Symptom Overview
Heparin-induced thrombocytopenia (HIT) is an immune-mediated thrombocytopenia and prothrombotic disorder that occurs in a minority of patients exposed to unfractionated or low-molecular-weight heparin (LMWH).1 HIT usually happens 5 to 10 days after the administration of heparin; rarely, it may occur acutely in patients who have received heparin within the past 3 months.1
The pathogenesis of HIT involves immune complex formation with heparin and platelet factor 4 (PF4). The heparin-PF4 complex crosslinks with circulating platelets secondary to immunoglobulin (Ig) G antibodies, leading to platelet activation, destruction, and overconsumption. This results in thrombocytopenia (50% reduction from baseline) and a propensity for venous and arterial thrombosis. HIT with thrombosis, which can be arterial in nature (ie, stroke, myocardial infarction, mesenteric ischemia) or venous (ie, deep-venous thrombosis or pulmonary embolus), causes the most morbidity and is the most common complication in patients with HIT.1-3
The diagnosis of HIT is established by clinical evaluation and is confirmed with antibody immunoassays.1 A clinical diagnosis with the 4Ts scoring system used for the diagnosis of HIT (score ≥4 indicates a very likely diagnosis) usually precedes the laboratory evaluation. The 4Ts scoring system evaluates the 4 components of the disease, including the magnitude of platelet fall, onset of platelet fall, thrombosis status, and the likelihood of other causes for thrombocytopenia. A 4Ts score of ≥4 is highly predictive of HIT and warrants treatment intervention, while awaiting laboratory confirmation.1
Epidemiology and Prognosis
Among the general population of people who have received heparin therapy, the incidence of HIT or HIT with thrombosis has been estimated as approximately 0.5%.2,3 In acutely ill hospitalized patients, the incidence of HIT increases up to 18%.4 Once a patient has HIT, the risk for in-hospital thrombosis is estimated to be approximately 30%.4
The risk for thrombosis increases by 5% daily if HIT is left untreated. HIT or HIT with thrombosis is also associated with increased rates of in-hospital mortality (10%) and in-hospital limb amputation (0.8%).4
Besides heparin or LMWH exposure, other risk factors for HIT include age, sex, surgery (particularly cardiac surgery), hemodialysis, and heparin type, dose, and duration.4 Additional information about risk factors for HIT is described in Table 1.1,5
Anticoagulant Drugs Management
The management of acute HIT or HIT with thrombosis involves the complete cessation of heparin or LMWH administration, including tube or catheter flushes and hemodialysis devices that use heparin.6,7 Parenteral anticoagulation with a nonheparin anticoagulant (ie, argatroban, bivalirudin, fondaparinux) is traditionally initiated for the treatment or prevention of thrombosis.6,7
Although nonheparin anticoagulants have been the standard of care for the management of acute HIT, challenges from several clinical studies are complicating the use of these anticoagulants in acutely ill patients. For example, bivalirudin and argatroban require monitoring every 2 to 4 hours with activated partial thromboplastin time (PTT), until the goal of activated PTT of 1.5 to 3 times the normal limit is maintained, which can be challenging in certain patient populations, such as those with liver disease or those with prolonged baseline coagulation profiles.1,6,7
Although some centers have adopted plasma-diluted thrombin time for direct thrombin inhibitor monitoring, this assay is not widely available, and its use in clinical practice is unclear.8,9 Furthermore, HIT or HIT with thrombosis may be associated with increased activated PTT. These factors often make monitoring and dose adjustment of parenteral nonheparin anticoagulation challenging in patients with acute HIT, which may result in subtherapeutic or supratherapeutic dosing.8,9
Vitamin K antagonists, although appealing as an alternative anticoagulant, are contraindicated during the early phases of HIT or HIT with thrombosis, because of the increased risks for thrombosis and skin necrosis if given in the acute setting.7,10 Warfarin can be initiated once the platelet count is normalized (to 150,000 platelets/mL) with parenteral anticoagulants.7 Warfarin may be continued for 3 months in patients with HIT with thrombosis and for 6 weeks in patients who do not have thrombosis.7
In the absence of contraindications, warfarin therapy requires parenteral anticoagulation overlap, frequent prothrombin time monitoring (goal international normalized ratio of 2.0-3.0), and the management of many drug–drug and drug–herb or diet interactions.6 For these reasons, long-term anticoagulation with warfarin would be cumbersome for most patients.
Parenteral nonheparin anticoagulation has been the mainstay treatment for acute HIT or HIT with thrombosis, with vitamin K antagonists reserved for the remainder of therapy; however, emerging data suggest that direct oral anticoagulants may be a safe, effective, and attractive alternative.11,12 Compared with warfarin, direct oral anticoagulants have fewer dietary restrictions, a lower number of drug–drug interactions, and do not require specific monitoring; thus, direct oral anticoagulants are considered a more convenient treatment option than warfarin.12-14 Rivaroxaban and apixaban can be safely administered for systemic anticoagulation in patients with venous thromboembolism (VTE), without the need for parenteral anticoagulation.14,15
Finally, several in vitro studies with apixaban, rivaroxaban, and dabigatran demonstrated a lack of cross-reactivity with HIT antibodies, which illustrates that direct oral anticoagulants do not exacerbate HIT.11,12 With these forethoughts in mind, the following sections outline the emerging clinical data indicating that direct oral anticoagulants may be safe and effective in patients with HIT or HIT with thrombosis.
The data from randomized studies about the use of direct oral anticoagulants for HIT or HIT with thrombosis treatment are limited. Several case reports and case series have addressed the use of direct oral anticoagulants for HIT or HIT with thrombosis treatment in various settings, leading to the more recent publication of systematic reviews on this topic.6,16
A 2018 systematic review evaluated 56 patients with acute HIT or HIT with thrombosis from different case reports, 2 retrospective case series, and 1 prospective trial.6 All patients had a 4Ts score of ≥4. Parenteral anticoagulation was pursued before the initiation of direct oral anticoagulants in 42 (75%) patients. The duration of follow-up for direct oral anticoagulants was approximately 4587 patient-days in 54 of the 56 patients. In terms of efficacy, 2 (4%) patients had recurrent thrombosis. The platelet counts normalized in 55 (98%) patients who received direct oral anticoagulants. Among the direct oral anticoagulants, rivaroxaban was the most frequently used (54%). In the context of actual thrombosis, direct oral anticoagulants were dosed as they are indicated for VTE treatment (15 mg twice daily for 21 days, followed by 20 mg once daily for rivaroxaban).6
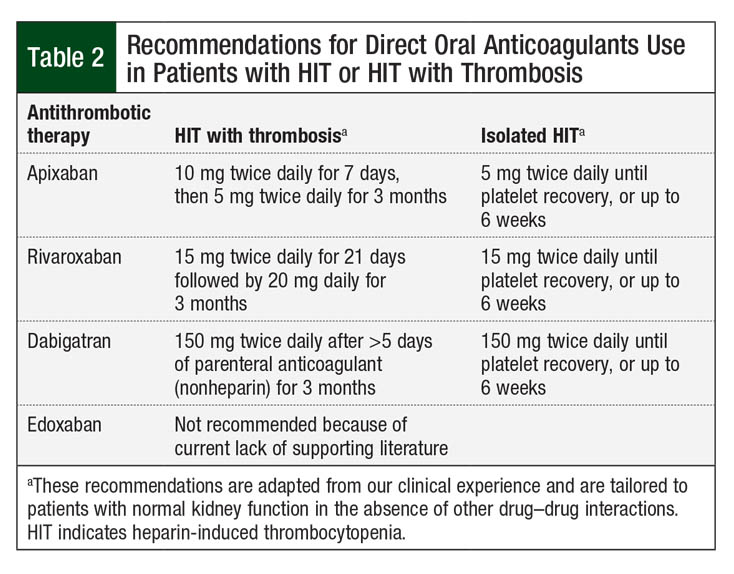
For the treatment of isolated HIT without thrombosis, less intensive oral regimens (apixaban 5 mg twice daily, dabigatran 150 mg twice daily) are proposed until platelets recover.7 The rationale for direct oral anticoagulant dosage recommendations is anecdotal and has not been evaluated in randomized controlled trials. Our proposed regimens for direct oral anticoagulants for the treatment of acute HIT or HIT with thrombosis are shown in Table 2.
A retrospective single-arm cohort of 12 patients with HIT or HIT with thrombosis (40% had HIT with thrombosis) evaluated direct oral anticoagulant therapy at various stages of HIT.17 This study included patients with a 4Ts score of ≥4 and a positive IgG-specific anti–PF4-heparin complex. Patients started treatment with apixaban (5-10 mg orally twice daily) or with rivaroxaban (15 mg orally twice daily).17
Parenteral anticoagulation preceded direct oral anticoagulant therapy in 58% of the study patients. The median time for parenteral argatroban exposure before direct oral anticoagulant initiation was approximately 206 hours. Of the patients who received apixaban or rivaroxaban, none had recurrent thrombosis or life-threatening bleeding during hospitalization.17 Despite the small sample size of the study, this retrospective review adds to the growing body of evidence supporting the use of direct oral anticoagulants for patients with HIT or HIT with thrombosis.
Similar to these findings, a recent report outlined a single center’s experience with the use of direct oral anticoagulants as an alternative therapy for HIT or HIT with thrombosis, either alone or after parenteral anticoagulation.18 A total of 16 patients with laboratory-confirmed HIT (positive IgG-specific enzyme immunoassay) and a 4Ts score of ≥4 received treatment with rivaroxaban (10-15 mg twice daily for 21 days followed by 20 mg once daily). Overall, 6 (37.5%) patients had HIT with thrombosis at the time of diagnosis. The study concluded that direct oral anticoagulants can be safe and effective for the treatment of HIT or HIT with thrombosis during the acute phase.18 The decision to start treatment with direct oral anticoagulants initially or after parenteral anticoagulation should be evaluated on a case-by-case basis.
Conclusion
The current evidence regarding the use of direct oral anticoagulants for HIT or HIT with thrombosis is limited and is relying on low-quality retrospective trials (mostly single-arm case reports or case series) with modest sample sizes (ie, <100 patients).
Despite the low quality of the evidence, direct oral anticoagulants offer similar effectiveness and safety to parenteral anticoagulants for the treatment of HIT or HIT with thrombosis. Whether direct oral anticoagulants (ie, rivaroxaban or apixaban) can be initiated without parenteral anticoagulation is unknown and requires further study to validate their use in this setting.
Direct oral anticoagulants were started without parenteral anticoagulation in approximately 50% of the patients across all the studies included in this review. These agents were dosed as indicated for VTE treatment during the acute phase of HIT with thrombosis. Less intensive dosing regimens were suggested for isolated HIT without thrombosis.
In the absence of more robust evidence, direct oral anticoagulants can be used for the treatment and prevention of HIT or HIT with thrombosis as an alternative to warfarin for long-term anticoagulation. Direct oral anticoagulants should be used with caution in the acute setting in patients who are newly diagnosed with HIT or HIT with thrombosis.
References
- Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373:252-261.
- Onwuemene O, Arepally GM. Heparin-induced thrombocytopenia: research and clinical updates. Hematology Am Soc Hematol Educ Program. 2016;2016:262-268.
- Arepally GM. Heparin-induced thrombocytopenia. Blood. 2017;129:2864-2872.
- Dhakal B, Kreuziger LB, Rein L, et al. Disease burden, complication rates, and health-care costs of heparin-induced thrombocytopenia in the USA: a population-based study. Lancet Haematol. 2018;5:e220-e231.
- Obeng EA, Harney KM, Moniz T, et al. Pediatric heparin-induced thrombocytopenia: prevalence, thrombotic risk, and application of the 4Ts scoring system. J Pediatr. 2015;166:144-150.e1.
- Tran PN, Tran MH. Emerging role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia. Clin Appl Thromb Hemost. 2018;24:201-209.
- Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392.
- Wanat MA, Hart SR, Putney D, et al. Alternative monitoring of argatroban using plasma-diluted thrombin time. Ann Pharmacother. 2013;47:e18.
- Love JE, Ferrell C, Chandler WL. Monitoring direct thrombin inhibitors with a plasma diluted thrombin time. Thromb Haemost. 2007;98:234-242.
- Srinivasan AF, Rice L, Bartholomew JR, et al. Warfarin-induced skin necrosis and venous limb gangrene in the setting of heparin-induced thrombocytopenia. Arch Intern Med. 2004;164:66-70.
- Walenga JM, Prechel M, Hoppensteadt D, et al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost. 2013;19:482-487.
- Krauel K, Hackbarth C, Fürll B, Greinacher A. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119:1248-1255.
- Pradaxa (dabigatran etexilate mesylate) capsules, for oral use [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; November 2019.
- Eliquis (apixaban) tablets, for oral use [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; November 2019.
- Xarelto (rivaroxaban) tablets, for oral use [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals; March 2020.
- Shatzel JJ, Crapster-Pregont M, Deloughery TG. Non-vitamin K antagonist oral anticoagulants for heparin-induced thrombocytopenia. A systematic review of 54 reported cases. Thromb Haemost. 2016;116:397-400.
- Davis KA, Davis DO. Direct acting oral anticoagulants for the treatment of suspected heparin-induced thrombocytopenia. Eur J Haematol. 2017;99:332-335.
- Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113.