Dr Butler is PGY-2 Oncology Resident, Dana-Farber Cancer Institute, Brighton, MA; Dr Waddell is Associate Professor, University of Tennessee Health Science Center, Knoxville, TN; Dr Crane is Clinical Pharmacist, Antimicrobial Stewardship, Blount Memorial Hospital, Maryville, TN; Dr Porter is Clinical Pharmacist, Blount Memorial Hospital, Maryville, TN.
The original version of this research was presented as a poster at the American Society of Health-System Pharmacist’s mid-year meeting in Las Vegas, NV, in December 2012; at the Tennessee Pharmacists Association meeting in Nashville, TN, in February 2013; and at the Hematology/Oncology Pharmacy Association meeting in March 2013. It was also presented orally at the South-eastern Residency Conference in April 2013 in Athens, GA.
Chemotherapy-induced febrile neutropenia (CIFN) is a life-threatening, costly complication that may develop in patients with cancer after receiving myelosuppressive chemotherapy.1 In 1991, filgrastim (Neupogen) was approved for the treatment of patients with CIFN, because it stimulates the production of neutrophils, which potentially treats and prevents febrile neutropenia.2,3 Filgrastim, pegfilgrastim (Neulasta), and sargramostim (Leukine) are classified as granulocyte colony-stimulating factors (G-CSFs). A 2005 study of the use of pegfilgrastim showed that the G-CSFs were effective in the prevention of febrile neutropenia.4 Despite this finding, the determination of which chemotherapy regimens require primary prophylaxis for CIFN with G-CSFs is still controversial.5
In 2012, the American Society of Clinical Oncology (ASCO) identified the appropriate use of G-CSFs as 1 of the top 5 recommendations to reduce expenditures for patients with cancer.1 In addition to a potential financial detriment, inappropriate prescribing of these medications may also lead to unnecessary adverse reactions, most often injection-site reactions, flu-like symptoms, and bone pain.2 The ASCO guidelines define inappropriate use as administering these medications to patients with a low, <20% risk for developing CIFN.1,6
A ≥20% risk for developing this complication after chemotherapy would be considered having a high risk for CIFN and would indicate the need to administer one of the G-CSFs for primary prophylaxis.1,6 It is important to note that ASCO also advises to continue to use clinical judgment in evaluating patients at risk for CIFN, which usually involves considering patient-specific factors, such as comorbidities, age, and intent of chemotherapy.6-8
Applying the ASCO recommendations consistently is challenging in clinical practice, because the rates of CIFN are not always reported in clinical trials, and the cumulative effects of multidrug regimens are rarely reported in a drug’s package insert or product information. The decision to prescribe one of the G-CSFs can be based on a variety of factors, including comorbidities, dated G-CSF guidelines, and even the patient’s type of insurance coverage.
A more thorough literature evaluation may lead to better prescribing patterns, but it may be tedious in the clinical practice setting. One trial by Fishman and colleagues demonstrated that prescribers were more likely to use G-CSFs appropriately when presented with the rates of febrile neutropenia and the updated National Comprehensive Cancer Network (NCCN) recommendations related to the use of G-CSFs.9 Of note, the NCCN Clinical Practice Guidelines differ from the ASCO guidelines by recommending that prescribers consider the use of G-CSFs for patients who have a 10% to 20% risk for CIFN.10
In this current study, we evaluated the use of G-CSFs and attempted to develop a reference guide to provide prescribers relevant CIFN rates associated with specific medications, in the hopes of optimizing the use of these medications at our institution.
Methods
We performed a retrospective chart review for all patients who received chemotherapy at our community institution, which has 300 inpatient beds and a 20-chair outpatient infusion clinic. The data were collected from December 1, 2010, to December 31, 2012 (ie, 25 months). No exclusion criteria were included in this review. Data collection included the type of malignancy, the chemotherapy regimen used, and the administration of G-CSFs. Patients’ charts were also reviewed to identify patients with a documented episode of febrile neutropenia.
The primary literature was extensively reviewed to determine the rates of CIFN in patients with cancer. PubMed and Ovid MEDLINE were the search engines used to locate the relevant clinical trials. The search was limited to phase 1, phase 2, or phase 3 clinical trials and included the chemotherapy drugs used in each of the regimens. Approximately 300 relevant articles and almost 200 regimens were found in our search of the literature. The trials found in this search were included in the analysis when documented rates of febrile neutropenia, neutropenic fever, or neutropenic sepsis were listed.
The rates of primary prophylaxis of CIFN in each trial and the related regimens were compiled into a pocket guide for clinician reference. The highest rate of febrile neutropenia in each trial would determine a patient’s risk level. Each chemotherapy regimen, the rate of febrile neutropenia, and the reference for every clinical trial included in this evaluation were listed in the pocket guide. The data analysis was conducted in the form of descriptive statistics using Microsoft Excel. Each chemotherapy regimen was evaluated using the ASCO guidelines,1 and the potential cost-savings were calculated based on average wholesale price and the elimination of unnecessary administrations of G-CSFs over the full study period.
Results
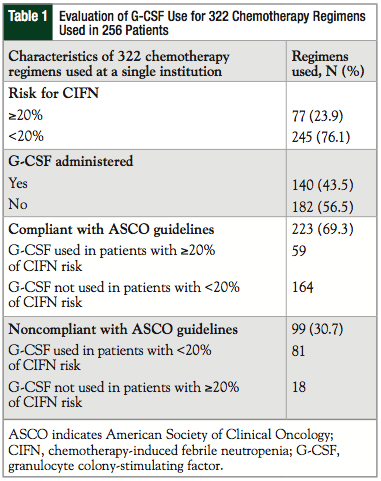
This evaluation included 256 patients who received 322 different chemotherapy regimens at our institution. The evaluation of the use of G-CSFs in these patients is described in Table 1. G-CSFs were administered after 140 (43.5%) of the 322 chemotherapy regimens. Only 77 (23.9%) regimens prescribed at our institution had a risk of ≥20% for developing CIFN. Of note, 25.5% of regimens administered were for hematologic malignancies (primarily acute myeloid leukemia and non-Hodgkin lymphoma) compared with 74.5% for solid tumors (primarily breast cancer and lung cancer). A total of 223 of the 322 (69.3%) regimens used at our institution were compliant with the ASCO guidelines with G-CSFs, and the other regimens were not compliant with the guidelines.
When G-CSFs were administered (N = 140), approximately 59 (42.1%) of the prescriptions were in compliance with the ASCO guidelines (Figure). In addition, 18 of the 77 (23.4%) high-risk regimens were not followed with prophylaxis for febrile neutropenia.
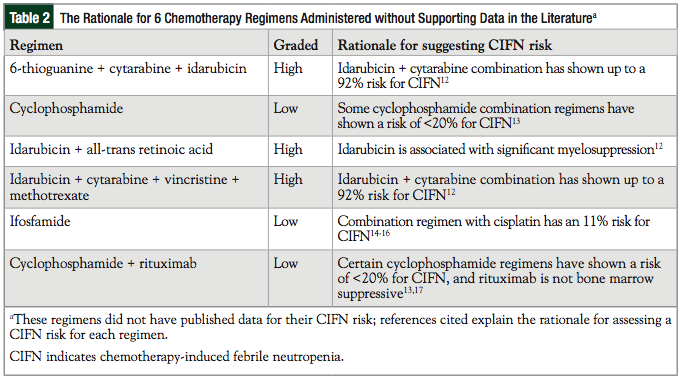
Of note, 6 chemotherapy regimens did not have published articles that reported the data for the rate of CIFN. Table 2 shows how these 6 cases were evaluated in our analysis for the risk of CIFN. Two regimens were administered G-CSFs for secondary prophylaxis of CIFN.
Based on these results, if all prescribers at our institution avoided the administration of G-CSFs in patients with a <20% risk for CIFN, more than $600,000 would have been saved during this study period.
The completed reference guide based on our review of the literature contained CIFN rates for 100 different chemotherapy regimens. Only 24 regimens showed a risk of >20% for developing this complication.
Discussion
The use of G-CSFs in our institution suggests that the prescribing of these medications is not always in compliance with current ASCO guidelines. Furthermore, there is some disagreement regarding the definition of low risk for CIFN between the ASCO guidelines and the NCCN recommendations.
Full compliance with the ASCO guidelines for G-CSFs use is not expected, because of a variety of factors, including treatment goals and patient comorbidities; however, it is concerning that in one representative (our) institution, 57.9% (81 of 140) of patients receiving these medications had a risk of <20% for CIFN. Furthermore, 39 of these 81 (48.1%) patients who received G-CSFs against the ASCO recommendations had a <10% risk for febrile neutropenia.
By comparison, Fishman and colleagues reported that 52 of 245 (21%) total units of pegfilgrastim were administered for patients at low risk for febrile neutropenia.9 The most common regimens administered against the ASCO guidelines at Blount Memorial Hospital were azacitidine and eribulin.
Noncompliance may also be explained by other factors. The rates of febrile neutropenia can be difficult to locate, including the challenges of gaining access to clinical trials or finding trials that actually studied or reported CIFN. Regimens included in this review required several hours to ascertain a clear clinical picture of whether G-CSFs were indicated for CIFN.
The G-CSF medications were not available until the early 1990s, so many clinical trials before that time did not place the same emphasis on febrile neutropenia rates after chemotherapy. Rates of neutropenia or leukopenia are usually well documented in clinical trials today, but they can potentially lead to a detrimental clinical impact (ie, infection, death). Also, there is not always a correlation between neutropenia and neutropenic fever for every chemotherapy regimen.
A more in-depth risk assessment tool is needed to ensure the optimal use of G-CSFs. The Multinational Association of Supportive Care in Cancer provides a risk assessment calculator for febrile neutropenia, but it is primarily used in practice to determine if a patient can be treated with outpatient antibiotics.11 It is unknown whether this tool can help clinicians with the prescribing of G-CSFs for primary prophylaxis.
These medications are costly, and represent an additional injection, as well as the potential for adverse events for patients with cancer; it is therefore important to correctly evaluate the need before administering them.
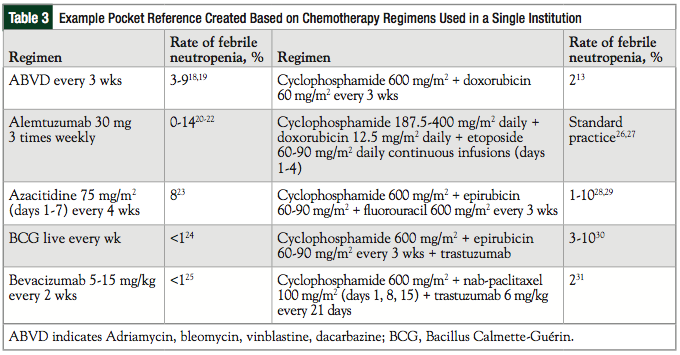
Table 3 provides the reference guide created for this study and lists the rates of CIFN associated with chemotherapy regimens at our institution based on clinical trial results. Providing prescribers with such a guide from institutional-specific chemotherapy regimens may aid in the evaluation of need, and can potentially eliminate the risk of not prescribing these medications when indicated, as well as decreasing the rate of unnecessary administrations of G-CSFs at institutions such as ours.
Limitations
A confounding factor in the current analysis is that previously documented cases of febrile neutropenia were not well recorded at our institution. We found 2 documented cases of previous febrile neutropenia in our review, and we hypothesize that more than 2 patients developed febrile neutropenia and required secondary prophylaxis with G-CSFs. Patients could also possibly have received G-CSFs at another site.
In addition, a weakness in the evaluations of risk for CIFN is that there are few available data on how to evaluate additional factors that may potentially affect the patient’s risk for CIFN. This leaves room for interpretation that may vary between prescribers.
Conclusion
Improving the optimal use of G-CSFs will require future research, including the evaluation of confounding factors associated with the evaluation of CIFN. A risk assessment tool needs to be developed and evaluated in a clinical trial to determine the exact impact of the many variables associated with febrile neutropenia, including the patient’s age and potential for bone marrow compromise. Until this research is completed, the first step is to increase awareness of the risk for CIFN with each regimen. Providing institution-specific reference guides may immediately help improve compliance rates, with the primary focus not only on limiting the inappropriate administrations, but also on making sure patients get these medications when indicated. Our institution will be reevaluated in the future to determine if the pocket reference improves compliance with ASCO guidelines and reduces the expenditure associated with the inappropriate use of G-CSFs.
Author Disclosure Statement
Dr Crane is Principal Investigator and Study Site Coordinator for the CAPTURE study for Cerexa, a subsidiary of Forest Pharmaceuticals. Dr Butler, Dr Waddell, and Dr Porter have reported no conflicts of interest.
References
1. Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30:1715-1724.
2. Neupogen (filgrastim) [prescribing information]. Thousand Oaks, CA: Amgen Inc; revised September 2013.
3. Glaspy JA, Bleecker G, Crawford J, et al. The impact of therapy with filgrastim (recombinant granulocyte colony-stimulating factor) on the health care costs associated with cancer chemotherapy. Eur J Cancer. 1993;29A(suppl 7):S23-S30.
4. Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23:1178-1184.
5. Lyman GH, Kuderer NM. The economics of the colony-stimulating factors in the prevention and treatment of febrile neutropenia. Crit Rev Oncol Hematol. 2004;
50:129-146.
6. Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187-3205.
7. Lyman GH, Lyman CH, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10:427-437.
8. Lyman GH, Kuderer NM, Crawford J, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer. 2011;117:1917-1927.
9. Fishman ML, Kumar A, Davis S, et al. Guideline-based peer-to-peer consultation optimizes pegfilgrastim use with no adverse clinical consequences. Am J Manag Care. 2012;18(5 special issue 2):e168-e172.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines (NCCN Guidelines®): myeloid growth factors. Version 2.2013. www.nccn.org/pro
fessionals/physician_gls/pdf/myeloid_growth.pdf. Accessed February 15, 2014.
11. Klastersky J, Paesmans M, Rubenstein EB, et al. The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000;18:3038-3051.
12. Chen YC, Lin SF, Yao M, et al. Induction therapy of newly diagnosed acute nonlymphocytic leukemia with idarubicin and cytosine arabinoside—the Taiwan experience. Semin Hematol. 1996;33(4 suppl 3):30-34.
13. Muss HB, Berry DA, Cirrincione C, et al; for the Cancer and Leukemia Group B Experience. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699-3704.
14. Mountzios G, Dimopoulos MA, Bamias A, et al. Randomized multicenter phase II trial of cisplatin and ifosfamide with or without paclitaxel in recurrent or metastatic carcinoma of the uterine cervix: a Hellenic Cooperative Oncology Group (HeCOG) study. Ann Oncol. 2009;20:1362-1368.
15. van den Bent MJ, Schellens JH, Vecht CJ, et al. Phase II study on cisplatin and ifosfamide in recurrent high grade gliomas. Eur J Cancer. 1998;34:1570-1574.
16. Vallejos C, Solidoro A, Gómez H, et al. Ifosfamide plus cisplatin as primary chemotherapy of advanced ovarian cancer. Gynecol Oncol. 1997;67:168-171.
17. Coiffier B, Osmanov EA, Hong X, et al; for the LYM-3001 Study Investigators. Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: a randomised phase 3 trial. Lancet Oncol. 2011;12:773-784.
18. Harker WG, Kushlan P, Rosenberg SA. Combination chemotherapy for advanced Hodgkin’s disease after failure of MOPP: ABVD and B-CAVe. Ann Intern Med. 1984;101:440-446.
19. Rueda A, Alba E, Ribelles N, et al. Six cycles of ABVD in the treatment of stage I and II Hodgkin’s lymphoma: a pilot study. J Clin Oncol. 1997;15:1118-1122.
20. Lundin J, Kimby E, Björkholm M, et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood. 2002;100:768-773.
21. Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:56165623.
22. Wierda WG, Kipps TJ, Keating MJ, et al; for the CLL Research Consortium. Self-administered, subcutaneous alemtuzumab to treat residual disease in patients with chronic lymphocytic leukemia. Cancer. 2011;117:116-124.
23. Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27:1850-1856.
24. Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124-1129.
25. Nagane M, Nishikawa R, Narita Y, et al. Phase II study of single-agent bevaciz-
umab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol. 2012;
42:887-895.
26. Sparano JA, Negassa A, Lansigan E, et al. Phase I trial of infusional cyclophosphamide, doxorubicin, and etoposide plus granulocyte-macrophage colony stimulating factor (GM-CSF) in non-Hodgkin’s lymphoma. Med Oncol. 2005;22:257-267.
27. Sparano JA, Wiernik PH, Strack M, et al. Infusional cyclophosphamide, doxorubicin, and etoposide in human immunodeficiency virus- and human T-cell leukemia virus type I-related non-Hodgkin’s lymphoma: a highly active regimen. Blood. 1993;81:2810-2815.
28. Edlund P, Ahlgren J, Bjerre K, et al. Dose-tailoring of FEC adjuvant chemotherapy based on leukopenia is feasible and well tolerated. Toxicity and dose intensity in the Scandinavian Breast Group phase 3 adjuvant Trial SBG 2000-1. Acta Oncol. 2011;50:329-337.
29. Martín M, Rodríguez-Lescure A, Ruiz A, et al; for the GEICAM 9906 Study Investigators. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by Paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805-814.
30. Untch M, Muscholl M, Tjulandin S, et al. First-line trastuzumab plus epirubicin and cyclophosphamide therapy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: cardiac safety and efficacy data from the Herceptin, Cyclophosphamide, and Epirubicin (HERCULES) trial. J Clin Oncol. 2010;28:1473-1480.
31. Yardley D, Burris H III, Peacock N, et al. A pilot study of adjuvant nanoparticle albumin-bound (nab) paclitaxel and cyclophosphamide, with trastuzumab in HER2-positive patients, in the treatment of early-stage breast cancer. Breast Cancer Res Treat. 2010;123:471-475.