The armamentarium of agents for the treatment of hematologic malignancies has grown exponentially in recent years. These newer therapies involve the use of non–chemotherapy-based mechanisms to target specific receptors or molecules that are secreted by malignant tumors. One of the newer mechanisms is to harness the power of T-lymphocytes to attack the aberrant cells. One of the first such methods was using interleukin (IL)-2 for the management of renal-cell carcinoma, and has since developed to involve antibodies that block the action of cytotoxic T-lymphocyte antigen 4, programmed cell death 1 (PD-1), or PD ligand 1 (PD-L1) receptors,1,2 which are known as immune checkpoint inhibitors.
These immune checkpoint inhibitors work to block the inhibition of T-cells and allow them to exert their cytotoxic effects. The benefit of using these agents to combat cancer cells is their ability to bypass resistance mechanisms associated with traditional chemotherapy.2
The newest method of harnessing T-cells, which is used in the chimeric antigen receptor (CAR) T-cell therapies, involves genetically modifying the T-cells to express certain receptors that are uniquely expressed on specific tumor cells.3 The CAR T-cell therapies have been approved by the US Food and Drug Administration (FDA) in the past few years to target various types of B-cell lymphomas, acute lymphoblastic leukemia (ALL), and multiple myeloma.3
The CD19 Receptor
The CD19 receptor is a type I transmembrane protein that belongs to the immunoglobulin family.4 The CD19 receptor is expressed on B-cells to a higher extent than other common B-cell receptors, such as CD20.3 CD19 serves as a signaling receptor for B-cell activation and expansion.3,4
The mechanism by which the CD19 receptor achieves B-cell activation is multimodal, involving the recruitment of signaling proteins to bind to the B-cell membrane, as well as mediating the advancement of B-cells through their life cycle from pre–B-cells to the development of mature B-cells.4,5 The CD19 receptor is limited to expression on B-cells and is retained on B-cell malignancies; therefore, it is an ideal target for CAR T-cell therapy because of a theoretical lack of collateral damage that could result from its expression on other cells.4
B-Cell Maturation Antigen
B-cell maturation antigen (BCMA) is a cell-surface receptor that belongs to the tumor necrosis factor superfamily.6 BCMA is preferentially expressed on mature B-cells and plasma cells, with minimal expression on immature B-cells and memory B-cells, and it lacks expression on non–B-cell lines.6,7 Multiple myeloma cells express BCMA to an elevated degree, and it is believed to mediate the development of multiple myeloma and progression to plasma-cell leukemia.7 The unique expression of BCMA on plasma cells, specifically on multiple myeloma cells, makes it a very appealing target for CAR T-cell therapy, because theoretically it would have less collateral damage than even CD19 receptors.
Mechanism of Action of CAR T-Cell Therapies
The mechanism by which CAR T-cells recognize and kill cancer cells is complex and uses the collective scientific knowledge of cytotoxic T-cell mechanisms. Although 5 CAR T-cell therapies are currently available, they all follow the same basic process. The first step in the process is the collection of cells via apheresis from the patient who will ultimately receive the therapy. Next, the cells are sent to one of the FDA-approved manufacturers, where the cells are modified via various vectors (often lentivirus or gammaretrovirus) to introduce genetic material coding for the desired receptor (presently CD19 or BCMA receptors),8 which is generically designated as the single-chain variable fragment. The other necessary component of CAR T-cell therapy is the incorporation of a co-stimulatory domain.
The current CAR T-cell therapies use a second-generation co-stimulatory domain, which consist of CD3-zeta plus either the CD28 or 4-1BB domain.3,9 Second-generation co-stimulatory domains lead to a much more robust expansion of T-cells and is better able to illicit an immune response than the first-generation domains, which consisted only of CD3-zeta.9
After second-generation co-stimulatory domains showed promise, investigators researched the third-generation domains, consisting of CD3-zeta plus 2 additional co-stimulatory domains.9 Although the use of third-generation domains did increase T-cell expansion, which was only seen in vitro, and to date, no additional benefit has been seen in vivo, additional studies are warranted.9
The second-generation co-stimulatory domains have significant differences in terms of the cellular kinetics of CAR T-cells. Perhaps the most notable is the differences in CAR T-cell persistence in the blood with the CD28 and 4-1BB domains.10,11 The 4-1BB domain has a lower peak concentration of expanded T-cells than the CD28 domain; however, the 4-1BB domain also lends to a longer persistence of these T-cells in the blood. Conversely, the CD28 domain has a higher peak concentration (and achieves this peak much faster) than the 4-1BB domain; however, the persistence of CD28 T-cells in the blood diminishes much faster than T-cells with the 4-1BB domain.10,11 Currently no clinical trials are comparing the superiority of one co-stimulatory domain versus another.
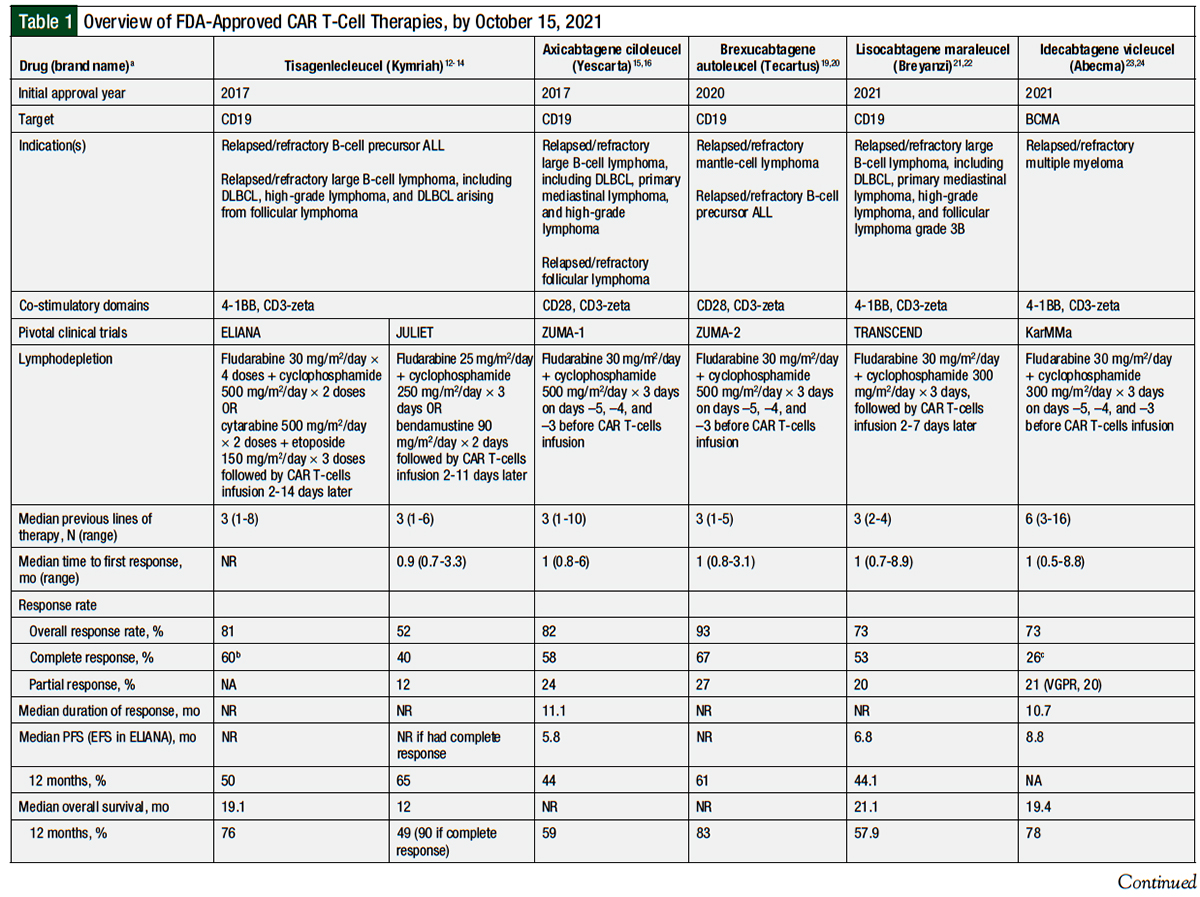
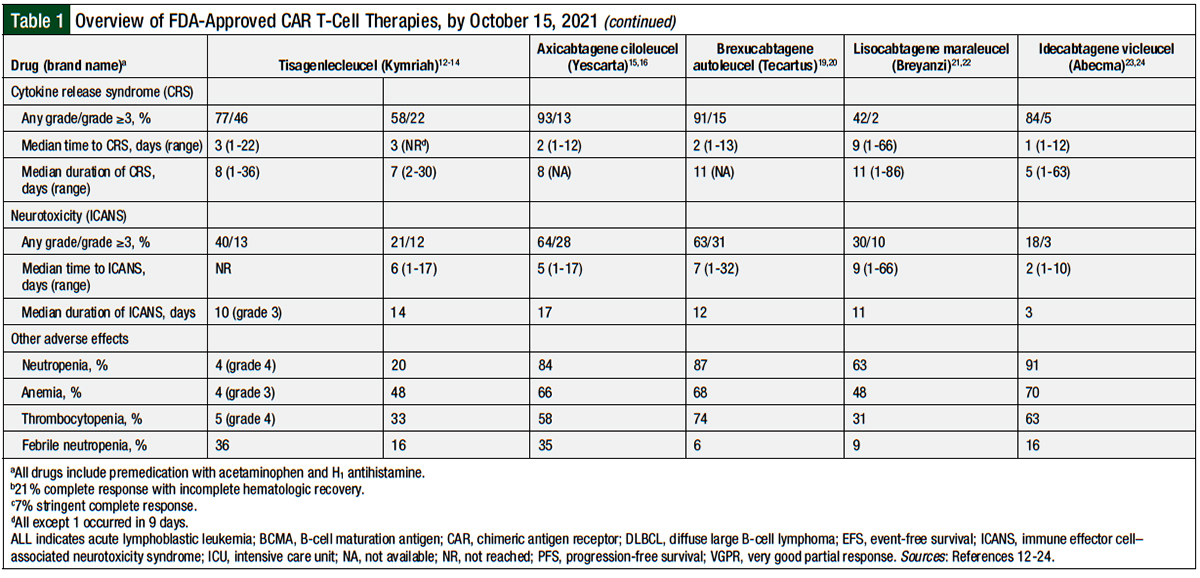
Table 1 provides an overview of the 5 currently available CAR T-cell therapies.12-24
Tisagenlecleucel
In 2017, tisagenlecleucel became the first CAR T-cell therapy to receive FDA approval. This approval was for the treatment of B-cell precursor ALL.12 This approval was based on the ELIANA study, a global, phase 1/2a, single-arm clinical trial of 75 patients.13 Patients were eligible for inclusion if they had at least 5% lymphoblasts in their bone marrow. The patients’ age had to be ≥3 years at screening and ≤21 years when diagnosed with ALL. Previous CD19-directed therapy was an exclusion criterion.12,13
The patients received lymphodepleting chemotherapy with either fludarabine 30 mg/m2 daily for 4 days and cyclophosphamide 500 mg/m2 daily for 2 days or with cytarabine 500 mg/m2 daily for 2 days, and etoposide 150 mg/m2 daily for 3 days, given 2 to 14 days before the infusion of CAR T-cells. The 2 lymphodepleting agents were initiated on the same day for each regimen. The patients were not required to have lymphodepletion if they had leukopenia. The median weight-adjusted dose of the CAR T-cells was 3.1 × 106 cells/kg, with a median total dose of 1 × 108 cells.12,13
At the preplanned interim analysis after the first 50 patients who received CAR T-cell therapy had completed 3 months of follow-up or have discontinued their study participation, the overall response rate (ORR) was 82% (95% confidence interval [CI], 69-91; P <.001).13 In the updated analysis with all 75 patients, the ORR was 81% (95% CI, 71-89). A total of 45 (60%) patients had a complete response, whereas 16 (21%) patients had a complete response with incomplete hematologic recovery.13
All patients with a complete response or a complete response with incomplete hematologic recovery were negative for minimal residual disease (MRD), with 58 (95%) patients being MRD-negative by day 28. In patients who had a response, the median duration of response was not reached, and the relapse-free survival rate was 80% at 6 months and 59% at 12 months. No patients had central nervous system (CNS) relapse during the primary follow-up. The event-free survival rate was 73% at 6 months and 50% at 12 months, and the median event-free survival not reached. The overall survival (OS) was 90% at 6 months and 76% at 12 months. The persistence of tisagenlecleucel in the blood was maintained for up to 20 months after infusion.13
A total of 58 (77%) patients had cytokine release syndrome (CRS) of any grade, including 35 (46%) patients with grade ≥3 CRS.13 The median time to CRS onset was 3 days (range, 1-22 days), with a median duration of 8 days (range, 1-36 days). A total of 35 (47%) patients were admitted to the intensive care unit (ICU) for the management of CRS, with a median length of stay of 7 days (range, 1-34 days). Overall, 19 (25%) patients received vasopressors, 33 (44%) received oxygen, 10 (13%) received mechanical ventilation, 7 (9%) had dialysis, and 28 (37%) received tocilizumab.13
Overall, 30 (40%) patients had immune effector cell-associated neurotoxicity syndrome (ICANS), including 10 (13%) patients with grade 3 ICANS; none had grade 4 ICANS. The specific ICANS symptoms were encephalopathy (11%), confusion (9%), agitation (7%), and somnolence (7%), with only 1 patient having a seizure. Most cases of ICANS occurred during CRS or shortly after CRS. For grade 3 ICANS, 50% of the cases resolved within 10 days and 75% resolved within 18 days. The management of ICANS consisted of supportive care measures.13 Other adverse events associated with tisagenlecleucel treatment are shown in Table 1.
In 2018, tisagenlecleucel received a second indication, for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after ≥2 lines of therapy. This indication includes DLBCL not otherwise specified, high-grade B-cell lymphoma (BCL2, BCL6, or MYC rearrangements; double- or triple-hit), and DLBCL transformed from follicular lymphoma.12
This second indication for tisagenlecleucel was based on the international phase 2 JULIET study of 93 patients.14 Patients were eligible for inclusion if they were aged ≥18 years, received ≥2 lines of therapy (including an anthracycline and rituximab), and were either ineligible for or had a relapse after an autologous hematopoietic-cell transplant (HCT).14
The study’s exclusion criteria included the presence of primary mediastinal DLBCL, active CNS involvement, a previous allogeneic HCT, or previous CD19-directed therapy. Lymphodepleting chemotherapy consisted of fludarabine 25 mg/m2 daily for 3 days and cyclophosphamide 250 mg/m2 daily for 3 days, or bendamustine 90 mg/m2 daily for 2 days, given 2 to 11 days before infusion. Lymphodepletion was not required for those with a white blood cell count of ≤1000 cells/mm3 within 1 week before infusion. The median dose of CAR T-cells infused was 3 × 108 cells.14
The study’s primary end point was the best ORR, which was 52% (95% CI, 41-62); this included 37 (40%) patients with a complete response and 11 (12%) with a partial response.14 The median duration of response was not reached, although the investigators estimated a 12-month relapse-free survival rate of 79% in patients with a complete response and 65% with a partial response. At 3 and 6 months, the ORR was 38% and 33%, respectively. The complete response rate at 3 and 6 months was 32% and 29%, respectively. At 12 months, the estimated progression-free survival (PFS) was 83% in patients who had a complete or partial response at 3 months, and the median PFS was not reached in those with a complete response. The estimated 12-month OS was 49% in all patients, but was 90% in those with a complete response. The median OS in all the patients was 12 months. Tisagenlecleucel persistence in the blood was observed for up to 2 years after infusion.14
Any-grade CRS occurred in 64 (58%) patients, and 24 (22%) patients had grade ≥3 CRS. The median onset of CRS was 3 days, with a median duration of 7 days (range, 2-30 days). Overall, 14% of the patients received tocilizumab, and 10% received tocilizumab plus steroids. This included 5% of patients who received 1 dose of tocilizumab and 9% who received 2 doses (no one received >2 doses of tocilizumab). In addition, 24% of the patients received oxygen, 7% were intubated, 6% received high-dose vasopressors, and 5% had dialysis. A total of 24% of the patients were admitted to the ICU.14
In the first 8 weeks of treatment, 23 (21%) patients had ICANS of any grade, and 13 (12%) patients had grade ≥3 ICANS. The median time to ICANS onset was 6 days (range, 1-17 days), and the median duration of ICANS was 14 days. The most common neurologic symptoms in the study were confusion (9%), encephalopathy (6%), and tremor (5%). No fatal cerebral edema was reported. The ICANS was managed via supportive care measures and corticosteroids. Other adverse events reported in this study are shown in Table 1.14
Axicabtagene Ciloleucel
Axicabtagene ciloleucel was the second CAR T-cell therapy approved by the FDA in 2017. It was initially approved for the treatment of patients with relapsed or refractory large B-cell lymphoma, including DLBCL, primary mediastinal B-cell lymphoma, or DLBCL arising from follicular lymphoma, who had received ≥2 lines of systemic therapy.15 This approval was based on the results of the phase 2 ZUMA-1 clinical trial of 101 patients who received this CAR T-cell therapy infusion.15,16 Patients were eligible for the study if they had DLBCL, primary mediastinal B-cell lymphoma, or transformed follicular lymphoma. All the patients had refractory disease, which was defined as progressive or stable disease as the best response to the most recent systemic treatment or as disease progression or relapse in the first 12 months after undergoing autologous HCT. Patients received lymphodepletion with fludarabine 30 mg/m2 daily and cyclophosphamide 500 mg/m2 daily, each for 3 days, followed by 2 days off, then the infusion of axicabtagene ciloleucel. The target dose of the CAR T-cells was 2 × 106 cells/kg.15,16
The primary end point of ORR was 82%, including 58% complete responses and 24% partial responses. The median duration of response was 11.1 months (95% CI, 3.9-not estimable). The 6-, 12-, and 15-month PFS rate was 49%, 44%, and 41%, respectively, with a median PFS of 5.8 months (95% CI, 3.3-not estimable). The 6-, 12-, and 15-month OS rate was 78%, 59%, and 52%, respectively, with a median OS not reached. The CAR T-cell levels peaked within 14 days, and most patients had detectable levels of CAR T-cells 180 days after infusion. The investigators noted that receiving tocilizumab or corticosteroids did not affect the efficacy of axicabtagene ciloleucel.16
Any-grade CRS occurred in 94 (93%) patients; only 13 (13%) patients had grade ≥3 CRS.16 The median time to CRS onset was 2 days (range, 1-12 days), and the median time to CRS resolution was 8 days. For the treatment of CRS, ICANS, or both, 43 (43%) patients received tocilizumab, and 27 (27%) patients received corticosteroids.16
ICANS occurred in 65 (64%) patients, including 28 (28%) patients with grade ≥3 events.16 The median time to ICANS onset was 5 days (range, 1-17 days), with a median resolution of neurologic events on day 17 after infusion. The most common grade ≥3 symptoms reported were encephalopathy (21%), confusion (9%), and somnolence (7%). The management of ICANS was similar to other studies. Other adverse events in the ZUMA-1 study are listed in Table 1.16
In addition to the ZUMA-1 study, Nastoupil and colleagues recently published the results of a retrospective review of patients who received axicabtagene ciloleucel at 17 US institutions.17 Of 275 patients who received infusion, the ORR was 82%, including 64% complete responses, which is similar to the results of the ZUMA-1 study. The median PFS was 8.3 months, and the median OS was not reached. Independent markers for decreased PFS and OS were Eastern Cooperative Oncology Group performance status of 2 to 4, and increased lactate dehydrogenase. Any-grade CRS occurred in 91% of the patients, including 7% grade ≥3. Neurotoxicity of any grade occurred in 69% of the patients, including 31% grade ≥3. A total of 62% of the patients received tocilizumab (median dose, 1). A total of 55% of the patients received corticosteroids for CRS, ICANS, or both.17 Similar to the efficacy results, these real-world data validated the results of the ZUMA-1 trial.16,17
In April 2021, axicabtagene ciloleucel received accelerated approval for the treatment of relapsed or refractory follicular lymphoma after ≥2 lines of systemic therapy.15 This approval was based on the results of the ZUMA-5 trial.18 A total of 104 patients were evaluated for efficacy, including 84 patients with follicular lymphoma. The patients had lymphodepletion and were given CAR T-cell therapy doses similar to the doses in the ZUMA-1 trial. The ORR was 92% (94% in patients with follicular lymphoma), including 76% complete responses (80% in patients with follicular lymphoma). The median duration of response, PFS, and OS were not reached. The 12-month PFS and OS rates were 73.7% and 92.9%, respectively. Grade ≥3 CRS and ICANS occurred in 7% and 19% of the patients (6% and 15%, respectively, of patients with follicular lymphoma).18 The full results of the ZUMA-5 study were not available at the time of this writing.
Brexucabtagene Autoleucel
Brexucabtagene autoleucel was approved in 2020 for the treatment of patients with relapsed or refractory mantle-cell lymphoma (MCL).19 This approval was based on the results of the phase 2 ZUMA-2 clinical trial of 74 patients who received an infusion of brexucabtagene autoleucel.20 Patients were eligible to participate if they were aged ≥18 years and had MCL with translocation t(11;14) or cyclin D1 overexpression, as well as if they had relapsed or refractory MCL after receiving up to 5 previous therapies. The previous therapies must have included an anti-CD20 monoclonal antibody, an anthracycline or bendamustine, and Bruton tyrosine kinase inhibition with ibrutinib or acalabrutinib. All patients received lymphodepletion with fludarabine 30 mg/m2 daily and cyclophosphamide 500 mg/m2 daily, each for 3 days, followed by 2 days off, then a single infusion of brexucabtagene autoleucel.19,20
The primary end point of ORR in the first 60 patients, with a minimum follow-up of 6 months, was 93% (95% CI, 84-98), including 67% complete responses and 27% partial responses.20 The median duration of response was not reached (95% CI, 9.2 months-not estimable). Of the 29 (39%) patients who were evaluated for MRD, 24 (83%) were MRD-negative (<1 in 100,000 cells) at week 4. Of the 24 MRD-negative patients, 19 had a complete response and 5 had a partial response. The PFS and OS rates at 12 months were 61% and 83%, respectively. The PFS was consistent even in the patients with TP53 mutation or with a Ki-67 proliferation index of ≥50%.20
A total of 62 (91%) patients had any-grade CRS, of which 52 (76%) had grade 1 or 2 CRS.20 The median time to onset of any-grade CRS was 2 days (range, 1-13 days), and the median time to resolution was 11 days. Of the patients with CRS, 59% received tocilizumab, 22% received corticosteroids, and 16% received vasopressors.20
Of the 43 (63%) patients who had ICANS, 22 (32%) patients had grade 1 or 2 and 21 (31%) had grade ≥3 ICANS.20 The median time to ICANS onset was 7 days (range, 1-32 days), with a median duration of 12 days. Overall, 26% of patients received tocilizumab, and 38% received corticosteroids. The most common ICANS symptoms were tremor (35%), encephalopathy (31%), and confusion (21%). One patient had grade 4 cerebral edema, which was resolved with aggressive supportive care, including a ventriculostomy. Other adverse events with brexucabtagene autoleucel are listed in Table 1.20
After the completion of this article, on October 1, 2021, the FDA approved a second indication for brexucabtagene autoleucel, for the treatment of patients with relapsed or refractory B-cell precursor ALL.19
Lisocabtagene Maraleucel
Lisocabtagene maraleucel was approved in 2021 for the treatment of adults with relapsed or refractory B-cell lymphoma after at least 2 lines of systemic therapy, including DLBCL not otherwise specified, primary mediastinal B-cell lymphoma, DLBCL arising from indolent lymphomas, high-grade B-cell lymphomas (double- or triple-hit), and follicular lymphoma grade 3B.21 This approval was based on the TRANSCEND clinical trial of 269 patients (256 in the primary analysis).21,22
Patients were eligible to participate if they had positron emission tomography–positive relapsed or refractory DLBCL after receiving at least 2 systemic therapies (including an anthracycline and anti-CD20 agents).22 Having had an autologous or an allogeneic HCT was not an exclusion criterion. Lymphodepletion with fludarabine 30 mg/m2 daily and cyclophosphamide 300 mg/m2 daily were each given once daily for 3 days, and the infusion of the CAR T-cell therapy given 2 to 7 days after lymphodepletion (the median time to infusion was 3 days after lymphodepletion). Of the 294 patients who received lisocabtagene maraleucel, 25 received a drug that did not meet the viability criteria but was deemed safe to infuse. Of the remaining 269 patients, 45 received dose level 1 (50 × 106 cells), 6 received dose level 1D (50 × 106 cells in 2 doses), 177 received dose level 2 (100 × 106 cells), and 41 received dose level 3 (150 × 106 cells). The median dose was 91 × 106 cells.21,22
The ORR was 73% (95% CI, 66.8-78), including 136 (53%) patients with a complete response and 50 (20%) patients with a partial response.22 The estimated duration of response at 1 year was 55% in the overall population and 65% in those with a complete response; the median duration of response was not reached. The 1-year PFS and OS rates were 44% and 58%, respectively, with a median of 6.8 months and 21.1 months, respectively.22
A total of 113 (42%) patients had CRS, and only 6 (2%) had grade ≥3 CRS.22 The median CRS onset was 5 days (range, 1-14 days), and the median time to resolution was 5 days (range, 1-17 days). In all, 27 (10%) patients received tocilizumab, 5 (2%) patients received corticosteroids, and 21 (8%) received both medications. A total of 7 (3%) patients received vasopressors, 1 received anakinra, and 1 received siltuximab.22
Overall, 80 (30%) patients had ICANS, of whom 27 (10%) had grade ≥3 ICANS.22 The median onset of ICANS was 9 days (range, 1-66 days), and the time to resolution was 11 days (range, 1-86 days). The most common ICANS symptoms were encephalopathy (21%), confusion (11%), and aphasia (10%). The management of ICANS was not reported, but no patients died from ICANS.22
Idecabtagene Vicleucel
Idecabtagene vicleucel is the most recent CAR T-cell therapy to gain FDA approval. It was approved in March 2021 for the treatment of relapsed or refractory multiple myeloma after at least 4 lines of therapy, including a proteasome inhibitor, an immunomodulator, and an anti-CD38 monoclonal antibody.23,24
This drug is the first BCMA-directed treatment ever approved by the FDA. The approval of idecabtagene vicleucel was based on the phase 2 KarMMa clinical trial of 128 patients with relapsed or refractory multiple myeloma. Patients were eligible to participate if they received at least 3 different treatment regimens, had disease progression within 60 days of their last dose of the last regimen, had measurable disease, and had adequate organ function. Lymphodepletion with fludarabine 30 mg/m2 daily and cyclophosphamide 300 mg/m2 daily was given daily for 3 days, followed by 2 days off before the idecabtagene vicleucel infusion.23,24
The ORR was 73% (95% CI, 66-81), including 42 (33%) complete responses or stringent complete responses. A total of 67 (52%) patients had a very good partial response or better. Of the 42 patients with a complete response or better, 33 (79%; 26% of the overall population) became MRD-negative. The median duration of response was 10.7 months (95% CI, 9-11.3). The median PFS was 8.8 months in the overall population and 20.2 months in those with a complete response or better. The median OS was 19.4 months in the overall population, and the 12-month OS was 78%.24
Any-grade CRS occurred in 107 (84%) patients, and only 7 (5%) patients had grade ≥3 CRS. The median onset of CRS was 1 day (range, 1-12 days), and the median duration of CRS was 5 days (range, 1-63 days). Tocilizumab was administered to 67 (52%) patients and corticosteroids to 19 (15%) patients. One patient in the study died from CRS-related complications.24 A total of 23 (18%) patients had any-grade ICANS, of whom 4 (3%) had grade ≥3 ICANS. The median time to ICANS was 2 days (range, 1-10 days), with a median duration of 3 days (range, 1-26 days). The frequency of corticosteroids or tocilizumab use was not specified.24
Serious Side Effects of CAR T-Cell Therapies
Cytokine Release Syndrome
CRS is triggered by the activation of the CAR T-cells, which leads to the release of inflammatory cytokines, such as IL-6 and interferon gamma.3,25 CRS often presents in the first week after the infusion of CAR T-cells, but it can also occur later.24 The common symptoms of CRS are fever, rigors, hypoxia, and hypotension.26
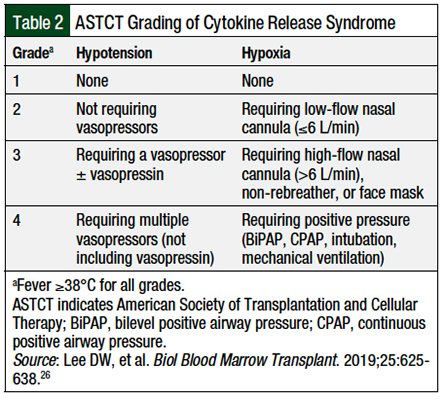
Several grading systems are available for the severity and management of CRS, including the grading systems or guidelines developed by the American Society of Transplantation and Cellular Therapy (ASTCT), the CAR T-Cell Therapy–Associated Toxicity (CARTOX), the Common Terminology Criteria for Adverse Events (CTCAE), and the grading system by Lee and colleagues, among others.27-30 Although the exact criteria differ between each grading system, they have many commonalities, such as the presence of fever, hypoxia, or hypotension.27-30 It is important to remember, however, that because of the differences in the CRS grading definitions, there may be some differences in the use of tocilizumab and corticosteroids, depending on the scale used.27 For example, the CARTOX guidelines have a slightly lower rate of capture of ICANS than the CTCAE guidelines.29,30
In light of these differences, the ASTCT recommendations were developed to standardize the definitions of CRS and ICANS.26 Different institutions may adopt their own grading scale or a preexisting grading scale. It is important for pharmacists to understand which CRS grading scale their institution uses, because this can help them better manage patients who have CRS.
The differences in grading scales were present in the various clinical trials.14,16,22 For example, the JULIET study for tisagenlecleucel in DLBCL used the University of Pennsylvania grading scale, which tends to increase the severity of grades 1 or 2 CRS to grades 2 or 3 CRS.14,29 Conversely, the ZUMA-1 and TRANSCEND studies used the CRS grading system by Lee and colleagues, which, like the CARTOX criteria, specifically address end-organ damage (ie, transaminitis).16,22,28 Although there is high concordance among the grading systems overall, it is still imperative to understand which specific grading scale is being used in clinical trials to understand the possible differences between the various therapies.29
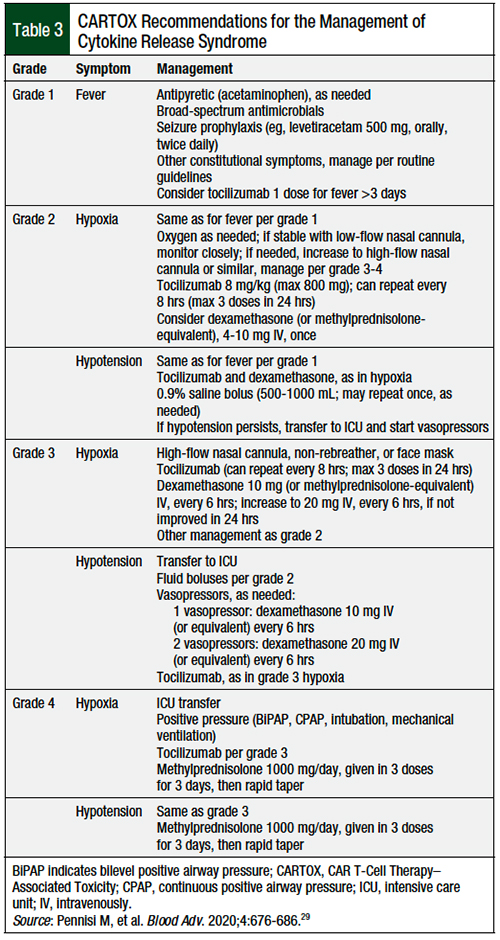
The management of CRS consists of supportive care, which involves normal saline boluses (500-1000 mL) for hypotension, acetaminophen or ibuprofen, and broad-spectrum antibiotics for fever.30 An antiepileptic drug, such as levetiracetam 500 mg twice daily, for seizure prophylaxis is recommended if the patient has fevers, because CRS can precipitate ICANS (including seizures). Supplemental oxygen should be used for patients with an oxygen saturation of <92% on room air.30
Tocilizumab, an anti–IL-6 receptor antibody, is recommended by the various guidelines for the management of CRS.29,30 The dosing is 8 mg/kg per dose (maximum 800 mg per dose) given no more than every 8 hours (maximum 3 doses daily, up to 4 doses total). Although the various grading criteria offer treatment recommendations, the prescribing information for each of the CAR T-cell therapies also offers treatment recommendations for the various grades of CRS. These recommendations follow the same general recommendations as the grading criteria, but they are not as explicit regarding specific definitions for symptoms such as hypotension.12,15,19,21,23,29,30
Pharmacists should be aware of their institution-specific definitions for the management of patients with CRS, and should refer to the specific prescribing information for each drug12,15,19,21,23 or the particular grading criteria for clarification.26,28-30 The ASTCT grading system is shown in Table 2.26 The CARTOX recommendations for the management of CRS are shown in Table 3.29
Immune Effector Cell and Neurotoxicity Syndrome
The pathophysiology of ICANS is less well-understood than that of CRS, but ICANS is also thought to result from an influx of inflammatory cytokines.25 ICANS often presents several days later than CRS, and CRS itself is a risk factor for ICANS.27 The symptoms of ICANS are varied and can include tremors, lethargy, headache, encephalopathy, seizures, and delirium, among others.26 The immune effector cell-associated encephalopathy (ICE) score is a validated scaling system used to assess a patient’s encephalopathy and is listed in the Appendix Table 1 (available at the top of the page).26
As with CRS, there are some differences in the various grading systems for ICANS, which may play a role in the determination of the severity of ICANS and its management.29 For example, the CARTOX scale may upgrade the severity of seizures, because all convulsive seizures are considered grade 4 in this scale unlike in other scales mentioned earlier.28-30 In addition, CARTOX, as well as the ASTCT scale, may underreport mild symptoms of ICANS (eg, headache or encephalopathy), but the patient would still score a 10 (ie, a perfect score) on the ICE scale.26,29
The management of lower-grade ICANS involves supportive care, such as aspiration precautions, including the avoidance of any oral intake and avoiding CNS depressants when possible.30 If seizures are absent, the CARTOX Working Group recommends continuing prophylactic antiepileptic drugs; however, if seizures manifest, then it is recommended to start patients with treatment such as lorazepam, treatment-dose levetiracetam, or phenobarbital.30
Because tocilizumab is not able to cross the blood–brain barrier, it is only recommended for the treatment of ICANS when patients also have concomitant CRS. Dexamethasone, however, is recommended for all grades of ICANS, with the dose ranging from 10 mg intravenously (IV) once for grade 1 ICANS to 20 mg IV every 6 hours for grade 3 ICANS. For grade 4 ICANS, it is recommended to start patients with methylprednisolone 1000 mg daily for several days, followed by a rapid taper if the patient improves.30
As for CRS, the prescribing information for each drug also offers treatment recommendations for each grade of ICANS.12,15,19,21,23,30 The ASTCT grading system of ICANS is outlined in the Appendix Table 226 (available at the top of the page). The CARTOX recommendations for the management of ICANS are shown in the Appendix Table 329 (available at the top of the page).
Place in Therapy
In current practice, all available CAR T-cell therapies are indicated for the treatment of relapsed or refractory hematologic malignancies, typically after at least 2 lines of therapy.
Ongoing studies of CAR T-cell therapies are underway for additional indications, such as for the treatment of adults with ALL, T-cell malignancies, or acute myeloid leukemia. In addition to these novel indications, research is also being done to introduce CAR T-cell therapy earlier in the course of treatment of various hematologic malignancies.31-35 The ZUMA-7 and ZUMA-12 clinical trials are currently exploring the use of axicabtagene ciloleucel in the second-line and first-line settings for patients with large B-cell lymphomas.31,32
In addition, the use of other medications to augment the CAR T-cell therapies is underway, such as in the ZUMA-6 trial, which is attempting to incorporate the PD-L1 inhibitor atezolizumab into the treatment plan after infusion of CAR T-cell therapy, to increase the levels and persistence of CAR T-cells.33
Finally, the use of allogeneic CAR T-cells are being harnessed in the experimental setting, in the hope of providing a benefit for the management of lymphomas and leukemias, using cells gathered from healthy donors that are infused into the patient. The current clinical trials of allogeneic CAR T-cell therapies include the CALM study in adults with ALL and the PALL study in pediatric patients with ALL.34,35
Conclusions
CAR T-cell therapy is a novel treatment approach for relapsed or refractory hematologic diseases. In the past 5 years, 5 CAR T-cell therapies have become available in the marketplace for a variety of indications. The high response rate with these novel drugs makes CAR T-cell therapy an attractive option for patients who often have chemorefractory disease. The efficacy of CAR T-cell therapy, however, is not without adverse events, notably CRS and ICANS.
With differences in the incidence and severity of each adverse event, it is paramount that oncology pharmacists become knowledegable about the management of these adverse reactions and the differences between the various drugs themselves. Pharmacists should also be aware of the specific grading criteria and treatment algorithms for CRS and ICANS.
Considering the additional ongoing studies and an expected expansion of the current indications for CAR T-cell therapies, this promising treatment strategy is likely to increase in use in the future. Pharmacists who are knowledegable in the management of patients who receive CAR T-cell therapy will be an important component in helping to serve the cancer care team and the patient.
Author Disclosure Statement
Dr Marjoncu has no conflicts of interest to report.
References
- Esfahani K, Roudaia L, Buhlaiga N, et al. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol. 2020;27(suppl 2):S87-S97.
- Lee L, Gupta M, Sahasranaman S. Immune checkpoint inhibitors: an introduction to the next-generation cancer immunotherapy. J Clin Pharmacol. 2016;56:157-169.
- June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64-73.
- Wang K, Wei G, Liu D. CD19: a biomarker for B-cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1:36.
- Carter RH, Wang Y, Brooks S. Role of CD19 signal transduction in B cell biology. Immunol Res. 2002;26:45-54.
- Shah N, Chari A, Scott E, et al. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34:985-1005.
- Tai YT, Anderson KC. Targeting B-cell maturation antigen in multiple myeloma. Immunotherapy. 2015;7:1187-1199.
- Smith AJ, Oertle J, Warren D, Prato D. Chimeric antigen receptor (CAR) T cell therapy for malignant cancers: summary and perspective. J Cell Immunother. 2016;2:59-68.
- Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388-398.
- Ying Z, He T, Wang X, et al. Parallel comparison of 4-1BB or CD28 co-stimulated CD19-targeted CAR-T cells for B cell non-Hodgkin’s lymphoma. Mol Ther Oncolytics. 2019;15:60-68.
- van der Stegen SJC, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14:499-509.
- Kymriah (tisagenlecleucel) suspension for intravenous infusion [prescribing information]. Novartis Pharmaceuticals Corporation; August 2021. www.novartis.us/sites/www.novartis.us/files/kymriah.pdf. Accessed October 12, 2021.
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439-448.
- Schuster SJ, Bishop MR, Tam CS, et al; for the JULIET investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45-56.
- Yescarta (axicabtagene ciloleucel) suspension for intravenous infusion [prescribing information]. Kite Pharma; April 2021. www.gilead.com/-/media/files/pdfs/medicines/oncology/yescarta/yescarta-pi.pdf. Accessed October 12, 2021.
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531-2544.
- Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38:3119-3128.
- Jacobson C, Chavez JC, Sehgal AR, et al. Primary analysis of Zuma-5: a phase 2 study of axicabtagene ciloleucel (axi-cel) in patients with relapsed/refractory (R/R) indolent non-Hodgkin lymphoma (iNHL). Blood. 2020;136(suppl 1):40-41.
- Tecartus (brexucabtagene autoleucel) suspension for intravenous infusion [prescribing information]. Kite Pharma; October 2021. www.gilead.com/-/media/files/pdfs/medicines/oncology/tecartus/tecartus-pi.pdf. Accessed October 21, 2021.
- Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331-1342.
- Breyanzi (lisocabtagene maraleucel) suspension for intravenous infusion [prescribing information]. Juno Therapeutics, a Bristol-Myers Squibb Company; February 2021. https://packageinserts.bms.com/pi/pi_breyanzi.pdf. Accessed October 12, 2021.
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839-852.
- Abecma (idecabtagene vicleucel), suspension for intravenous infusion [prescribing information]. Celgene Corporation, a Bristol-Myers Squibb Company; March 2021. https://packageinserts.bms.com/pi/pi_abecma.pdf. Accessed October 12, 2021.
- Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705-716.
- Santomasso B, Bachier C, Westin J, et al. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. 2019;39:433-444.
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625-638.
- Gust J, Ponce R, Liles WC, et al. Cytokines in CAR T cell–associated neurotoxicity. Front Immunol. 2020;11:577027. doi: 10.3389/fimmu.2020.577027.
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188-195. Errata in: Blood. 2015;126:1048; Blood. 2016;128:1533.
- Pennisi M, Jain T, Santomasso BD, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020;4:676-686.
- Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47-62.
- ClinicalTrials.gov. Efficacy of axicabtagene ciloleucel compared to standard of care therapy in subjects with relapsed/refractory diffuse large B cell lymphoma (ZUMA-7). Identifier: NCT03391466. https://clinicaltrials.gov/ct2/show/NCT03391466. Accessed October 12, 2021.
- ClinicalTrials.gov. Efficacy and safety of axicabtagene ciloleucel as first-line therapy in participants with high-risk large B-cell lymphoma (ZUMA-12). Identifier: NCT03761056. https://clinicaltrials.gov/ct2/show/NCT03761056. Accessed October 12, 2021.
- ClinicalTrials.gov. Safety and efficacy of KTE-C19 in combination with atezolizumab in adults with refractory diffuse large B-cell lymphoma (DLBCL) (ZUMA-6). Identifier: NCT02926833. https://clinicaltrials.gov/ct2/show/NCT02926833. Accessed October 12, 2021.
- ClinicalTrials.gov. Efficacy and safety of two treatment algorithms in adults with moderate to severe Crohn’s disease (CALM). Identifier: NCT01235689. https://clinicaltrials.gov/ct2/show/NCT01235689. Accessed October 12, 2021.
- ClinicalTrials.gov. Study of UCART19 in pediatric patients with relapsed/refractory B acute lymphoblastic leukemia (PALL). Identifier: NCT02808442. https://clinicaltrials.gov/ct2/show/NCT02808442. Accessed October 12, 2021.