Molecular drugs are derived by means of chemical synthesis, resulting in small structures (<1000 daltons) that are simple, well-defined, and easily characterized.1 Conversely, biologic drugs are derived from living-cell cultures, resulting in large structures (>10,000 daltons) that are complex and are typically defined by the manufacturing process.1 Biologics consist of a wide range of drugs that include (but are not limited to) vaccines, clotting factors, enzymes, antibodies, and other recombinant therapeutic proteins, all of which are intended to prevent, treat, or cure a disease.1,2
A biosimilar drug is a biologic medicine that is highly similar to, but not identical to, the originator or the reference biologic drug.3 The US Food and Drug Administration (FDA)’s regulatory definition of biosimilar drugs requires that they be highly similar to the reference drug, with no clinically meaningful differences in the safety, purity, and potency of the drug, and only allows for minor differences in the clinically inactive components.4
Demonstrating this biosimilarity is a stringent process, with FDA guidance recommending a stepwise approach consisting of a variety of studies that include structural and functional characterization, animal testing, human pharmacokinetic and pharmacodynamic data, and clinical immunogenicity assessments.5,6
Granulocyte colony-stimulating factor (G-CSF) drugs are one such example of biologic medicines that have available biosimilar drugs.7,8 As a therapeutic agent, G-CSF mimics endogenous G-CSF, which acts on the myeloid lineage of hematopoietic progenitors, such as neutrophils, to stimulate their proliferation and differentiation.9 G-CSF is primarily indicated for the prevention of chemotherapy-induced myelosuppression with or without fever.9,10 G-CSF is also used to mobilize stem cells from bone marrow for collection and use in stem-cell transplants.10-12
In Europe, the first biosimilar of the reference G-CSF drug filgrastim was approved in 2008 by the European Medicines Agency.13 In the United States, however, the first biosimilar of filgrastim was only approved by the FDA in 2015.13 The main advantage of using these biosimilar G-CSF drugs in place of the reference drugs is the potential for an up to 80% decrease in costs.13,14
Despite the significant cost advantages of biosimilars and their theoretical ability to maintain therapeutic efficacy and safety, biosimilar drugs are not without controversy. Although the FDA requires there to be no clinically meaningful difference between the reference and the biosimilar drugs in terms of safety, purity, and potency, concerns remain regarding their interchangeability.1,5,15
Biosimilarity does not equate to interchangeability, which requires the biosimilar drug to produce the same clinical result as the reference drug in any given patient.16 Under the Biologics Price Competition and Innovation Act, biosimilar drugs that meet this definition can be designated “interchangeable,” allowing the biosimilar drug to be freely interchanged with the reference drug, without the prescriber’s intervention.2,4,17 By May 2022, only 2 biosimilars (Semglee and Cyltezo) have met this definition of interchangeability.
For any biosimilar drug, interchangeability is of concern, because of the inherent heterogeneity associated with the different manufacturing processes between the reference and the biosimilar drugs, which may affect these clinical results.1,15 Tied to this concern is the issue of extrapolation, a process through which the biosimilar drug will also gain approval for all the indications of the reference drug once biosimilarity is demonstrated, regardless of whether the biosimilar drug was clinically studied in those specific conditions.1,5
Like other biosimilar drugs, biosimilar G-CSF drugs share these concerns of manufacturing heterogenicity, interchangeability, and indication extrapolation. For the prevention of chemotherapy-induced neutropenia, many phase 1, 2, and 3 clinical trials are indicating the efficacy and safety equivalency of the biosimilar and the reference G-CSF drugs, which helps to alleviate these concerns for this particular use.18-21
Data are lacking about the use of biosimilar G-CSF for stem-cell mobilization, which is one of the extrapolated indications. The limited available evidence suggests that a biosimilar G-CSF drug is equivalent to the reference G-CSF drug for stem-cell mobilization purposes.22-34 By contrast, one study showed that biosimilar G-CSF drugs were less effective in poor mobilizers.26 The majority of current studies are descriptive, have limited sample sizes, and/or are conducted in a practice setting outside of the United States.22-34 In all, 2 studies conducted were in the United States, including one that was published as a correspondence and examined mobilization outcomes with filgrastim-sndz in 1 institution,35 and the other studied tbo-filgrastim, which was approved by the FDA before the availability of the FDA’s current biosimilar drug approval pathway.36
The objective of our retrospective study was to compare the stem-cell mobilization outcomes of biosimilar G-CSF drugs versus their reference G-CSF drugs.
Methods
This single-center, retrospective cohort study design was conducted to compare the reference G-CSF drug filgrastim (Neupogen) with the biosimilar G-CSF drug filgrastim-aafi (Nivestym). On September 1, 2020, Mayo Clinic Enterprise formally incorporated the use of biosimilar drugs into clinical practice through the implementation of a therapeutic interchange workflow. Accordingly, the study included 2 groups based on the period the patient had undergone mobilization; the 2 periods were defined as a preimplementation period, from September 1, 2019, to February 28, 2020, in which patients received the reference G-CSF drug class, and the postimplementation period, from September 1, 2020, to February 28, 2021, in which patients received the biosimilar G-CSF drug class.
The 6-month study windows were assigned with the intent to minimize the potential impact of the COVID-19 pandemic; that is, the low numbers of patients undergoing autologous stem-cell transplant (ASCT) between March 2020 and August 2020. This study was approved by the Institutional Review Board and complied with all applicable regulations for the protection of human subjects.
Adults aged ≥18 years with a diagnosis of multiple myeloma, immunoglobulin light-chain amyloidosis, non-Hodgkin lymphoma (including primary central nervous system), or solid tumors (specifically germ-cell tumors) who were undergoing mobilization and treatment with ASCT at Mayo Clinic Arizona were eligible for this study. Patients had to have received a reference G-CSF drug during the preimplementation period (preimplementation group) or a biosimilar G-CSF drug during the postimplementation period (postimplementation group) to be included in this study. Patients aged <18 years, those who had mobilization at an outside facility, or those who otherwise did not meet the inclusion criteria were excluded from this study.
The reference G-CSF drug was filgrastim (Neupogen), and the biosimilar G-CSF drug was filgrastim-aafi (Nivestym), which was our institution’s preferred formulary drug. All patients were eligible to receive any biosimilar G-CSF drug per the payer-specific requirements, but filgrastim-aafi was the only biosimilar drug used during the study period.
The reference and the biosimilar drugs were each dosed at 10 mcg/kg daily, which was rounded down to the nearest vial size of 300 mcg or 480 mcg. Every subcutaneous dose was repeated on a daily basis until the collection parameters were met or the patient’s stem cells did not mobilize. The exceptions to this dosing regimen were patients with a diagnosis of amyloidosis, who received G-CSF at a twice-daily dosing of 5 mcg/kg, in accordance with our institutional protocol.
If the patient did not meet the stem-cell collection parameters, which were defined by a first-day collection of <1.5 × 106 CD34-positive cells/kg or a subsequent-day collection of <0.5 × 106 CD34-positive cells/kg, a hematopoietic stem-cell mobilizer, plerixafor, was added on day 4 of mobilization. Plerixafor was dosed at 0.24 mg/kg, with a maximum dose of 24 mg. Plerixafor was dose-adjusted for renal function, using a dose of 0.16 mg/kg for creatinine clearance of <50 mL/min.
The study’s primary outcome was the percent of patients with successful mobilization, which was defined by the total collection of ≥2 × 106 CD34-positive cells/kg body weight by day 7, regardless of the use of plerixafor or the number of apheresis collections. The secondary outcomes included the total number of CD34-positive cells collected (× 106/kg), the total G-CSF dose (mcg/kg daily), and the number of days to absolute neutrophil count (ANC) and platelet engraftment. Per the Center for International Blood and Marrow Transplant Research, neutrophil engraftment was defined as the first of 3 consecutive days, at least 24 hours apart, where ANC is ≥0.5 × 109/L.37
Patients were allowed to receive growth factors for this engraftment definition. Per the Center for International Blood and Marrow Transplant Research, platelet engraftment was defined as the first of 3 consecutive days, at least 24 hours apart, in which the platelet count is ≥20 × 109/L and without a platelet transfusion in the previous 7 days.37 Additional secondary outcomes included the total number of apheresis collections and the total use of plerixafor (mg/kg).
The demographic data collected for the evaluation of the patients’ baseline characteristics included age, sex, diagnosis, treatment regimen, height (in cm), weight (in kg), and body surface area (in m2). We compared the patients’ demographics and clinical characteristics between the reference drug and the biosimilar drug groups, using the chi-square test for categorical variables and a Wilcoxon rank-sum test for continuous measures.
The mobilization success was compared between the 2 groups using a one-sided z-test based on a noninferiority margin of 11%, and a 97.5% confidence interval of the difference in mobilization success between the reference drug and the biosimilar drug groups was constructed. The noninferiority margin should be based on clinical and statistical reasoning. In a comparable study to our study, the researchers selected a noninferiority margin of 20%22; however, we decided that this margin was not one in which the groups would be truly similar.
In our analysis, multiple collections for a single patient were treated as independent for the primary analysis. A confirmatory analysis was also conducted using 1 record per patient, and removing duplicate records for a given patient. For patients with unsuccessful stem-cell collection, the record associated with the unsuccessful collection was used; for patients with successful stem-cell collection, the first collection was used.
Assumed sample sizes of 70 patients in each group achieves 85% power, for a noninferiority margin of 11%. Under the null hypothesis, we assumed that the reference drug group success rate was 95%, and the biosimilar drug group success rate was 84%.
R Statistical Software version 4.0.4 (the R Foundation for Statistical Computing; Vienna, Austria) was used to conduct the analysis.
Results
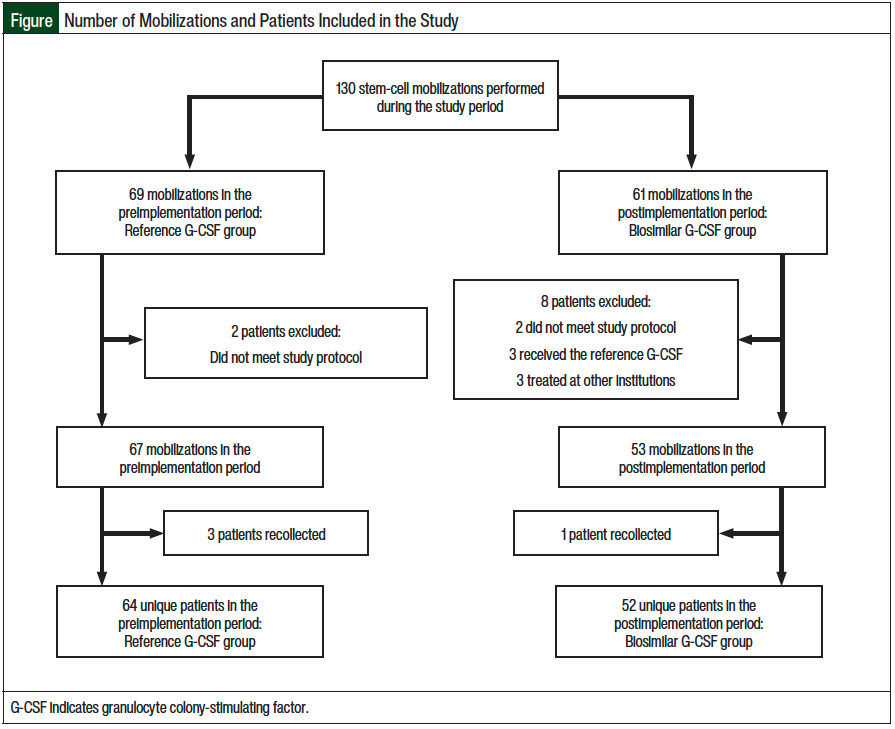
A total of 130 mobilizations met the study’s inclusion criteria, including 69 mobilizations (with the reference G-CSF) in the preimplementation period and 61 mobilizations (with the biosimilar G-CSF) in the postimplementation period. Overall, 2 patients were excluded from the preimplementation group, and 8 patients were excluded from the postimplementation group. Because some patients who did not meet the stem-cell mobilization goals had a repeated mobilization, a total of 67 collections were done for 64 unique patients in the preimplementation group and 53 collections for 52 unique patients in the postimplementation group. A total of 116 unique patients were ultimately included in this study, with 64 receiving the reference G-CSF drug and 52 receiving the biosimilar G-CSF drug. The Figure summarizes the number of mobilizations and the number of patients included in the study.
Table 1 outlines the patient baseline characteristics for the 2 patient groups; a P value is given for each univariate test, with a P value of <.05 considered statistically significant. None of the P values is significantly small, suggesting that the preimplementation and the postimplementation groups were sufficiently similar. The majority of the patients were male (69%), had a mean age of 60 years, were diagnosed with multiple myeloma (65.5%), and received melphalan as a treatment regimen (68.1%). In addition, 29.3% of the patients had non-Hodgkin lymphoma, and 27.6% of the patients received a conditioning regimen with carmustine, etoposide, cytarabine, and melphalan.
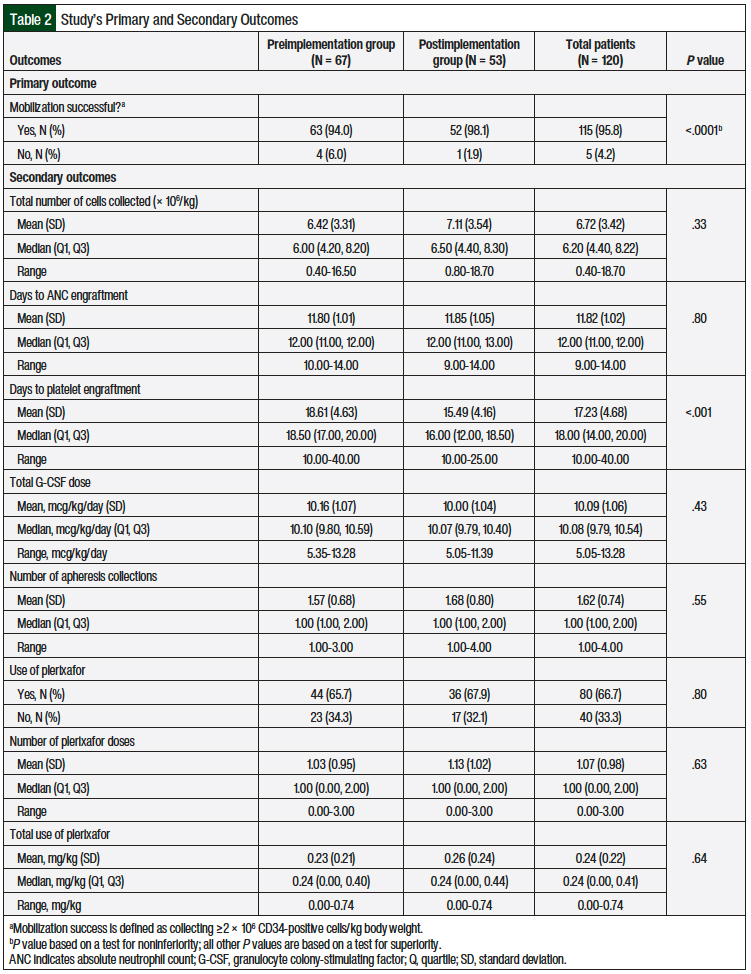
The primary study outcome was the percentage of stem-cell mobilization success, which was defined as the collection of ≥2 × 106 CD34-positive cells/kg of body weight by day 7. Across the pre- and postimplementation groups, the overall mobilization success rate was 95.8%, and only 5 mobilizations among the 120 mobilizations that were evaluated did not meet the collection parameters (Table 2). The mobilization success rate was 94% for the preimplementation group (the reference G-CSF) and 98.1% for the postimplementation group (the biosimilar G-CSF), an increase of 4.1% in the point estimate.
Based on a one-sided 97.5% confidence interval, the difference in the percentage of mobilization success between the preimplementation and the postimplementation groups is <4.4%, so we concluded that the biosimilar drug was noninferior to the reference drug, based on a noninferiority margin of 11%.
We also examined 8 additional secondary outcomes that are thought to be related to the clinical efficacy of G-CSF drugs in stem-cell mobilization and ASCT (Table 2). These outcomes included the total number of CD34-positive cells collected (× 106/kg), the number of days to ANC and platelet engraftment, and the G-CSF dose (mcg/kg daily). Other secondary outcomes included the number of apheresis collections and the total use of plerixafor (mg/kg). For all the secondary outcomes, we found no significant differences between the 2 groups, with the exception of days to platelet engraftment, where platelet engraftment was achieved more quickly in the postimplementation group (15.49 days) than in the preimplementation group (18.61 days), on average.
Discussion
The National Comprehensive Cancer Network (NCCN) guidelines on hematopoietic growth factors state that an FDA-approved biosimilar drug is an appropriate substitute for filgrastim for the mobilization of stem cells in the ASCT setting.10 This recommendation is driven by the rigorous FDA approval pathway, according to which the bioequivalence of biosimilar drugs must be demonstrated through structural characterization, pharmacokinetics, pharmacodynamics, clinical immunogenicity, and other various safety and efficacy outcomes in clinical studies. Such clinical trials for biosimilars, however, have been conducted under the indication of the prophylaxis and treatment of febrile neutropenia.
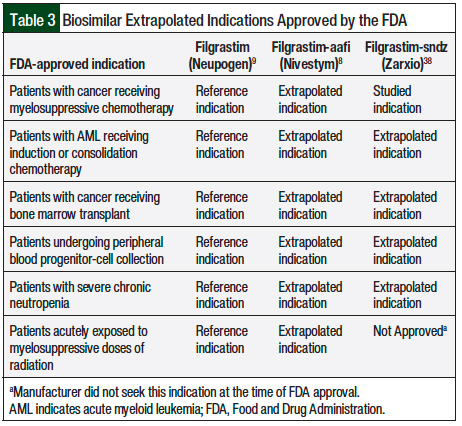
The FDA-approved indication of G-CSF biosimilars for stem-cell mobilization was an extrapolated indication, for which the supporting data are limited (Table 3).8,9,38 The NCCN guidelines acknowledge this limitation, emphasizing that long-term outcomes are essentially unknown.10 Furthermore, the NCCN guidelines advise the clinician to be cognizant of any complications from biosimilar mobilization discussed in the literature or presented in their patients, and the reporting of these variations in clinical outcomes is of critical importance going forward.10
As mentioned earlier, data are lacking for the use of biosimilar G-CSF for its extrapolated indication of stem-cell mobilization for ASCT. The majority of the current supporting studies on this topic are descriptive in nature, have small sample sizes, and/or were conducted in a practice setting outside of the United States.22-34 Of the 2 studies conducted in the United States, one was presented as a correspondence and examined mobilization outcomes with filgrastim-sndz,35 and the other examined mobilization outcomes with tbo-filgrastim,36 which was approved before the existence of the conventional biosimilar approval pathway.
Abdallah and colleagues studied 370 patients undergoing stem-cell mobilization for ASCT with a reference or a biosimilar G-CSF in the United States.35 Similar to our study, that study was a single-center, retrospective review that compared the clinical outcomes associated with the use of a biosimilar G-CSF drug and a reference G-CSF drug. The primary outcome was the use of plerixafor, a reversible antagonist of chemokine receptor type 4 that increases stem-cell yield when used in conjunction with G-CSF. The results showed no statistical difference in plerixafor use or in any of the other clinical outcomes (ie, CD34-positive cells collected, number of apheresis days, need for second mobilization) between the groups using the biosimilar or the reference G-CSF drug.35
Similarly, our study showed that the biosimilar and the reference drugs were similar in terms of mobilization success. In addition, with the exception of days to platelet engraftment, we did not find a significant difference in the secondary outcomes between the 2 groups. The biosimilar G-CSF group had a faster time to platelet engraftment than that of the reference G-CSF group (mean, 15.49 days vs 18.61 days; P <.001).
We attribute this difference in the time to platelet engraftment to the potential limitations associated with retrospective chart reviews or to other confounding factors that are not fully elucidated by this study. In addition, although the secondary outcome of platelet engraftment may be of clinical interest, it may not be truly reflective of the clinical efficacy of G-CSFs used in stem-cell mobilization because of other factors affecting this metric, such as individual patient characteristics, that are not accounted for in this study.
We only examined biosimilar outcomes for filgrastim-aafi and not for filgrastim-sndz (Zarxio), the only other short-acting biosimilar G-CSF that is available in the United States. The data generated in our study support the use of filgrastim-aafi for stem-cell mobilization and may not be generalizable to filgrastim-sndz. This study was designed to address concerns of manufacturing heterogenicity, interchangeability, and extrapolated indications between reference and biosimilar drugs. To state that our data are generalizable to other biosimilar G-CSFs that have not been studied circumvents the aims of our study, because these concerns still exist among biosimilar drugs themselves.
A major driver for the adoption of biosimilars is their potential for an up to 80% cost-savings for the healthcare institution using these drugs.13,14 Along with clinical and safety outcomes, pharmacoeconomics must be considered when implementing the use of biosimilars into clinical practice. The wholesale acquisition cost for the reference G-CSF Neupogen 480 mcg is $501.33 per vial compared with $350.40 per vial of the biosimilar Nivestym, and $438.98 per syringe of the biosimilar Zarxio.39
Comparing the costs between the pre- and postimplementation cohorts in our study, we saw an approximate 57% reduction in the drug costs to our institution. For patients, this would translate to a reduction in charges billed through their medical benefit, when biosimilar drugs are used in place of reference drugs.
Furthermore, under the 340B drug discount program, biosimilar drugs have been granted pass-through status and are reimbursed at a rate of average sale price (ASP) plus 6%.40 Comparatively, reference drugs are reimbursed at ASP minus 22.5%, making them far more costly to use at 340B sites. Depending on the volume of patients undergoing mobilization for stem-cell collection, this can represent a significant cost-savings for the stem-cell transplant program.
Limitations
As a single-center study, these results may not be generalizable to other stem-cell transplant centers that may use different mobilization protocols. Ideally, our study would be expanded to a multicenter study using other Mayo Clinic Enterprise locations, as well as to other institutions. The final study sample size was smaller than what was originally planned; however, the margin of difference was minimal between the G-CSF reference drug cohort and the G-CSF biosimilar cohorts.
In addition, as a retrospective cohort study design, the quantity and quality of the data are limited to what has been documented in our electronic medical record system. This limitation pertains to the safety data as well. Safety outcomes were not reported in this study, because a prospective study would be needed to ascertain these data reliably instead of relying on what may be reported in a patient’s chart.
Another study limitation is that we only evaluated the G-CSF biosimilar filgrastim-aafi, although there are currently 6 biosimilar G-CSFs (including pegylated G-CSFs) that are approved in the United States. The biosimilar interchange workflow at the Mayo Clinic allows the selection of a biosimilar based on payer demand, but no other biosimilar drugs were administered during the postimplementation study period.
Conclusions
Concerns associated with the use of biosimilars include manufacturing heterogenicity, interchangeability, and indication extrapolation. This is especially true for biosimilar G-CSFs that are used for stem-cell mobilization, because this particular indication is extrapolated, and has few supporting studies. The results of our single-center, retrospective analysis indicate that the use of a biosimilar G-CSF drug for stem-cell mobilization in ASCT is noninferior to the reference G-CSF drug in terms of mobilization success, which was the primary outcome in this study. Furthermore, no significant differences were observed between the 2 groups in the majority of the secondary outcomes, with the exception of days to platelet engraftment.
This study adds to the limited body of data regarding the use of biosimilar G-CSF drugs for the extrapolated indication of stem-cell mobilization in the US practice setting. Additional prospective studies are warranted to determine the clinical mobilization outcomes in patients receiving a biosimilar G-CSF drug versus a reference G-CSF drug. In addition, studies are needed to examine the clinical mobilization outcomes with biosimilar G-CSFs other than filgrastim-aafi and to ascertain the safety outcomes.
Funding Source
The Mayo Clinic Department of Pharmacy provided funding for biostatistician support for data analysis and statistical writing for this study.
Author Disclosure Statement
Dr Bartels, Dr Klanderman, Ms Rogers, Ms Gray, Ms Kosiorek, and Dr Coughlin have no conflicts of interest to report.
References
- Declerck PJ. Biologicals and biosimilars: a review of the science and its implications. Generics Biosimilars Initiat J. 2012;1:13-16.
- Kozlowski S, Woodcock J, Midthun K, Behrman Sherman R. Developing the nation’s biosimilars program. N Engl J Med. 2011;365:385-388.
- Santos SB, Sousa Lobo JM, Silva AC. Biosimilar medicines used for cancer therapy in Europe: a review. Drug Discov Today. 2019;24:293-299.
- Regulation of biological products, 42 USC §262 (2011). www.govinfo.gov/content/pkg/USCODE-2011-title42/html/USCODE-2011-title42-chap6A-subchapII-partF-subpart1-sec262.htm. Accessed December 31, 2020.
- McKinnon RA, Cook M, Liauw W, et al. Biosimilarity and interchangeability: principles and evidence: a systematic review. BioDrugs. 2018;32:27-52.
- US Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. April 2015. www.fda.gov/media/82647/download. Accessed April 1, 2022.
- US Food and Drug Administration. Simple search results for: Neupogen. Purple Book. 2020. https://purplebooksearch.fda.gov/results?query=filgrastim&title=Neupogen. Accessed December 31, 2020.
- Nivestym (filgrastim-aafi) injection, for subcutaneous or intravenous use [prescribing information]. Pfizer; November 2021. https://labeling.pfizer.com/ShowLabeling.aspx?id=10899. Accessed April 1, 2022.
- Neupogen (filgrastim) injection, for subcutaneous or intravenous use [prescribing information]. Amgen; February 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/neupogen/neupogen_pi_hcp_english.pdf. Accessed April 1, 2022.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Hematopoietic Growth Factors. Version 1.2022. December 22, 2021. www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed April 1, 2022.
- Hosing C. Hematopoietic stem cell mobilization with G-CSF. In: Kolonin MG, Simmons PJ, eds. Stem Cell Mobilization: Methods and Protocols. New York, NY: Humana Press; 2012:37-47. Methods in Molecular Biology, vol 904.
- Anasetti C, Logan BR, Lee SJ, et al; for the Blood and Marrow Transplant Clinical Trials Network. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487-1496.
- Schulz M, Bonig H. Update on biosimilars of granulocyte colony-stimulating factor-when no news is good news. Curr Opin Hematol. 2016;23:61-66.
- Publicover A, Richardson DS, Davies A, et al. Use of a biosimilar granulocyte colony-stimulating factor for peripheral blood stem cell mobilization: an analysis of mobilization and engraftment. Br J Haematol. 2013;162:107-111.
- Chow SC. Assessing biosimilarity and interchangeability of biosimilar products. Stat Med. 2013;32:361-363.
- Christl LA, Woodcock J, Kozlowski S. Biosimilars: the US regulatory framework. Annu Rev Med. 2017;68:243-254.
- US Food and Drug Administration. Implementation of the Biologics Price Competition and Innovation Act of 2009. February 12, 2016. www.fda.gov/drugs/guidance-compliance-regulatory-information/implementation-biologics-price-competition-and-innovation-act-2009. Accessed April 4, 2022.
- Waller CF, Bronchud M, Mair S, Challand R. Comparison of the pharmacodynamic profiles of a biosimilar filgrastim and Amgen filgrastim: results from a randomized, phase I trial. Ann Hematol. 2010;89:971-978.
- Sörgel F, Schwebig A, Holzmann J, et al. Comparability of biosimilar filgrastim with originator filgrastim: protein characterization, pharmacodynamics, and pharmacokinetics. BioDrugs. 2015;29:123-131.
- Yao HM, Ottery FD, Borema T, et al. PF-06881893 (Nivestym), a filgrastim biosimilar, versus US-licensed filgrastim reference product (US-Neupogen): pharmacokinetics, pharmacodynamics, immunogenicity, and safety of single or multiple subcutaneous doses in healthy volunteers. BioDrugs. 2019;33:207-220.
- Yang J, Yu S, Yang Z, et al. Efficacy and safety of supportive care biosimilars among cancer patients: a systematic review and meta-analysis. BioDrugs. 2019;33:373-389. Erratum in: BioDrugs. 2019;33:589-594.
- Chew C, Ng HY. Efficacy and safety of Nivestim versus Neupogen for mobilization of peripheral blood stem cells for autologous stem cell transplantation. Sci Rep. 2019;9:19938. doi: 10.1038/s41598-019-56477-w.
- Schmitt M, Hoffmann JM, Lorenz K, et al. Mobilization of autologous and allogeneic peripheral blood stem cells for transplantation in haematological malignancies using biosimilar G-CSF. Vox Sang. 2016;111:178-186.
- Reményi P, Gopcsa L, Marton I, et al. Peripheral blood stem cell mobilization and engraftment after autologous stem cell transplantation with biosimilar rhG-CSF. Adv Ther. 2014;31:451-460.
- Schmitt M, Publicover A, Orchard KH, et al. Biosimilar G-CSF based mobilization of peripheral blood hematopoietic stem cells for autologous and allogeneic stem cell transplantation. Theranostics. 2014;4:280-289.
- Parody R, Sánchez-Ortega I, Ferrá C, et al. Mobilization of hematopoietic stem cells into peripheral blood for autologous transplantation seems less efficacious in poor mobilizers with the use of a biosimilar of filgrastim and plerixafor: a retrospective comparative analysis. Oncol Ther. 2020;8:311-324.
- Pahnke S, Egeland T, Halter J, et al; for the Working Group Medical of the World Marrow Donor Association. Current use of biosimilar G-CSF for haematopoietic stem cell mobilisation. Bone Marrow Transplant. 2019;54:858-866.
- Nasillo V, Paolini A, Riva G, et al. Effectiveness of originator (Neupogen) and biosimilar (Zarzio) filgrastim in autologous peripheral blood stem cell mobilization in adults with acute myeloid leukemia: a single-center retrospective study. Leuk Lymphoma. 2018;59:225-228.
- Bonig H, Becker PS, Schwebig A, Turner M. Biosimilar granulocyte–colony-stimulating factor for healthy donor stem cell mobilization: need we be afraid? Transfusion. 2015;55:430-439.
- Lefrère F, Brignier AC, Elie C, et al. First experience of autologous peripheral blood stem cell mobilization with biosimilar granulocyte colony-stimulating factor. Adv Ther. 2011;28:304-310.
- Antelo ML, Zabalza A, Sánchez Antón MP, et al. Mobilization of hematopoietic progenitor cells from allogeneic healthy donors using a new biosimilar G-CSF (Zarzio). J Clin Apher. 2016;31:48-52.
- Manko J, Walter-Croneck A, Jawniak D, et al. A clinical comparison of the efficacy and safety of biosimilar G-CSF and originator G-CSF in haematopoietic stem cell mobilization. Pharmacol Rep. 2014;66:239-242.
- Lanza F, Saraceni F, Pezzi A, et al; for GITMO (Italian Society for Transplantation). A comparative analysis of biosimilar vs. originator filgrastim in combination with plerixafor for stem cell mobilization in lymphoma and multiple myeloma: a propensity-score weighted multicenter approach. Am J Hematol. 2017;92:E557-E559.
- Wicherska-Pawłowska K, Rybka J, Prajs I, et al. The comparison of effectiveness and safety between different biosimilars of G-CSF in the mobilization of peripheral blood stem cells (PBSCs) for autologous transplantation (autologous peripheral blood stem cell transplantation, auto-PBSCT). J Clin Apher. 2020;35:4-8.
- Abdallah N, Kim S, Ayash L, et al. Does use of biosimilar G-CSF change plerixafor utilization during stem cell mobilization for autologous stem cell transplant? Bone Marrow Transplant. 2020;55:1655-1657.
- Eplin DD, Jackson AD, Smith AM, et al. Use of biosimilar granulocyte colony-stimulating factor for mobilization in autologous and allogeneic hematopoietic stem cell transplantation. Clin Hematol Int. 2019;1:229-233.
- Center for International Blood and Marrow Transplant Research. Transplant Essential Data Manuals. Q17–18: initial platelet recovery. Updated October 29, 2021. https://www.cibmtr.org/manuals/fim/1/en/topic/q12-14-initial-platelet-recovery. Accessed April 18, 2022.
- Zarxio (filgrastim-sndz) injection, for subcutaneous or intravenous use [prescribing information]. Sandoz; March 2021. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=c0d1c22b-566b-4776-bdbf-00f96dad0cae&type=display. Accessed April 4, 2022.
- Medi-Span Price Rx. Wolters Kluwer Clinical Drug Information. 2021. www.wolterskluwer.com/en/solutions/medi-span/price-rx. Accessed October 28, 2021.
- Hagen T. CMS payment policy plays role in biosimilar uptake. The Center for Biosimilars. January 30, 2020. www.centerforbiosimilars.com/view/cms-payment-policy-plays-role-in-biosimilar-uptake. Accessed October 23, 2021.