was no significant difference in PFS. The lack of association between IPI and outcome suggests that the cell kill profile of the DA-EPOCH regimen is inherently different from that of the CHOP regimen. Overexpression of BCL-2 was the only factor associated with treatment failure.4
Dose intensity was maintained in patients who received DA-EPOCH, and the toxicity profile was no worse than that of CHOP. The targeted degree of neutropenia was achieved in 49% of cycles (absolute neutrophil count, 0.1-0.5 K/µL), and hospitalization for febrile neutropenia occurred in only 8% of cycles. The achieved dose intensity for vincristine was 96%, and no serious long-term neurologic side effects occurred.4 However, a prophylactic bowel regimen is now recommended to prevent severe constipation. It is noteworthy that cardiac toxicity was not observed with continuous infusion; hence, the EPOCH regimen continues to be an option for patients with decreased LVEF.
Rituximab was added to the DA-EPOCH regimen (DA-EPOCH-R), which was evaluated in 2 phase 2 clinical trials, one in a large elderly population and the other in patients with high IPI scores (Table 4).3,18,20 In the NCI-based study (28% aged ≥60 years; 40% with high-intermediate or high IPI), PFS was 80%, and OS was 79% at the median follow-up time of 5 years after completion of DA-EPOCH-R.20 In a multicenter, phase 2 study conducted by CALGB, the DA-EPOCH-R regimen was evaluated in patients with previously untreated DLBCL (43% aged ≥60 years; 40% with high-intermediate or high IPI).18 The reported 5-year time to disease progression and OS were 81% and 84%, respectively.18
The results of these studies are comparable to those of the R-CHOP regimen with regard to OS and toxicity. In addition, these studies report that the remission rates obtained with DA-EPOCH-R are durable through 5 years.18,20
To our knowledge, there has been no head-to-head comparison of the R-CHOP and the DA-EPOCH-R regimens. A phase 3 clinical trial in which the 2 regimens are being compared within the GCB and the ABC molecular subtypes of DLBCL to ascertain definitive differences is currently underway (CALGB 50303; NCT00118209).
Distinct Subtypes of DLBCL and Treatment Considerations
As technology has progressed, subpopulations of NHL that have poor outcomes with R-CHOP have been identified. Approximately 40% of all B-cell lymphomas are characterized by the presence of a recurrent reciprocal chromosomal translocation. Lymphomas previously classified as DLBCL or Burkitt-like with recurrent chromosomal breakpoints activating multiple oncogenes, including MYC, are now called double-hit lymphomas (DHLs).
DHLs account for 5% to 10% of DLBCL cases, and the majority have germinal center phenotype and express BCL-2 (BCL-2+/MYC+). A small subset of DHLs express BCL-6 (BCL-6+/MYC+) or express both BCL-2 and BCL-6 and are called triple-hit lymphomas (BCL-2+/BCL-6+/MYC+). Patients with DHLs often have poor prognostic features, including elevated lactate dehydrogenase, high IPI score, and involvement of the bone marrow or the central nervous system.
Because data for DHLs are continuously emerging, no definitive clinical information is driving therapy decisions, and previous reports are based on a collection of case series.21,22 Although the optimal regimen for DHLs is not known, it should be noted that patients who received R-CHOP–like regimens have a poor prognosis, with a median OS of ≤12 months.23
In a retrospective review, data were combined for 23 academic medical institutions in the United States.23 Overall, 311 newly diagnosed patients with DHL, defined by fluorescence in situ hybridization (BCL-2+/MYC+ or BCL-6+/MYC+), received either R-CHOP or a more intensive regimen, including DA-EPOCH-R; rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone/methotrexate, and cytarabine; rituximab, cyclophosphamide, vincristine, doxorubicin, methotrexate/ifosfamide, etoposide, and cytarabine; or “other.” The overall PFS improved with the more intensive regimens compared with R-CHOP (median PFS 7.8 vs 21.6 months; P = .001). However, no difference was observed when the intensive regimens were compared.23
Of note, the DA-EPOCH-R regimen resulted in significantly higher rates of CR than the other intensive regimens. Consolidation with autologous stem-cell transplantation in first CR (n = 39) was not associated with better OS than observation alone (P = .14).23 The 2-year OS for non–stem-cell transplantation in patients who achieved CR (n = 112) was ≥75%,23 suggesting that the main predictor of improved outcome is achievement of CR with an intensive induction regimen, such as DA-EPOCH-R; however, the role of consolidation with stem-cell transplantation should be explored further.
PMBL, another subtype of DLBCL, represents 10% of DLBCL cases and is clinically and biologically related to nodular sclerosing Hodgkin lymphoma, which also arises from thymic B-cells. PMBL is aggressive and manifested by a localized, mediastinal mass. It predominantly affects young women (median age, 35 years), and most patients have mutations in the BCL-6 gene. Standard immunochemotherapy is not effective, and most patients require mediastinal radiation, which may lead to late adverse effects. Because of the small number of reported cases, few prospective studies have been conducted, leading to lack of a treatment standard; however, retrospective data suggest that more intensive chemotherapy regimens appear to be more effective than R-CHOP.
In a retrospective analysis (N = 63), the failure rate for induction with R-CHOP was 21%,24 suggesting that alternative therapies are needed. In a single-center phase 2 study at NCI, DA-EPOCH-R was evaluated prospectively among 51 newly diagnosed patients, and findings were compared with retrospective DA-EPOCH-R data from Stanford to independently verify outcomes.25
In the prospective-evaluation arm, the EFS was 93%, and the OS was 97% at 5 years. Similarly, in the retrospective cohort, 100% of patients were alive and event-free at 37 months of follow-up. Moreover, no patient had late morbidity or cardiotoxicity. To assess cardiac effects, 42 patients received follow-up for up to 10 years following treatment. All 42 patients had normal LVEF throughout follow-up, and no significant relationship between ejection fraction and cumulative doxorubicin dose or time since treatment was reported.25
Patients with AIDS-related NHL are another subpopulation that may benefit from alternative chemotherapy regimens. Most clinical trials in which CHOP-like regimens were evaluated in patients with AIDS-related NHL took place before the era of highly active antiretroviral therapy (HAART). Outcomes were generally poor when compared with patients with no AIDS-related NHL patients; CR rates ranged from 30% to 50%, and 2-year OS was <20%.26
Outcomes for patients with AIDS-related NHL are improving, and most of the mortality benefit is attributable to HAART secondary to immune reconstitution. In the post-HAART era, the safety and efficacy of rituximab in addition to combination chemotherapy have been documented in multiple phase 2 and 1 phase 3 study, which suggests that rituximab therapy should now be standard care for most patients with CD4 counts of ≥50 cells/mm3.26-28
Phase 2 trials of the efficacy of R-CHOP in patients with AIDS-related NHL have demonstrated CR rates of 77% and 69%.27,28 The French National Research Agency estimated the 2-year OS at 75%,27 and the Spanish group, Programa para el Tratamiento do Hemopatías Malignas, estimated a 3-year OS of 56%.28 Although these studies demonstrated better outcomes than those obtained by CHOP, they also showed that the R-CHOP regimen could be safely combined with HAART, as evidenced by the acceptable toxicity profile.
In an attempt to overcome resistance caused by P-glycoprotein overexpression, which is reportedly more common in patients with AIDS-related NHL, liposomal doxorubicin was substituted for conventional doxorubicin in R-CHOP in a phase 2 clinical trial. Forty patients were assessed, and the 2-year PFS and OS rates were 52% and 61.6%, respectively.29,30
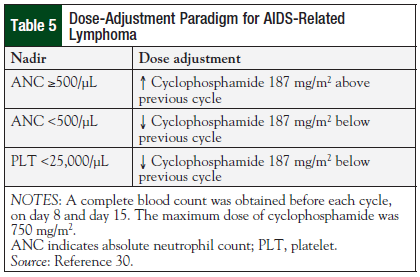
DA-EPOCH for AIDS-related NHL is dosed different from traditional EPOCH, as shown in Table 5.30 The cyclophosphamide dose is initially based on CD4 count, and then adjusted for absolute neutrophil count and platelet nadir. The initial study of DA-EPOCH in patients with AIDS-related NHL (N = 39; 79% with DLBCL; 18% with Burkitt lymphoma) demonstrated a CR rate of 74%, and an OS of 60% at the median follow-up of 53 months.19
The investigators delayed initiation of antiretrovirals until after completion of chemotherapy because there was concern at the time that drug interactions between antiretrovirals and chemotherapy would result in unacceptable toxicity. A multivariate analysis showed that the only factors significantly associated with decreased OS were CD4 counts <100 cells/mm3, and involvement of the central nervous system.19
A phase 2, randomized trial was conducted by the AIDS Malignancy Consortium (AMC034) to evaluate the addition of rituximab to DA-EPOCH and HAART to prospectively assess sequential versus concurrent administration of rituximab.31 The primary efficacy end point, CR, was met only for the concurrent-administration arm (n = 48; DLBCL, 69%; Burkitt lymphoma, 31%), and, at 24 months, resulted in a CR rate of 73%, and OS of 70%. These rates were lower (55% and 67%, respectively) for the sequential-administration arm (n = 53; DLBCL, 80%; Burkitt lymphoma, 20%), demonstrating the effectiveness of concurrent administration of rituximab.31
Data were pooled from 2 consecutive studies: R-CHOP versus CHOP (AMC010) and concurrent versus sequential DA-EPOCH-R (AMC034).26,31,32 Results for patients in the R-CHOP arm and the concurrent DA-EPOCH-R arm were evaluated retrospectively. The objective was to determine whether the perceived benefit of infusional EPOCH persisted after adjustment for covariates known to be associated with inferior outcomes (ie, high-risk IPI and CD4 count <100) and to identify patients at high risk for lethal toxicity during treatment with rituximab plus chemotherapy.
When compared with R-CHOP, DA-EPOCH-R significantly improved OS (hazard ratio [HR], 0.38; P <.001) and EFS (HR, 0.4; P <.001). Treatment-related deaths caused by infection occurred more frequently in patients whose CD4 counts were <50 cells/mm3 (37% vs 6%; P = .01), secondary to sepsis, cryptosporidiosis, and John Cunningham virus, which suggests that rituximab should not be used in these patients. When stratified for age-adjusted IPI, EPOCH was favored for patients with low- or high-risk IPI scores.26,31,32
Overall, DA-EPOCH-R significantly improves EFS and OS rates among patients with AIDS-related NHL, and also improves the duration of response. To promote immune reconstitution and decrease the risk for death from infection, all patients with AIDS-related NHL should be started on antiretrovirals at the time AIDS-related NHL is diagnosed.
Conclusion
Although DLBCL is a common form of NHL in adults, this subtype has a variety of biologically distinct differences. Treatment options currently are driven not only by pathologic subtype but also by several biologic characteristics that contribute to the clinical behavior of the disease. For many patients with DLBCL, the R-CHOP regimen remains a viable initial treatment option and can produce durable responses. However, patients with distinct subtypes of DLBCL, such as DHL, PMBL, or AIDS-related NHL, may benefit from alternative regimens, such as DA-EPOCH.
Author Disclosure Statement
Dr Curry has no conflicts of interest to report; Dr Liewer has received consulting fees from Bristol-Myers Squibb, Seattle Genetics, and Spectrum Pharmaceuticals.
References
1. National Cancer Institute. SEER cancer statistics factsheets: non-Hodgkin lymphoma. http://seer.cancer.gov/csr/1975_2013/results_merged/sect_19_nhl.pdf. Accessed March 7, 2016.
2. Wilson WH. Treatment strategies for aggressive lymphomas: what works? Hematology Am Soc Hematol Educ Program. 2013;2013:584-590.
3. Roschewski M, Dunleavy K, Wilson WH. Moving beyond rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone for diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55:2428-2437.
4. Wilson WH, Grossbard ML, Pittaluga S, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002;99:2685-2693.
5. Yalowich JC. Effects of microtubule inhibitors on etoposide accumulation and DNA damage in human K562 cells in vitro. Cancer Res. 1987;47:1010-1015.
6. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1002-1006.
7. Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26:2258-2263.
8. Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) group. Lancet Oncol. 2011;12:1013-1022.
9. Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d&rsqo;Etudes des Lymphomes de l&rsqo;Adulte. Blood. 2010;116:2040-2045.
10. Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121-3127.
11. Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104:634-641.
12. Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:1817-1826.
13. Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013;14:525-533.
14. Limat S, Daguindau E, Cahn JY, et al. Incidence and risk-factors of CHOP/R-CHOP-related cardiotoxicity in patients with aggressive non-Hodgkin's lymphoma. J Clin Pharm Ther. 2014;39:168-174.
15. Boslooper K, Kibbelaar R, Storm H, et al. Treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone is beneficial but toxic in very elderly patients with diffuse large B-cell lymphoma: a population-based cohort study on treatment, toxicity and outcome. Leuk Lymphoma. 2014;55:526-532.
16. Pfreundschuh M, Poeschel V, Zeynalova S, et al. Optimization of rituximab for the treatment of diffuse large B-cell lymphoma (II): extended rituximab exposure time in the SMARTE-R-CHOP-14 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group. J Clin Oncol. 2014;32:4127-4133.
17. Vaidya R, Witzig TE. Prognostic factors for diffuse large B-cell lymphoma in the R(X)CHOP era. Ann Oncol. 2014;25:2124-2133.
18. Wilson WH, Jung SH, Porcu P, et al. A cancer and leukemia group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. 2012;97:758-765.
19. Little RF, Pittaluga S, Grant N, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653-4659.
20. Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26:2717-2724.
21. Tomita N. BCL2 and MYC dual-hit lymphoma/leukemia. J Clin Exp Hematop. 2011;51:7-12.
22. Petrich AM, Nabhan C, Smith SM. MYC-associated and double-hit lymphomas: a review of pathobiology, prognosis, and therapeutic approaches. Cancer. 2014;120:3884-3895.
23. Petrich AM, Gandhi M, Jovanovic B. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124:2354-2361.
24. Soumerai JD, Hellmann MD, Feng Y, et al. Treatment of primary mediastinal B-cell lymphoma with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone is associated with a high rate of primary refractory disease. Leuk Lymphoma. 2014;55:538-543.
25. Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368:1408-1416.
26. Kaplan LD. HIV-associated lymphoma. Best Pract Res Clin Haematol. 2012;25:101-117.
27. Boué F, Gabarre J, Gisselbrecht C, et al. Phase II trial of CHOP plus rituximab in patients with HIV-associated non-Hodgkin's lymphoma. J Clin Oncol. 2006;24:4123-4128.
28. Ribera JM, Oriol A, Morgades M, et al. Safety and efficacy of cyclophosphamide, adriamycin, vincristine, prednisone and rituximab in patients with human immunodeficiency virus-associated diffuse large B-cell lymphoma: results of a phase II trial. Br J Haematol. 2008;140:411-419.
29. Levine AM, Tulpule A, Espina B, et al. Liposome-encapsulated doxorubicin in combination with standard agents (cyclophosphamide, vincristine, prednisone) in patients with newly diagnosed AIDS-related non-Hodgkin's lymphoma: results of therapy and correlates of response. J Clin Oncol. 2004;22:2662-2670.
30. Levine AM, Noy A, Lee JY, et al. Pegylated liposomal doxorubicin, rituximab, cyclophosphamide, vincristine, and prednisone in AIDS-related lymphoma: AIDS Malignancy Consortium Study 047. J Clin Oncol. 2013;31:58-64.
31. Sparano JA, Lee JY, Kaplan LD, et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115:3008-3016.
32. Barta SK, Lee JY, Kaplan LD, et al. Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma. Cancer. 2012;118:3977-3983.