Infusion-related reactions present a daily challenge to any provider involved in the administration of chemotherapy. These reactions range from mild to life-threatening and may significantly alter the planned course of therapy for patients with cancer. Epidemiologic studies of infusion reactions are lacking, but these are much needed to identify the risk factors and patterns of the reactions.1
In the medical literature, the terms “anaphylaxis” and “anaphylactoid reactions” are used to describe infusion-related hypersensitivity reactions.2 Anaphylaxis is a severe and immediate systemic response to an antigen-antibody reaction involving immunoglobulin (Ig) E and mast cells. Alternately, anaphylactoid reactions are cytokine-mediated.2 Despite their pathologic differences, the clinical manifestations and management of anaphylaxis and anaphylactoid reactions are similar.2
Taxanes, such as paclitaxel and docetaxel, are often associated with non–IgE-mediated reactions; these events typically occur with the first or second dose of the chemotherapy.3 Most reactions present with type 1 hypersensitivity-like symptoms, including bronchospasm, hypotension, and rash that result from smooth muscle contraction and capillary dilation.4
Reactions to taxanes occur within only a few minutes of infusion. Conversely, reactions to platinum compounds, such as carboplatin and oxaliplatin, are generally IgE-mediated, which are acquired and observed after multiple cycles of chemotherapy.4 These reactions occur at variable times with respect to the infusion start time. In particular, reactions to oxaliplatin are not easily prevented with premedications, such as antihistamines or corticosteroids.5 Rechallenging patients who have mild reactions to oxaliplatin is an option, but severe reactions require the discontinuation of oxaliplatin therapy, because of the near-inevitability of a recurring reaction.5
Reactions to monoclonal antibodies, such as rituximab, typically occur within a few minutes of the start of the infusion during the first or second dose.6 Rituximab is associated with an incidence rate of up to 77% of any-grade reaction during the first infusion.6 Reactions to rituximab are largely nonallergic in nature, and represent symptoms of cytokine release syndrome,7 with rigors as a common complication.
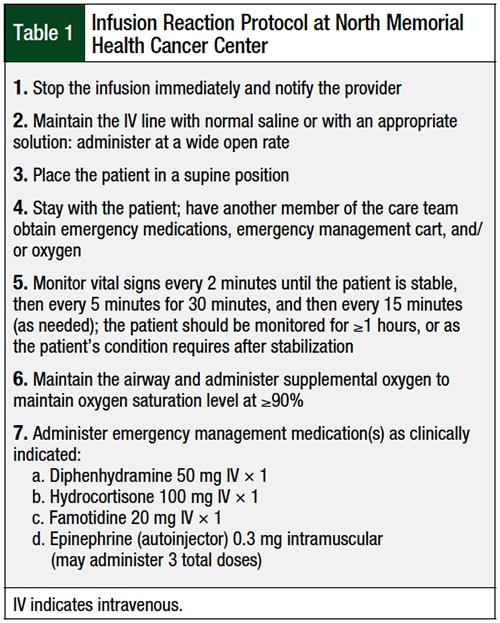
North Memorial Health Cancer Center follows a strict protocol when an infusion-related reaction occurs, and all interventions are documented. A medication kit containing hydrocortisone, diphenhydramine, famotidine, epinephrine, atropine (for the management of reactions to irinotecan), and nebulized albuterol is rushed to the patient, and a multidisciplinary team of nurses, pharmacists, advanced practice providers, and physicians promptly attend to the patient. The steps of our protocol are listed in Table 1.
The order, selection, and dosing of emergency medications is determined at the time of the infusion reaction by the physician in collaboration with the pharmacist. The decision to restart the infusion, consider a future rechallenge on a different day, adjust the infusion rate of the offending agent in the future, or include additional premedications with future chemotherapy cycles is determined on a case-by-case basis, depending on the reaction type and severity.
The objective of this study was to evaluate the incidence of infusion reactions at our cancer center and assess how we manage these reactions. Hard-copy documentation regarding the details of each infusion reaction was retained since September 2009, creating a complete record of all infusion reactions that occurred.
To our knowledge, this is the first epidemiologic report of all infusion reactions at a single cancer center over the course of a decade.
Methods
We conducted a retrospective review of every infusion reaction to intravenously administered agents in our cancer center, which is located in the upper-midwestern United States, between September 2009 and May 2018. Using our center’s electronic medical records, we recorded the premedications, drug inciting the reaction, dose, number of previous exposures for that patient to the inciting agent, number of minutes to the onset of reaction symptoms, description of the reaction per nursing dictation, outcome, reaction severity per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.3 (published on May 28, 2009), drugs and doses administered for reaction management, as well as the patient’s age, sex, diagnosis, and the number of documented allergies in the medical records.
Summary statistics were used to report the demographics and characteristics of the patients who had an infusion-related reaction. To determine the incidence of infusion reactions, we obtained the total number of dispenses of each medication annually from the center’s dispensing record.
Premedications for docetaxel, as a part of breast cancer treatment regimens, included oral dexamethasone 8 mg for 2 doses the day before chemotherapy, and oral dexamethasone 8 mg, in combination with intravenous (IV) ondansetron 12 mg, given 30 minutes before the infusion. Patients who were nonadherent to oral dexamethasone the day before receiving chemotherapy received IV dexamethasone, which was added as a premedication.
For patients with lung or prostate cancer, IV dexamethasone 12 mg was given before docetaxel. Patients receiving paclitaxel were premedicated with IV dexamethasone 20 mg, IV famotidine 20 mg, and IV diphenhydramine 25 mg to 50 mg.
Premedication with rituximab included the standard combination of oral acetaminophen 650 mg and IV diphenhydramine 50 mg (which was always given intravenously for the first exposure to rituximab). Approximately 50% of the patients also received a corticosteroid.
All patients who received platinum agents were given a corticosteroid and an antiemetic drug, and many patients who received carboplatin were also given an antihistamine during their infusion visit.
Our community cancer center actively participates in clinical trials; the data reported here include only infusions given outside of clinical trials. Because it is not a major gynecologic center, we do not maintain a standard carboplatin desensitization protocol, as is common practice at institutions that routinely manage patients with gynecologic malignancies.
Results
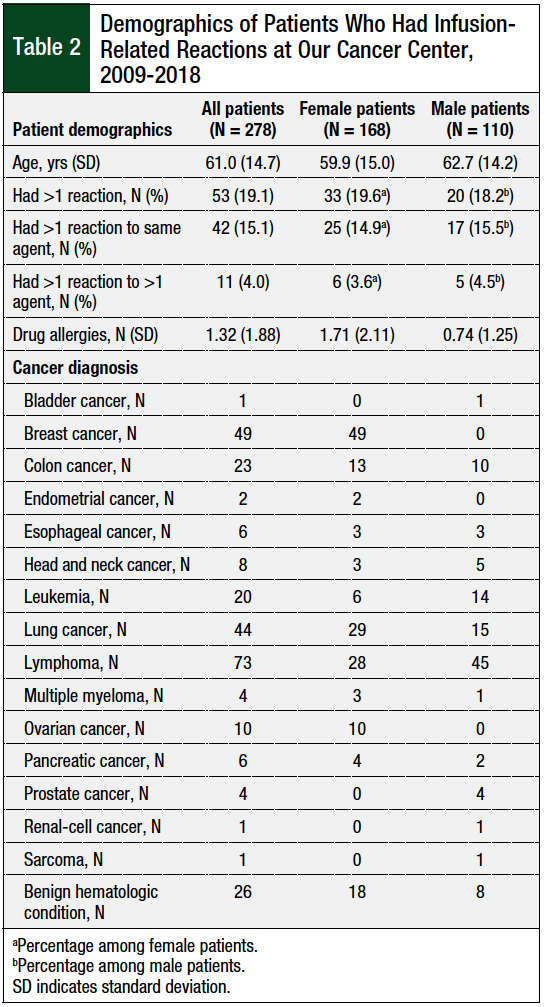
The study patients’ demographics are shown in Table 2. Nearly 50% of all patients who had an infusion-related reaction were receiving treatment for breast cancer or for lymphoma, which is reflective of our patient population, as well as the agents that most often caused reactions at our center. On average, female patients had more than twice the number of documented medication allergies in the medical record than male patients (Table 2).
Of the 278 patients in this study, 53 (19.1%) patients reacted to a medication more than once (Table 2); 42 of these patients had duplicate reactions to the same drug, and the other 11 patients reacted to 2 or more different agents at various encounters. Patients who reacted to 1 agent over the study period had notably fewer drug allergies listed in the medical record (average, 1.2 drugs) compared with patients who had multiple reactions (average, 1.8 drugs).
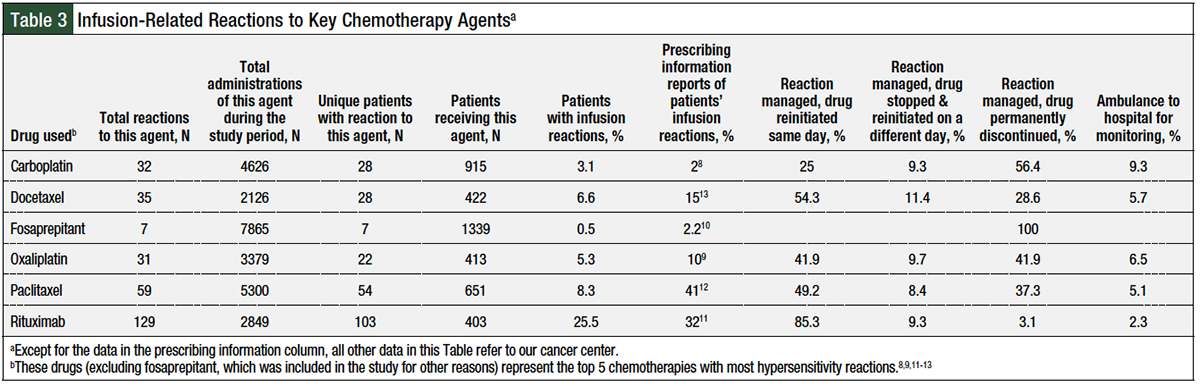
A total of 349 infusion reactions occurred at our institution between September 2009 and May 2018; Table 3 lists the data only for the 5 top chemotherapies with most frequently occurring reactions—carboplatin, docetaxel, oxaliplatin, paclitaxel, and rituximab—as well as for fosaprepitant, which was of particular interest for this study. Of the total of 349 reactions, 325 (93%) reactions were grade 1 or 2, and 24 (7%) reactions were grade 3 or 4, based on CTCAE version 4.3 criteria.
The vast majority (81%) of the reactions at our institution occurred in response to 1 of these 5 agents: rituximab, paclitaxel, docetaxel, carboplatin, or oxaliplatin. Our calculated reaction incidences based on the number of administrations and the number of unique patients who had a reaction at our center were lower than those reported in the prescribing information for all these agents, except carboplatin (Table 3).8-13
Doses of rituximab were the reference drug, which were infused intravenously and were titrated using 50 mg per hour as the starting rate, with 50 mg per hour incremental increases every 30 minutes, to a maximum infusion rate of 400 mg per hour. Of the 2849 doses of rituximab administered during the study period, 129 reactions occurred (incidence rate, 4.5%); those 2849 doses were administered to 403 unique patients, 103 of whom had some type of reaction (incidence rate, 25% per patient, all grades). The average rate of infusion for rituximab was 164 mg per hour at the onset of symptoms.
One patient with head and neck cancer reacted to paclitaxel once (grade 2, no rechallenge attempted), to carboplatin once (grade 2, no rechallenge attempted), and to docetaxel twice (grade 2, successful rechallenge). Another patient with prostate cancer reacted to docetaxel once (grade 2, unsuccessful rechallenge attempted), to doxorubicin once (grade 2), and to liposomal doxorubicin once (grade 2). Several patients reacted to irinotecan and to oxaliplatin at separate times, but none of the reactions required hospitalization for management.
On average, 2 interventional medications were administered for all infusion reactions, except for reactions to irinotecan, in which an average of 3 medications were given (likely because of the addition of atropine in 89% of patients who had a reaction to irinotecan). IV hydrocortisone was administered in 86% of patients who had infusion-related reactions, diphenhydramine in 53% of patients, and famotidine in 41% of all cases.
A pattern observed often was an attempt to abate less-severe infusion reactions with single-agent hydrocortisone; if no improvement was achieved, antihistamine agents were promptly administered. For severe reactions, multiple agents were administered upfront. Meperidine was used for the treatment of rigors in 41% of the reactions to rituximab.
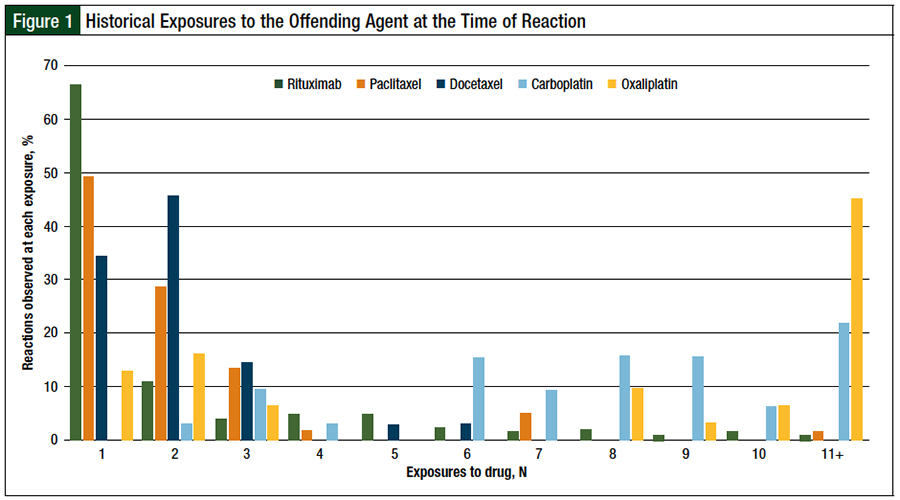
Nearly all reactions to rituximab occurred with the first (66%) or second (11%) exposure to the drug (Figure 1). Reactions to paclitaxel or docetaxel occurred at a median of 2 exposures, with nearly 80% of all reactions to taxanes occurring in cycle 1 or cycle 2. By contrast, carboplatin and oxaliplatin reactions occurred at a median of 8 exposures; 75% of reactions to platinum agents occurred in cycle 6 or later.
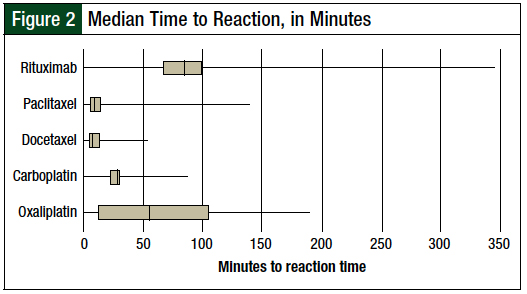
The time that lapsed between the start of the infusion and the onset of the reaction varied significantly between the different agents (Figure 2).
The main complaints during the reaction depended on the eliciting agent. Paclitaxel predominantly caused shortness of breath, whereas docetaxel caused chest tightness. Cutaneous reactions, primarily itching, were predominant with carboplatin. Flushing occurred in 65% of taxane reactions. Reactions to irinotecan presented as swelling of the lips or tongue in 63% of patients, along with burning eyes and sweating, reflecting the cholinergic pathway involved in reactions to irinotecan.
In 59% of the patients, the offending chemotherapy agent was reinitiated and the infusion was completed on the same day as the reaction. However, in 30% of all cases, the offending agent was permanently discontinued, illustrating the profound effect that hypersensitivity reactions have on the course of therapy, with patients often not receiving a potentially beneficial treatment.
Most (85%) of the rituximab infusions were completed on the same day as the reaction occurred. For reactions to platinum agents, 49% of the cases resulted in permanent discontinuation of the drug compared with 34% of patients who had a reaction to taxanes.
Furthermore, although not a chemotherapy agent, 7 reactions (all severe) to fosaprepitant occurred of the 7865 doses administered to 1339 different patients during the time of this study (incidence, 0.05%). Nearly all the reactions were in young female patients, many of whom had a documented allergy to sulfonamide antibiotics.
Among these 7 patients who had a reaction to fosaprepitant, 6 received fosaprepitant for the first time. Although the incidence of infusion reactions to fosaprepitant is greater with peripheral venous administration than with central venous administration,14 6 of these 7 patients received the drug by central venous access. Fosaprepitant rechallenge was never attempted, because these reactions were particularly severe.
Discussion
Our observed infusion reaction rates were lower than the rates in the drugs’ prescribing information,8-13 with the exception of carboplatin8 (2% vs 3.1%, respectively). Much higher reaction incidences have been reported in the literature for carboplatin (2.6%-7.9%,15 depending on the patient population) compared with 2% as reported in carboplatin’s prescribing information.8
Most infusion reactions in our center were manageable with interventional medications and did not progress to grade 3 or 4 reactions. Reactions to rituximab were common, as expected, especially with the first exposure, but they were mild and resulted in treatment discontinuation in only 5.4% of cases. Reactions to taxanes occurred shortly after the initiation of the infusion, and were mostly rechallenged successfully. The majority of reactions to platinum agents occurred after the sixth exposure, and treatment with carboplatin was permanently discontinued in nearly 70% of patients who had a reaction to the drug.
Our multidisciplinary approach to addressing these events is critical in the acute setting, as well as in the overall course of cancer treatment for the patient. When oncologists directly witness their patients’ infusion reactions, they are armed with important data and are best suited to determine whether to rechallenge the patient with the offending agent. Our providers almost always added additional premedications to future infusions to attempt to prevent recurrent reactions.
In 2012, we began infusing rapid rituximab, which is a 90-minute expedited infusion of rituximab for patients who have previously tolerated standard infusion of rituximab.16 Of 273 rapid infusions, a total of 4 reactions occurred in 2 patients. One patient reported a temporary rash during 2 separate rapid infusions, which resolved.
The second patient was short of breath with the first rapid infusion (her sixth time receiving rituximab in her lifetime), but with the second rapid infusion, rituximab was permanently discontinued after the patient became hypoxic, was short of breath, and required hospitalization. This case highlights the importance of strict patient selection criteria for rapid rituximab infusions.
The incidence rate of treatment-related reactions to monoclonal antibodies at our center was contrary to what we expected based on data from the prescribing information17-19 and reports in the literature.20,21 Despite a boxed warning in the prescribing information of panitumumab for dermatologic toxicities and a warning about severe infusion reactions that occurred in 1% of patients,17 we witnessed no reactions to panitumumab among the 304 doses that were administered during the study period.
The prescribing information for pertuzumab requires observation for 30 to 60 minutes after infusion.18 Of the hundreds of doses that we administered, we witnessed 1 reaction to pertuzumab, which occurred during the infusion, not after the infusion.
We postulated that the low frequency of reactions to cetuximab we observed may be geographically related to our upper-midwestern US location, because the reaction rates to cetuximab are highest in the southern and eastern United States.20 Reactions to cetuximab are more common in patients with head and neck cancer21; all the reactions to cetuximab in our patient population occurred in patients with head and neck cancer.
The 4 reactions to daratumumab were grade 2 and occurred with the first infusion; the infusion of daratumumab was completed in all 4 cases.
Reactions to fosaprepitant are thought to be related to the polysorbate-80 formulation content22; 1 patient in our study who had a severe reaction within minutes of receiving an infusion of fosaprepitant had a history of a severe reaction to docetaxel.
Based on our results, we proposed no changes to our cancer center’s protocol for the prevention and management of infusion-related reactions.
Although 25% of all patients who received rituximab in our study had an infusion-related reaction, 94.6% of the patients with a reaction continued to use this agent as part of their treatment regimen. Taxane reactions typically occurred in early cycles, and 66% of the patients using taxane therapy were able to continue using it.
Platinum-related reactions present a unique challenge, because they occur in later cycles, often unpredictably; infusion reactions resulted in permanent discontinuation of platinum therapy in 51% of the patients with platinum-related reactions, highlighting the profound effect of hypersensitivity reactions on the course of cancer treatment.
For all infusion reactions to fosaprepitant, docetaxel, or paclitaxel, we strongly recommend adding polysorbate-80 and the offending medication to the patient’s allergy list to avoid preventable future adverse reactions.
Limitations
This study has several limitations. First, because this study describes a decade of experience at a single community cancer center, the ability to extrapolate these data to larger academic centers, especially those that routinely use desensitization protocols to chemotherapies, is limited.
In addition, the descriptions of patients’ symptoms during the infusion reactions were recorded by nurses in a free-form note filed in the electronic medical record; therefore, some documentation may not have comprehensively included every detail.
Furthermore, the validity of the number of documented drug allergies may be limited, because we strictly counted each entry in the electronic medical record rather than interpreting the entries as side effects, intolerances, or true medication allergies.
Finally, because this study spans the years 2009 through 2018, our data reflect reactions to reference drugs, and we cannot compare the reaction incidences to available biosimilars. This should be elucidated in future studies at community cancer centers and academic centers alike.
Conclusion
Adverse reactions to chemotherapy at our cancer center showed predictable timing for rituximab, taxanes, and platinum agents. Because the rates of most adverse reactions were lower than the data reported in the drugs’ prescribing information, we proposed no immediate changes to our current premedication practices.
The results of this study suggest that routine premedication strategies as described are largely effective in infusion reaction prevention for chemotherapies often administered in the community oncology setting. Furthermore, a thorough and specific protocol to activate in the event of an infusion reaction is essential in reaction management.
Although clinical trial data have primarily supplied anticipated incidences of infusion and hypersensitivity reactions to chemotherapy agents, this epidemiologic community oncology–based study provides reassurance that routine premedication practices are largely effective in preventing infusion-related reactions to chemotherapy. Hypersensitivity reactions, particularly to platinum agents, may lead to permanent omission of the offending agent from cancer treatment plans, highlighting the importance of multidisciplinary reaction preparedness, awareness, and management.
Further studies with matched patients and multivariate regression analyses performed at other community cancer centers and larger academic centers are needed to determine which patient-, chemotherapy-, and premedication-related factors are positively associated with infusion reactions.
Author Disclosure Statement
Dr Hayes, Dr Whalen, Dr Schutz, and Dr Levine have no conflicts of interest to report.
References
- Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2005;5:309-316.
- Cmelak AJ, Lordick F, Borner M, et al. Management of infusion reactions in clinical trials and beyond: the US and EU perspectives. Oncology (Williston Park). 2009;23(suppl 1):18-25.
- Boulanger J, Boursiquot JN, Cournoyer G, et al. Management of hypersensitivity to platinum- and taxane-based chemotherapy: CEPO review and clinical recommendations. Curr Oncol. 2014;21:e630-e641.
- Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601-609.
- Siu SWK, Chan RTT, Au GKH. Hypersensitivity reactions to oxaliplatin: experience in a single institute. Ann Oncol. 2006;17:259-261.
- Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol. 2009;102:179-187; quiz 187-189, 222.
- Vogel WH. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs. 2010;14:E10-E21.
- Carboplatin injection [prescribing information]. Irvine, CA: Teva Parenteral Medicines; October 2011.
- Eloxatin (oxaliplatin) injection, for intravenous use [prescribing information]. Bridgewater, NJ: sanofi-aventis US; October 2015.
- Emend (fosaprepitant) for injection, for intravenous use [prescribing information]. Whitehouse Station, NJ: Merck & Co; April 2018.
- Rituxan (rituximab) injection, for intravenous use [prescribing information]. South San Francisco, CA: Genentech and Biogen; January 2019.
- Taxol (paclitaxel) injection [prescribing information]. Princeton, NJ: Bristol-Myers Squibb; April 2011.
- Taxotere (docetaxel) injection, for intravenous use [prescribing information]. Bridgewater, NJ: sanofi-aventis US; October 2018.
- Chau E, Lundberg J, Phillips G, et al. Updated report on incidence of infusion-site reactions associated with peripheral intravenous administration of fosaprepitant. J Oncol Pharm Pract. 2019;25:1053-1057.
- Navo M, Kunthur A, Badell ML, et al. Evaluation of the incidence of carboplatin hypersensitivity reactions in cancer patients. Gynecol Oncol. 2006;103:608-613.
- Patel J, Ho M, Ho V, et al. Rapid infusion rituximab for maintenance therapy: is it feasible? Leuk Res Treatment. 2013;2013:629283.
- Vectibix (panitumumab) injection, for intravenous use [prescribing information]. Thousand Oaks, CA: Amgen; June 2017.
- Perjeta (pertuzumab) injection, for intravenous use [prescribing information]. Thousand Oaks, CA: Amgen; June 2017.
- Erbitux (cetuximab) injection, for intravenous use [prescribing information]. Indianapolis, IN: Eli Lilly and Company; November 2020.
- O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644-3648.
- Palomar Coloma V, Bravo P, Lezghed N, et al. High incidence of cetuximab-related infusion reactions in head and neck patients. ESMO Open. 2018;3:e000346.
- Lundberg JD, Crawford BS, Phillips G, et al. Incidence of infusion-site reactions associated with peripheral intravenous administration of fosaprepitant. Support Care Cancer. 2014;22:1461-1466.