Hypercalcemia associated with malignancy is a serious complication of a variety of cancers. Typically occurring in patients with advanced cancer, hypercalcemia associated with malignancy is estimated to affect as many as 20% to 30% of patients with cancer.1,2 Hypercalcemia associated with malignancy is most frequently linked to humoral hypercalcemia that results from tumor cells’ ectopic production of parathyroid hormone–related peptide, which in turn reduces osteoblast activity, increases osteoclast activity, and promotes renal tubular calcium reabsorption.3,4 Typically, mild and chronic hypercalcemia can be asymptomatic or can present only with mild and nonspecific symptoms, including fatigue, constipation, dehydration, anorexia, and mental impairment.2,3
A rapid increase of serum calcium level in acute hypercalcemia associated with malignancy is often linked to significant neurologic symptoms and acute renal insufficiency and volume depletion.1,2 If left untreated, excessive serum calcium level can lead to more severe complications, such as renal failure, coma, and death.1 A retrospective study of malignancy-related hypercalcemia showed that the condition is associated with poor prognosis that results in an up to 50% mortality rate within 30 days of diagnosis.5
The management of hypercalcemia associated with malignancy typically involves aggressive hydration and intravenous (IV) bisphosphonate therapy.2,4,6 The second-generation bisphosphonates zoledronic acid and pamidronate are approved by the US Food and Drug Administration (FDA) for the treatment of hypercalcemia associated with malignancy. Bisphosphonates inhibit osteoclastic bone resorption and bone turnover, leading to decreased release of calcium from the bones into the bloodstream.7
Zoledronic acid and pamidronate are administered as IV infusions, ranging in infusion time from 15 minutes to 6 hours. Zoledronic acid is generally preferred over pamidronate based on 2 randomized controlled trials that demonstrated a higher rate of complete response, that is, corrected serum calcium to ≤10.8 mg/dL, by day 10, and a longer duration of response in the zoledronic acid group.7 The rate of normalization of serum calcium levels by day 10 in the zoledronic acid 4-mg group was 88.4% compared with 69.7% in the patients who received pamidronate.7
Zoledronic acid and pamidronate have similar toxicity profiles, with the most common adverse event being acute-phase reactions that manifest as mild-to-moderate flulike symptoms in the first few days after the infusion.8,9 In patients who receive zoledronic acid, nephrotoxicity occurs in up to 11% of patients with previously normal creatinine levels and in up to 40% of patients with previously abnormal renal function. By contrast, in patients who receive pamidronate, nephrotoxicity occurs in up to 12% of those with normal baseline creatinine level and in up to 20% of those with abnormal renal function.8
Although pamidronate nephrotoxicity typically manifests as nephrotic syndrome and glomerular lesions, such as collapsing focal segmental glomerulosclerosis, zoledronic acid nephrotoxicity is mainly associated with tubular injury that results in acute tubular necrosis.9
Bisphosphonates remain a standard of care for the treatment of hypercalcemia associated with malignancy, but denosumab is an alternative bone resorptive agent with a novel mechanism of action that has been used for the treatment of patients with malignancies. Denosumab is a fully humanized antibody that targets the receptor activator of nuclear factor-kappa (RANK) ligand (RANKL), a bone resorption mediator.10
Tumor cells release cytokines that bind to RANK, a transmembrane protein that is involved in the regulation of osteoclast differentiation and activation. By binding to RANKL, denosumab prevents osteoclast activation and reduces bone turnover, thereby reducing the release of calcium into the bloodstream.10 In contrast to the bisphosphonates, denosumab has a lower incidence of nephrotoxicity10 and may be more convenient to administer as a subcutaneous injection than an IV infusion, which is necessary for bisphosphonates.
Denosumab is currently approved by the FDA for the treatment of bisphosphonate-refractory hypercalcemia associated with malignancy based on a single-arm, open-label study that included 33 patients with malignancy-related hypercalcemia refractory to IV bisphosphonates.10,11 In that study, bisphosphonate-refractory was defined as a corrected serum calcium level of ≥12.5 mg/dL, despite treatment with IV bisphosphonate that was administered within the previous 7 to 30 days. The patients received subcutaneous injections of denosumab 120 mg on days 1, 8, and 15, for the first 28-day cycle, followed by monthly injections. In all, 64% of the patients reached a corrected serum calcium level of ≤11.5 mg/dL by day 10, and 36% of patients had a complete response, with a corrected serum calcium level of ≤10.8 mg/dL and an estimated median duration of response of 104 days.11
In addition, many case reports have been published that describe the efficacy of denosumab in patients with hypercalcemia associated with malignancy that is refractory to bisphosphonates and other standard treatments, or in patients with severe renal dysfunction.12-20
Denosumab’s efficacy in the second-line therapy setting provides a rationale to consider denosumab in the front-line treatment setting for patients with malignancy-related hypercalcemia. Anecdotally, denosumab has been used in the first-line outpatient management of hypercalcemia associated with malignancy at our institution, although there are currently no prospective studies that can validate this practice.
We sought to evaluate and characterize our institution’s use of denosumab in the first-line treatment of hypercalcemia associated with malignancy.
Methods
This retrospective cohort study was conducted at the University of North Carolina Medical Center in Chapel Hill, NC, after Institutional Review Board approval. All adults aged >18 years who received denosumab 120 mg subcutaneously in the outpatient oncology clinics between April 1, 2014, and September 15, 2017, were identified, using a centralized data warehouse.
Patients were included if they had received denosumab for the first-line treatment of hypercalcemia associated with malignancy, had a solid tumor or multiple myeloma, and had a corrected serum calcium level of >11 mg/dL. Patients were excluded if they did not have a repeated serum calcium laboratory test within 8 weeks of denosumab administration, had a previous episode of hypercalcemia, or received denosumab or zoledronic acid for any other indication, including for the prevention of skeletal-related events.
The patient demographic data collected included sex, age, and race. The study’s primary outcome was the normalization of serum calcium levels within 8 weeks of the first denosumab administration for hypercalcemia. The normalization of corrected serum calcium was defined as a serum calcium level of <10.5 mg/dL.
The corrected serum calcium level was calculated using the following formula:
Corrected calcium = {0.8 × [normal albumin (4 g/dL) – patient’s albumin level]} + serum calcium.
We defined the severity of a patient’s hypercalcemia based on serum calcium level, as mild (11-11.9 mg/dL), moderate (12-13.9 mg/dL), or severe (≥14 mg/dL). The baseline symptoms of hypercalcemia that were assessed included altered mental status, fatigue, weakness, gastrointestinal symptoms (ie, nausea, vomiting, constipation, decrease in appetite), electrocardiogram changes as documented in physician visit notes, and impaired renal function, defined as an increase in serum creatinine of 1.5 times the patient’s baseline before a hypercalcemia of malignancy event.
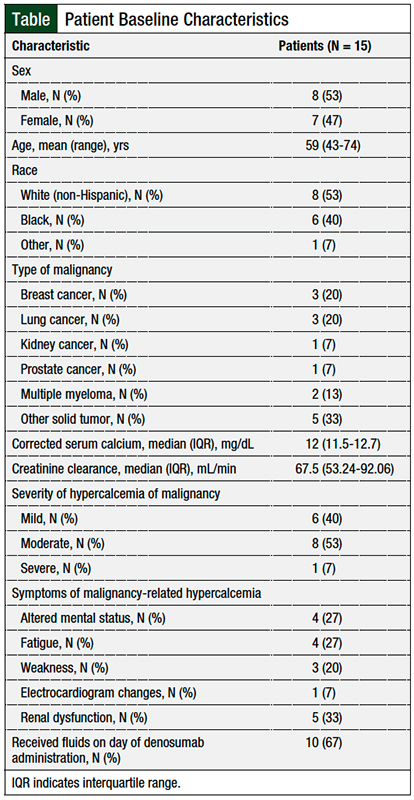
Other baseline data collected included administration of IV fluid (ie, 0.9% sodium chloride, 5% dextrose, lactated Ringer’s solution) on the day of denosumab’s administration, serum sodium, serum potassium, and serum creatinine. Creatinine clearance (CrCl) was calculated using the Cockcroft-Gault formula. All baseline data were collected from the date the patient presented with hypercalcemia of malignancy.
Our primary outcome was the rate of normalization of corrected serum calcium levels (ie, <10.5 mg/dL) within 8 weeks of denosumab administration. We chose this end point to ensure that patients had at least 1 clinic follow-up visit, because of the diversity of practice at our institution. The secondary outcomes included the time to corrected serum calcium normalization; the rates of relapse of hypercalcemia, which were defined as the corrected serum calcium returning to levels of >11 mg/dL during the 8-week follow-up period; and the adverse events after the administration of denosumab.
The adverse events were evaluated based on Common Terminology Criteria for Adverse Events version 5,21 and included a grade ≥2 increase in serum creatinine (ie, >1.5-fold increase from baseline level), hypocalcemia of any grade (ie, serum calcium <8.5 mg/dL), hypophosphatemia of any grade (serum phosphorous less than lower limit of normal), and electronic health record documentation of osteonecrosis of the jaw.
All the adverse events, with the exception of osteonecrosis of the jaw, were followed for 8 weeks after the administration of denosumab therapy. The patients’ charts were reviewed for any occurrence of osteonecrosis of the jaw, and if found, were reviewed to determine whether the incidence was related to the administration of denosumab.
Statistical Analysis
We used descriptive statistics, including percentages, medians, and interquartile ranges. Fisher’s exact and Wilcoxon rank-sum tests were used to compare the rates of normalization of corrected serum calcium for categorical and continuous variables, respectively. The Kaplan–Meier method was used to calculate the time to normalization of corrected serum calcium using the time from the index denosumab treatment. All analyses were conducted using SAS statistical software version 9.4 (SAS Institute; Cary, NC).
Results
A total of 2363 patients were identified who had received denosumab in the outpatient clinics during the data collection period. The majority of the patients were receiving denosumab for the prevention of skeletal-related events and thus were excluded from our study. A total of 15 patients met our inclusion criteria and were included in this analysis. All 15 patients received denosumab 120 mg as first-line treatment for an initial episode of hypercalcemia of malignancy in the outpatient setting.
The Table summarizes the patients’ baseline characteristics. In all, 8 patients were male and 7 were female, 53% of patients were non-Hispanic white; the patients’ mean age was 59 years. The most common malignancies were breast cancer, lung cancer, and other solid tumors. The median baseline CrCl level was 67.5 mL/min.
At baseline presentation, 1 (7%) patient was classified as having severe hypercalcemia of malignancy, 8 (53%) patients had moderate hypercalcemia of malignancy, and 6 (40%) patients had mild hypercalcemia of malignancy. The most common symptoms of hypercalcemia in these patients were altered mental status, fatigue, increased serum creatinine, and weakness. One patient had a noted electrocardiogram change.
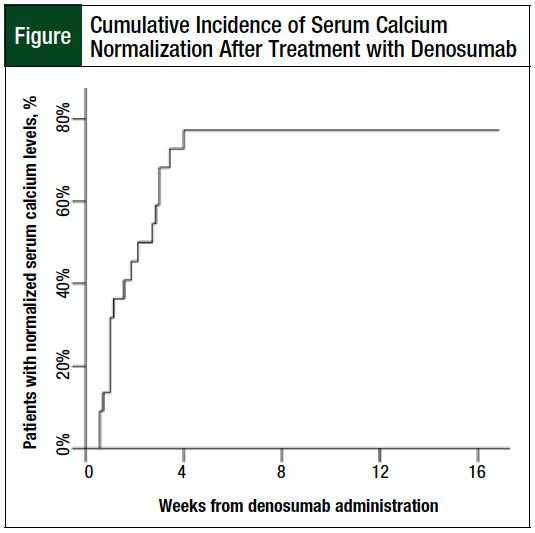
After receiving denosumab 120 mg subcutaneously, 12 (80%) patients achieved corrected serum calcium normalization within 8 weeks. The serum calcium of the patients who responded to treatment normalized within 28 days of denosumab administration (Figure).
The estimated median time to normalization of corrected serum calcium, including for the nonresponding patients, is 8 days (95% confidence interval, 3-21) with a mean corrected serum calcium level of 9.26 mg/dL on the date of resolution. Only 1 of the responding patients had a relapse within the 8-week follow-up period.
When evaluated by hypercalcemia of malignancy severity, the patient with severe hypercalcemia of malignancy, as well as 7 of the 8 (88%) patients with moderate hypercalcemia of malignancy and 4 of the 6 (67%) patients with mild hypercalcemia of malignancy, achieved a corrected serum calcium level of <10.5 mg/dL within the study period.
No patients required additional doses of denosumab treatment within the initial 28-day period after the index denosumab administration. A total of 10 (67%) patients received a single dose of denosumab, and 5 (33%) patients received an additional dose of denosumab within the 8-week follow-up period. The 5 patients who received an additional dose of denosumab continued with monthly treatments of 120 mg for the prevention of skeletal-related events rather than for the treatment of hypercalcemia associated with malignancy.
In all, 3 patients did not respond to treatment with denosumab. Overall, 2 patients had corrected serum calcium levels of 10.54 mg/dL and 10.52 mg/dL within the 8-week period of initial denosumab administration, but the serum calcium level did not normalize per our definition of serum calcium level of <10.5 mg/dL. The third nonresponding patient had a new diagnosis of multiple myeloma. Although this patient did not have a response to denosumab in the 8-week follow-up, the patient’s corrected serum calcium level decreased to 10.52 mg/dL 76 days after receiving the initial dose of denosumab. The patient required additional treatment for hypercalcemia of malignancy, using a dose of zoledronic acid, as well as cancer-directed therapy.
A total of 5 (33%) patients had grade 1 hypocalcemia, and 2 (13%) patients had grade 2 hypocalcemia. In addition, 3 patients had grade 1 hypophosphatemia within 8 weeks after receiving denosumab. There were no episodes of edema, headache, or nausea noted in any of the patients in the 8 weeks after treatment. No episodes of osteonecrosis of the jaw were identified in any patient from the time of treatment to the time of data collection, which included at least 1 year of follow-up data.
In all, 3 patients had documented increases in creatinine levels after the administration of denosumab, but these were classified as grade ≤2 and were unrelated to the denosumab treatment (2 cases were associated with volume depletion, and 1 case was associated with a relapse of hypercalcemia of malignancy that was classified as severe).
Discussion
This study was designed to evaluate the efficacy and safety of a single dose of denosumab as a potential agent for the initial treatment of hypercalcemia of malignancy. The observed normalization of corrected serum calcium rate of 80%, with a median response time of 8 days in our cohort, is comparable with historical data for first-line bisphosphonate treatment, where 88% of patients who received zoledronic acid had normalization of corrected serum calcium levels by day 10.8
Although our prespecified end point was normalization of serum calcium levels within 8 weeks, all the patients who responded to denosumab therapy achieved corrected serum calcium normalization by day 28, with a mean time of 8 days to response. Of note, none of the patients who responded to denosumab treatment required a repeated dose within the initial 8-week period because of relapse of hypercalcemia associated with malignancy, which suggests a sustained response to therapy and leads to the question of whether repeat doses on days 8 and 15 in the first 28-day period, as is recommended for refractory hypercalcemia associated with malignancy, would be needed if denosumab is used in the first-line setting.13
The safety profile of denosumab observed in our cohort was consistent with previous experience with the drug, with the most common adverse events being hypocalcemia and hypophosphatemia.10 It is important to consider that these electrolyte abnormalities could be more pronounced in patients with baseline poor renal function, although only 1 of the 7 patients in our cohort who had hypocalcemia had a CrCl level of <60 mL/min.16
Larger studies that directly compare denosumab and zoledronic acid for the prevention of skeletal-related events suggest that these drugs have a similar overall incidence of adverse effects, but that zoledronic acid is associated with an increased risk for acute-phase reactions and more severe renal toxicity. Denosumab was associated with an increased risk for hypocalcemia compared with zoledronic acid.22
It is notable that the rate of hypocalcemia reported in our study is higher than what is reported in patients who received treatment for the prevention of skeletal-related events, although we did not observe any grade 3 or 4 events that required calcium supplementation or additional treatment. One possible explanation for the higher rate of low-grade hypocalcemia is the hesitation to initiate calcium and vitamin D supplementation in patients who are receiving treatment for hypercalcemia of malignancy, which was routinely done in larger studies that evaluated the use of denosumab treatment for other indications.10,22 Given the mild severity of hypercalcemia, we believe that denosumab is a safe treatment option for hypercalcemia of malignancy, with no unique safety events observed in our small cohort.
Although prospective studies in hypercalcemia of malignancy use normalization of calcium levels at 10 days as the primary outcome, we designed our study to look at 8 weeks to ensure that a follow-up visit had occurred, although all patients did receive a repeated calcium supplementation within 28 days of the administration of denosumab.
Our study cohort was small, but the observed efficacy and considerations for adverse events and convenience suggest that denosumab may be a therapeutic option in place of a bisphosphonate for the first-line treatment of hypercalcemia of malignancy.
The use of a single dose of subcutaneous denosumab in patients with hypercalcemia of malignancy who may otherwise be managed in the outpatient setting could provide a benefit in certain patient populations, including patients who are receiving treatment with palliative intent and desire to minimize the time spent in the healthcare environment, patients who are receiving treatment at home or at a long-term care facility that lacks IV infusion capabilities, or patients who have issues with access to IV administration. In certain patients, denosumab could also provide a quality-of-life benefit, by reducing the time spent in the clinical setting.
Limitations
Our study has several limitations, including the differences in the frequencies of calcium and symptom assessments in our population.
There is also an inherent selection bias in our study, because this is a retrospective analysis and we only included 1 patient with severe hypercalcemia of malignancy.
The low number of patients identified in our cohort with severe hypercalcemia is likely a result of the supportive care needed in patients who have a more severe presentation and because outpatient management with denosumab in cases of severe hypercalcemia of malignancy may not be appropriate.
Our analysis prompts additional questions that require further study. Because we limited our study to patients who received treatment in the outpatient setting, we did not have their serum calcium levels available, as would be the case in the inpatient setting. Therefore, we were unable to determine if patients who were characterized as nonresponders had an early response that was not captured, and their hypercalcemia had already relapsed at the time of follow-up. Furthermore, there was not a standard follow-up time for these patients because of individual provider practice.
Although our analysis did not include a cost-benefit analysis, treating hypercalcemia of malignancy in a timely fashion in the clinic would eliminate the need for the coordination of bisphosphonate infusion appointments and would prevent progression to severe hypercalcemia of malignancy that requires hospitalization; consequently, timely treatment would reduce healthcare costs, resource utilization, as well as the indirect costs to patients and their families, such as time spent at infusion appointments and lost time from work.
Conclusion
Our characterization of denosumab for the treatment of hypercalcemia associated with cancer supports its use as a convenient strategy with favorable safety and efficacy profiles. Our findings suggest that single-dose denosumab is effective for the first-line treatment of hypercalcemia of malignancy when outpatient management is appropriate. We observed response rates that are comparable to standard treatment with IV bisphosphonates, with minimal clinically relevant adverse events associated with denosumab therapy. Future studies comparing the outcomes of patients receiving zoledronic acid and denosumab for hypercalcemia of malignancy, as well as the pharmacoeconomic impact of each therapy, will help determine which patient population may derive more benefit with each agent.
Author Disclosure Statement
Dr Morgan has received honoraria from MJH Lifesciences and Medscape. Dr Weiss has received research funding from AstraZeneca, Boehringer Ingelheim, G1, Immunicum, and Loxo; is Consultant to or on the Advisory Board of AstraZeneca, EMD Serono, Genentech, Inivata, G1, Jounce, AbbVie, Azitra, Eli Lilly, Blueprint, Pfizer, Saatchi, and Jazz; and owns stocks in Nektar and Vesselon. Ms Sun, Ms Deal, and Dr Cipriani have no conflicts of interest to report.
References
- Stewart AF. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373-379.
- Goldner W. Cancer-related hypercalcemia. J Oncol Pract. 2016;12:426-432.
- Malangone S, Campen CJ. Hypercalcemia of malignancy. J Adv Pract Oncol. 2015;6:586-592.
- Thomas SA, Chung SH. Management of hypercalcemia of malignancy. J Hematol Oncol Pharm. 2016;6(1):18-21.
- Ralston SH, Gallacher SJ, Patel U, et al. Cancer-associated hypercalcemia: morbidity and mortality: clinical experience in 126 treated patients. Ann Intern Med. 1990;112:499-504.
- Feldenzer KL, Sarno J. Hypercalcemia of malignancy. J Adv Pract Oncol. 2018;9:496-504.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558-567.
- Zometa (zoledronic acid) injection, for intravenous use [prescribing information]. Novartis Pharmaceuticals Corporation; December 2018.
- Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74:1385-1393.
- Xgeva (denosumab) injection, for subcutaneous use [prescribing information]. Amgen; 2020.
- Hu MI, Glezerman IG, Leboulleux S, et al. Denosumab for treatment of hypercalcemia of malignancy. J Clin Endocrinol Metab. 2014;99:3144-3152.
- Bech A, de Boer H. Denosumab for tumor-induced hypercalcemia complicated by renal failure. Ann Intern Med. 2012;156:906-907.
- Boikos SA, Hammers HJ. Denosumab for the treatment of bisphosphonate-refractory hypercalcemia. J Clin Oncol. 2012;30:e299.
- Freeman A, El-Amm J, Aragon-Ching JB. Use of denosumab for renal cell carcinoma-associated malignant hypercalcemia: a case report and review of the literature. Clin Genitourin Cancer. 2013;11:e24-e26.
- Adhikaree J, Newby Y, Sundar S. Denosumab should be the treatment of choice for bisphosphonate refractory hypercalcaemia of malignancy. BMJ Case Rep. 2014;2014:bcr2013202861. doi:10.1136/bcr-2013-202861.
- Cicci JD, Buie L, Bates J, van Deventer H. Denosumab for the management of hypercalcemia of malignancy in patients with multiple myeloma and renal dysfunction. Clin Lymphoma Myeloma Leuk. 2014;14:e207-e211.
- Vellanki P, Lange K, Elaraj D, et al. Denosumab for management of parathyroid carcinoma-mediated hypercalcemia. J Clin Endocrinol Metab. 2014;99:387-390.
- Fountas A, Andrikoula M, Giotaki Z, et al. The emerging role of denosumab in the long-term management of parathyroid carcinoma-related refractory hypercalcemia. Endocr Pract. 2015;21:468-473.
- Dietzek A, Connelly K, Cotugno M, et al. Denosumab in hypercalcemia of malignancy: a case series. J Oncol Pharm Pract. 2015;21:143-147.
- Ashihara N, Nakajima K, Nakamura Y, et al. Denosumab is effective for controlling serum calcium levels in patients with humoral hypercalcemia of malignancy syndrome: a case report on parathyroid hormone-related protein-producing cholangiocarcinoma. Intern Med. 2016;55:3453-3457.
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed May 17, 2021.
- Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082-3092.