Symptom Overview
Patients undergoing hematopoietic stem-cell transplantation (HSCT) often receive highly emetogenic, multiday conditioning chemotherapy. Many patients experience nausea and vomiting, which may negatively affect quality of life, despite receiving triple therapy with dexamethasone, a neurokinin-1 (NK-1) receptor antagonist, and serotonin receptor antagonists (5-HT3).1 The data are limited supporting the use of a definitive antiemetic prophylaxis regimen in patients receiving high-dose conditioning chemotherapy for HSCT, which is broadly considered highly emetogenic.
As recommended by multiple standard-dose chemotherapy guidelines, olanzapine may be added to triple therapy for chemotherapy-induced nausea and vomiting (CINV) prophylaxis with highly emetogenic chemotherapy regimens. However, little guidance exists as to the optimal strategy for incorporating olanzapine-based regimens into HSCT.2,3
This overview highlights available data and considerations on the use of olanzapine in patients undergoing HSCT who are receiving highly emetogenic chemotherapy.
Etiology
The conditioning chemotherapy used for patients undergoing HSCT is often highly emetogenic and is therefore associated with dose-limiting nausea and vomiting. For patients undergoing HSCT, the presence of CINV from previous chemotherapy, a history of poorly controlled CINV, the use of alkylating agents, dimethyl sulfoxide cryopreservation of peripheral blood stem cells, total-body irradiation, the intensity of chemotherapy regimen (ie, myeloablative vs reduced intensity), and multiday conditioning therapy preceding the transplant may further increase the risk for CINV.4
For these reasons, elucidating the best antiemetic strategies to use with HSCT conditioning is of critical importance.
Treament Options
Olanzapine antagonizes the dopamine D2 receptor and is approved by the US Food and Drug Administration as an antipsychotic agent.5 This drug may be used off-label for the treatment of delayed or refractory nausea and vomiting in patients receiving highly emetogenic chemotherapy. Olanzapine modulates various pathways involved in nausea and vomiting, including dopaminergic, serotonergic, histaminergic, muscarinic, and adrenergic receptors, and has shown benefit in the prophylactic, refractory, and rescue settings with a variety of chemotherapy regimens.5-7
Two meta-analyses identified a statistically higher proportion of patients achieving a complete response (ie, no emesis or use of as-needed antiemetic medications) with the addition of olanzapine to triple therapy compared with triple therapy alone.8,9 The paucity of data about HSCT may be caused by several factors, including the relative rarity of HSCT and the complexity and variability of HSCT conditioning regimens among institutions.
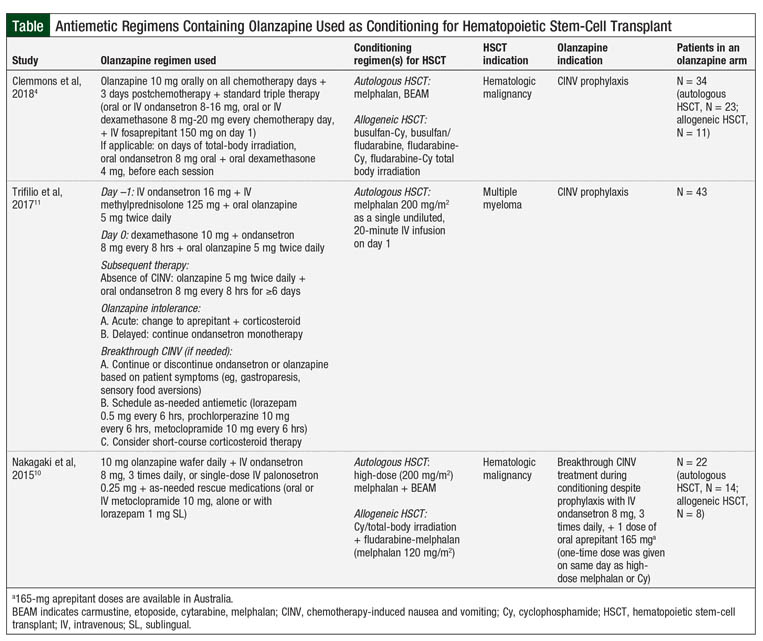
The Table provides the results of 3 studies of antiemetic regimens with olanzapine for HSCT conditioning.4,10,11
A randomized clinical trial assessed the benefit of olanzapine in autologous and allogeneic HSCT–associated CINV, showing that olanzapine in combination with ondansetron is more effective in treating breakthrough CINV than intravenous (IV) ondansetron 32 mg daily over 24 hours or 1 dose of IV palonosetron 0.25 mg.10 The primary end point was the composite outcome of no emesis, no use of rescue antiemetics, and reduced nausea severity by ≥50% compared with nausea severity at the time of randomization. At 24 and 48 hours, olanzapine was more effective than ondansetron (P = .01 and P = .0002, respectively) and palonosetron at 48 hours (P = .005) at achieving the primary end point (Table).10
Subsequently, Trifilio and colleagues retrospectively compared olanzapine versus aprepitant and fosaprepitant for CINV in patients with multiple myeloma who are receiving high-dose melphalan conditioning for autologous HSCT (Table).11 The results of this study showed that patients receiving oral olanzapine 5 mg twice daily for 5 days, starting on the day before the transplant, had significantly less acute nausea (P <.0001 vs olanzapine; P <.0318 vs fosaprepitant) or delayed nausea (P <.0004 vs olanzapine; P <.1519 vs fosaprepitant). Furthermore, patients receiving olanzapine required less rescue medication for acute-onset and delayed-onset CINV (P <.0643 and P <.0024, respectively).11 This study offers insight into the use of olanzapine as an NK-1 antagonist–sparing strategy for CINV; however, it is limited in identifying the potential benefit of quadruple prophylaxis regimens.
To date, the most robust data regarding CINV prophylaxis available are from the study by Clemmons and colleagues.4 This prospective, randomized clinical trial evaluated the use of olanzapine for CINV prophylaxis in patients receiving conditioning therapy for HSCT. The researchers compared the effectiveness of olanzapine added to the 3-drug regimen of fosaprepitant, ondansetron, and dexamethasone (FOND) plus olanzapine (FOND-O) 10 mg once daily on all chemotherapy days, and 3 days after chemotherapy versus triple therapy with the FOND regimen.4
In the FOND-O study, the researchers found a benefit in complete response, defined as no emesis and minimal nausea (<25 mm on a 100-mm visual analog scale) in patients receiving the FOND-O regimen in the overall and delayed phases (P = .003 and P = .001, respectively), although no difference was seen in the acute phase.4 Among secondary outcomes, significantly more patients receiving the FOND-O regimen achieved minimal nausea in the overall (P = .001) and delayed (P = .0002) phases, as well as in having fewer overall mean emesis counts (P = .05) compared with those receiving the FOND regimen. Although a benefit was seen in the overall population, the subgroup analyses showed no difference between triple and quadruple therapy in patients undergoing allogeneic HSCT (Table).4
Although olanzapine may have a benefit in preventing CINV, some concerns exist about the associated adverse effects, notably QT prolongation and somnolence.2,5,12-14 Metabolic abnormalities have been reported with olanzapine but these are generally not considered relevant when used for CINV in the acute setting.13 Studies evaluating olanzapine for the prevention of CINV in patients undergoing HSCT showed few to no patients who required early discontinuation of therapy as a result of adverse effects.4,11
Clinical Practice Recommendations
The addition of olanzapine to triple therapy with an NK-1 receptor antagonist, a 5-HT3 antagonist, and dexamethasone should be considered for adults who are receiving highly emetogenic conditioning therapy for HSCT, especially those undergoing an autologous transplant. Healthcare providers should take into consideration patient-specific factors and comorbidities when determining if the patient is a candidate for olanzapine therapy.
As suggested by the FOND-O study, the recommended olanzapine dose is 10 mg orally once daily with chemotherapy, continued for an additional 3 days after completion of chemotherapy, and it may be modified on a patient-specific basis.4
Given olanzapine’s long half-life of 21 to 54 hours, once-daily dosing may be appropriate,13 and may provide improved adherence, especially in the outpatient HSCT setting. A lower dose of 5 mg may be considered in elderly patients or in patients who experience excessive sedation with the 10-mg dose. In addition, the dose may be further reduced to 2.5 mg in those whose sedation is excessive with the 5-mg dose.2 Furthermore, lower doses should be considered in patients who are debilitated or are pharmacodynamically sensitive, are predisposed to hypotensive reactions, or have the potential for slower drug metabolism.13
Patients should be monitored for adverse effects, including sedation and QT interval prolongation, although major adverse effects have not been seen to date. Sedation, however, may be beneficial in alleviating nausea and vomiting,14 and administering doses each night at bedtime may prevent daytime sedation.
QT interval is of concern, because of the risk for life-threatening arrhythmias, and should be monitored at baseline and periodically through HSCT. Providers should be cautious when using concomitant drug therapies that may further increase the risk for overlapping adverse events. Finally, it is important to provide patient counseling regarding the use of an antipsychotic agent as an antiemetic to alleviate possible patient concerns.
Conclusion
Despite the use of triple therapy with dexamethasone, an NK-1 receptor antagonist, and a 5-HT3 for CINV prophylaxis, patients receiving highly emetogenic chemotherapy for HSCT often have persistent nausea and vomiting. Olanzapine added to the triple- therapy regimen may be efficacious in preventing CINV in this specific patient population. Currently, limited data exist, and current guidelines do not discuss the benefit of olanzapine in this specific setting.
Published data are largely specific to the autologous HSCT setting, and the only prospective study conducted did not show significant benefit in allogeneic HSCT. Therefore, further prospective studies, as well as real-world retrospective analyses and multi-institutional evaluations, are needed to provide insight into the optimal use of olanzapine for CINV prophylaxis in patients undergoing HSCT, and should be encouraged to establish standard practices.
Author Disclosure Statement
Dr Denson, Dr Wiggins, Dr Martin, and Dr Arnall have no conflicts of interest to report.
References
- Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines. NCCN Guidelines–Antiemesis. Version 1.2020. February 19, 2020. www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed September 17, 2019.
- Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:3240-3261.
- Clemmons AB, Orr J, Andrick B, et al. Randomized, placebo-controlled, phase III trial of fosaprepitant, ondansetron, dexamethasone (FOND) versus FOND plus olanzapine (FOND-O) for the prevention of chemotherapy-induced nausea and vomiting in patients with hematologic malignancies receiving highly emetogenic chemotherapy and hematopoietic cell transplantation regimens: the FOND-O Trial. Biol Blood Marrow Transplant. 2018;24:2065-2071.
- Tan L, Liu J, Liu X, et al. Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res. 2009;28:131.
- Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134-142.
- Chiu L, Chow R, Popovic M, et al. Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer. 2016;24:2381-2392.
- Yang T, Liu Q, Lu M, et al. Efficacy of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting: a meta-analysis. Br J Clin Pharmacol. 2017;83:1369-1379.
- Yoodee J, Permsuwan U, Nimworapan M. Efficacy and safety of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;112:113-125.
- Nakagaki M, Barras M, Curley C, et al. A randomized trial of olanzapine and palonosetron versus infused ondansetron for the treatment of chemotherapy induced nausea and vomiting in patients undergoing hematopoietic stem cell transplantation. Support Care Cancer. 2017;25:607-613.
- Trifilio S, Welles C, Seeger K, et al. Olanzapine reduces chemotherapy-induced nausea and vomiting compared with aprepitant in myeloma patients receiving high-dose melphalan before stem cell transplantation: a retrospective study. Clin Lymphoma Myeloma Leuk. 2017;17:584-589.
- Petersen AB, Andersen SE, Christensen M, Larsen HL. Adverse effects associated with high-dose olanzapine therapy in patients admitted to inpatient psychiatric care. Clin Toxicol (Phila). 2014;52:39-43.
- Zyprexa (olanzapine) tablet for oral use [prescribing information]. Indianapolis, IN: Lilly USA; 2019.
- Tendas A, Marchesi F, Annibali O, et al; for the Rome Transplant Network. Chemotherapy-induced nausea and vomiting prophylaxis in high-dose melphalan and autologous stem cell transplantation [Letter to the editor]. Clin Lymphoma Myeloma Leuk. 2017;18:161-162.