Many approaches for improving outcomes in patients with acute myeloid leukemia (AML) have been investigated over the years. AML is molecularly complex, and its multiple genetic aberrations are critical pathophysiologic and prognostic features that can influence therapy. Mutations in the FMS-like tyrosine kinase 3 (FLT3) gene, identified in approximately 30% of adults with AML, likely confer the largest single-gene impact on overall survival; the FLT3 internal tandem duplication (ITD) mutation is an independent predictor of a high relapse rate and poor overall survival.1,2
The FLT3 receptor has been explored as a therapeutic target for years. Midostaurin, the first therapy approved by the US Food and Drug Administration that targets mutations in the FLT3 gene, is an orally administered multikinase inhibitor of FLT3, CD117/KIT, platelet-derived growth factor receptor, vascular endothelial growth factor receptor 2, Gardner-Rasheed feline sarcoma viral oncogene, proto-oncogene tyrosine-protein kinase, spleen-associated tyrosine kinase (SYK), and protein kinase C.3-6 The recent publication by Stone and colleagues shows that midostaurin added to chemotherapy and as a single-agent maintenance therapy significantly prolonged overall survival and event-free survival in adults with FLT3 mutation–positive AML, which may represent a major turning point in how we treat AML in adults.5
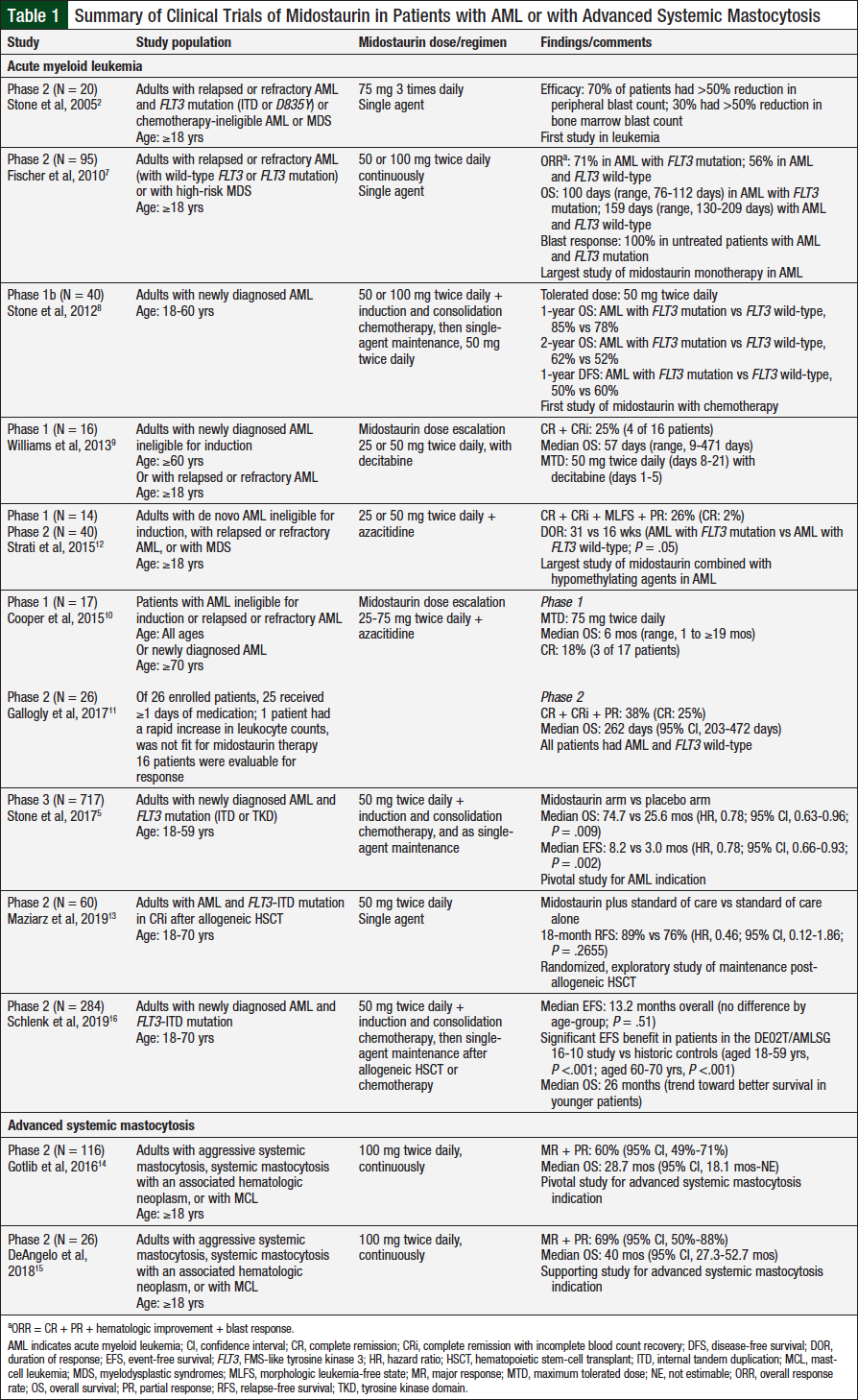
Midostaurin was initially evaluated in the relapsed or refractory setting, with a focus on elderly patients with AML or with myelodysplastic syndromes (MDS). Later, midostaurin was added to standard induction regimens for AML and is currently being evaluated in the post–allogeneic hematopoietic stem-cell transplant (HSCT) setting, in combination with a variety of drugs for the treatment of AML in adults and for pediatric patients with leukemia, as well as for the treatment of advanced systemic mastocytosis (Table 1).2,5,7-16
This article reviews the pharmacology of midostaurin, summarizes the clinically relevant safety and efficacy data, and highlights the salient points to providing appropriate care for patients.
Pharmacology and Pharmacokinetics
FLT3 mutation is the most common mutation in adults with AML and confers a poor prognosis.5,17 The most common FLT3 mutation is ITD, which occurs in approximately 25% of cases.17 FLT3-ITD mutations inhibit the ability of the FLT3 receptor to downregulate, creating a constitutively active receptor.18 Approximately 7% of patients with AML have activating mutations within the tyrosine kinase domain, with mutations at aspartate 835 (D835 or “activating loop” mutations) being the most common.19,20 The tyrosine kinase SYK is a regulator of FLT3 that plays an integral role in the pathogenesis of FLT3 mutation–positive leukemia and in resistance to anti-FLT3 therapy.21
The dual inhibition of FLT3 and SYK, among other targets, is an important differentiating feature of midostaurin compared with other FLT3 inhibitors, such as quizartinib.21 FLT3 mutations have been associated with decreased cellular uptake of cytarabine, the backbone of many AML induction regimens, through the decreased production of equilibrative nucleoside transporter 1.22 Midostaurin has been studied in dosages ranging from 25 mg once daily to 75 mg 3 times daily in patients with hematologic malignancies (Table 1).
Midostaurin is absorbed within 1 to 3 hours.3 If taken with food, the exposure to midostaurin increases; however, peak serum concentration is inversely affected by food intake.3 Midostaurin has a large volume of distribution because it is highly (99.8%) bound to plasma alpha-1-acid glycoprotein.3 With continuous dosing, midostaurin reaches a maximum concentration after approximately 7 days, and the concentration then decreases with the increased presence of metabolic enzymes.3
The primary metabolism of midostaurin is via cytochrome P450 3A4 (CYP3A4), resulting in the active metabolites CGP52421 and CGP62221.3 CGP62221 is highly active against FLT3, retaining equal activity to that of midostaurin, whereas CGP52421 is 2 to 4 times less active than midostaurin or CGP62221.3 The half-life of elimination for midostaurin is 19 hours and for CGP62221 is 32 hours, with no long-term accumulation, whereas CGP52421 has a half-life of 482 hours because of its increased affinity for binding to plasma.3 Midostaurin and its metabolites are 95% excreted in feces, with the remaining 5% excreted in urine.3 The metabolites of midostaurin constitute 91% of the eliminated compounds.3
Clinical Trials in AML
Single-Agent Midostaurin
The first study of midostaurin in leukemia was a phase 2 clinical trial in patients with relapsed or refractory AML and FLT3 mutation (ITD or D835Y) or patients with advanced MDS who were ineligible for induction chemotherapy.2 Among 20 patients who received midostaurin 75 mg 3 times daily for at least 2 weeks, 14 achieved a more than 50% reduction in peripheral blasts (7 had a 2-log reduction [99%] that lasted at least 4 weeks) and 6 achieved a more than 50% reduction in bone marrow blasts (3 had fewer than 5% blasts).2
The pivotal phase 2b clinical trial of midostaurin included 95 patients with FLT3 mutation and patients with FLT3 wild-type.7 A 50% or more reduction in blasts was achieved in 71% of patients with the FLT3 mutation, 49% of whom reached a 2-log (99%) reduction. All patients who did not previously receive treatment had a blast response compared with 69% of those who had received previous therapy. These responses were more impressive than those achieved by patients with FLT3 wild-type, of whom 42% achieved a blast response. No apparent differences were observed between the groups regarding adverse events.7
Midostaurin plus Hypomethylating Agents
A phase 1 clinical trial evaluated decitabine in combination with a dose escalation of midostaurin.9 Among 16 patients, 14 had the FLT3 wild-type mutation and 57% achieved stable disease during the study. Overall, 36% of the evaluable patients achieved complete remission (CR) or CR with incomplete blood count recovery (CRi), with a median duration of response of 107 days (range, 28-331 days). The midostaurin 50-mg twice-daily cohorts had dose-limiting toxicities, including 2 patients who died as a result of pulmonary toxicity. The maximum tolerated dose of midostaurin was 50 mg twice daily given on days 8 to 21 plus decitabine (20 mg/m2) given on days 1 to 5 of each 28-day cycle.9
A phase 1 dose-escalation study evaluated the combination of azacitidine and escalating doses of midostaurin.10 None of the 17 patients enrolled had an FLT3 mutation; 3 patients achieved CR, and 2 had hematologic improvement.10 The most frequent nonhematologic adverse events were febrile neutropenia, pneumonia, hepatotoxicity, and fever.10 The maximum tolerated dose was midostaurin 75 mg twice daily (days 8-21) in combination with azacitidine (75 mg/m2 intravenously, days 1-7) in each 28-day cycle.10
A phase 2 extension study enrolled 26 patients, none of whom had FLT3-ITD mutations.11 Among 16 patients who were evaluable for response, 4 achieved CR, 1 achieved CRi, and 1 achieved a partial response. The most common treatment-emergent adverse events were nausea, diarrhea, constipation, and fatigue. The most frequent cause for treatment discontinuation was infection, and 3 patients died during the study as a result of infection.11
A larger, phase 1/2 clinical trial evaluated the combination of azacitidine and midostaurin.12 Midostaurin was administered on days 8 to 21 of the first cycle and continuously in subsequent cycles in combination with azacitidine (days 1-7). Among 54 enrolled patients, 74% had FLT3 mutations; 14 patients and 40 patients were included in the phase 1 and 2 portions of the study, respectively. The overall response rate (ORR) was 26%, with 7 patients achieving either CR or CRi. The most common any-grade nonhematologic adverse events were infections and nausea or vomiting. Midostaurin 50 mg twice daily was demonstrated to be safe in combination with azacitidine.12
Midostaurin plus Standard Chemotherapy
Stone and colleagues conducted a phase 1b clinical trial of midostaurin added to induction therapy with daunorubicin and cytarabine and consolidation therapy with high-dose cytarabine; patients could continue single-agent midostaurin as maintenance therapy.8 The original midostaurin dosage was 100 mg twice daily on days 1 to 28 or on days 8 to 28. The schedule was amended to limit midostaurin treatment to 50 mg twice daily for 14 days in each 28-day cycle (concomitantly, days 1-7 and 15-21; sequentially, days 8-21) because of toxicity concerns. Of 40 patients who received the 50-mg dose, 74% of patients with FLT3 wild-type and 92% with FLT3 mutations achieved CR. Kaplan-Meier overall survival probabilities at 2 years were similar between the patients with FLT3 wild-type and those with FLT3 mutations (0.52; 95% confidence interval [CI], 0.33-0.71; and 0.62; 95% CI, 0.35-0.88, respectively). Three patients received >2 cycles of midostaurin 50 mg twice daily as maintenance therapy, and all patients remained in CR at the end of the study follow-up. The most common nonhematologic adverse events were gastrointestinal (GI)-related. Because of increased rates of grade 3 or 4 GI adverse events and discontinuations with the 100-mg dose,8 midostaurin 50 mg twice daily given sequentially with induction and consolidation chemotherapies was chosen for evaluation in the pivotal phase 3 clinical trial.5
The phase 3 RATIFY clinical trial demonstrated the superiority of midostaurin over placebo in patients with AML when combined with standard daunorubicin and cytarabine induction therapy and high-dose cytarabine consolidation therapy followed by single-agent midostaurin maintenance therapy in patients who remained in remission after consolidation.5 This double-blind randomized study included 717 adults with untreated AML and FLT3 mutation; patients were randomized in a 1:1 ratio to midostaurin or to placebo. The overall survival was significantly longer in patients receiving midostaurin than in patients receiving placebo, with a median overall survival of 74.7 months versus 25.6 months, respectively (P = .009). Midostaurin combined with standard chemotherapy was well-tolerated compared with placebo. The rates of grade ≥3 adverse events were not different between the midostaurin and placebo arms, except for rash/desquamation (14% vs 8%, respectively) and anemia (93% vs 88%, respectively). The most common nonhematologic grade ≥3 adverse events were febrile neutropenia, infection, and diarrhea.5 Nausea rates were 10% in the placebo group versus 6% in the midostaurin group.5
Evidence of the efficacy of midostaurin in first-line therapy in older patients with AML comes from the DE02T/AML Study Group (AMLSG) 16-10 clinical trial.16 Unlike RATIFY, DE02T/AMLSG 16-10 was a single-arm study, with allogeneic HSCT being the preferred postremission therapy; the patients were older (aged 60-70 years) and received midostaurin maintenance therapy after consolidation with chemotherapy or with allogeneic HSCT. The study results suggest a clinical benefit when adding midostaurin to therapy versus historical controls, regardless of age (younger, 18-59 years; older, 60-70 years).16
Of the 284 patients who received treatment with midostaurin (198 younger patients and 86 older patients), 217 (76%) achieved a CR/CRi. Overall, 207 (73%) patients proceeded to an allogeneic HSCT (134 patients after achieving CR/CRi during the study), and 97 (34%) patients received maintenance therapy (75 patients after allogeneic HSCT and 22 patients after consolidation chemotherapy). The median event-free survival was 13.2 months and was not significantly different between the younger and older age-groups (P = .51).16 An analysis comparing patients in the DE02T/AMLSG 16-10 study with historical controls showed a significant (P <.001) improvement with midostaurin overall and in older patients. The median overall survival was 26 months. The most common grade 3 or 4 adverse events during the first induction cycle were infection, febrile neutropenia, GI events, and metabolic adverse events.16
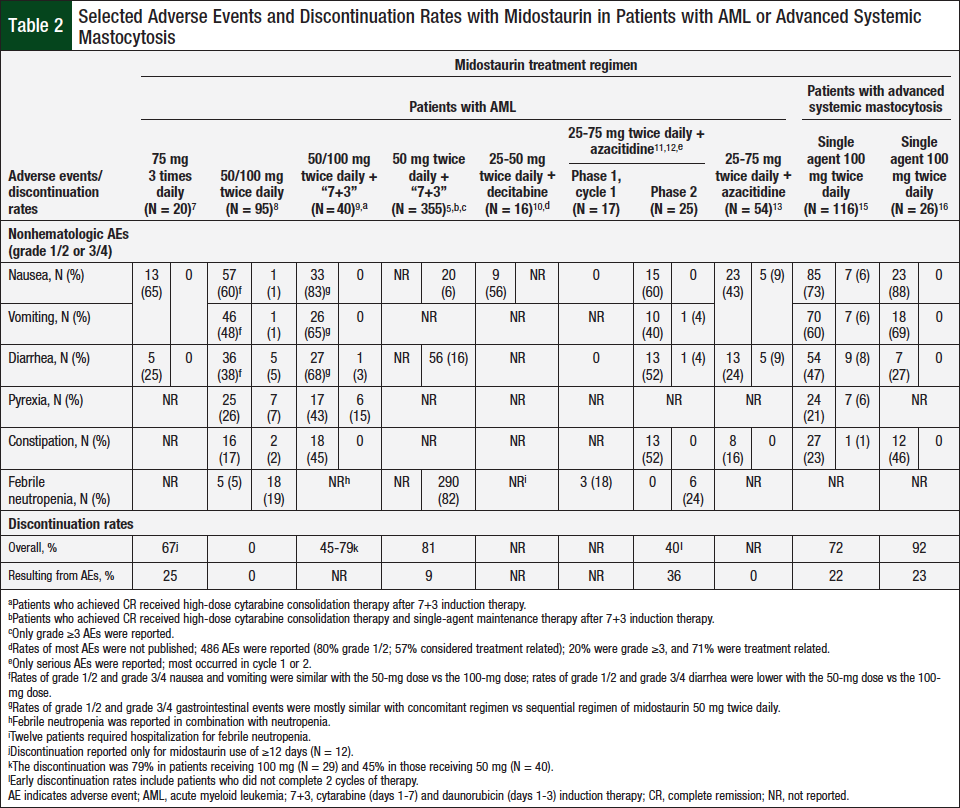
The targeting of specific high-risk mutations in AML provides a valuable therapeutic option for patients with a historically poor prognosis.1,2 Midostaurin has a tolerable safety profile as a single agent and in combination with established chemotherapy regimens and in a wide variety of patient demographics (Table 2).5,7-13,15
The efficacy of midostaurin has been well-established in the first-line setting in combination with standard chemotherapy,2,5,8,9 but additional late-phase studies of midostaurin in combination with other regimens and in different indications are needed to determine its full potential in the AML therapeutic landscape.
Clinical Trials in Advanced Systemic Mastocytosis
Advanced systemic mastocytosis is a disease in which abnormal mast cells accumulate in tissues, including in the bone marrow, spleen, liver, and skin. It has 3 subtypes per the World Health Organization (WHO): aggressive systemic mastocytosis, systemic mastocytosis with an associated hematologic neoplasm, and mast-cell leukemia.14 KIT mutations are present in approximately 90% of advanced systemic mastocytosis cases, and midostaurin has activity against these mutations.14,15,23,24
Midostaurin showed positive results in 2 open-label, single-arm, phase 2 clinical trials evaluating midostaurin 100 mg twice daily in patients with advanced systemic mastocytosis, including aggressive systemic mastocytosis, systemic mastocytosis with an associated hematologic neoplasm, or mast-cell leukemia.3,14,15 Among 89 patients who were evaluable for response in the D2201 study, the ORR was 60% (95% CI, 49%-70%), and 45% of patients had a major response. The median overall survival was 28.7 months.14 The observed reversal of organ damage was particularly noteworthy. For example, 40% of patients who were initially dependent on red blood cell transfusions became independent, and 100% of patients became platelet transfusion–independent.14 The most frequent nonhematologic adverse effects were nausea, vomiting, and diarrhea.14
In the A2213 study, 26 patients were evaluated.15 The ORR in the first 12 cycles was 69% (95% CI, 50%-88%), with 50% of patients achieving a major response. As in the D2201 study, the reversal of organ damage and other improvements were observed. It is important to note that with a longer follow-up, the quality of responses also improved.15
In a rare disease such as advanced systemic mastocytosis, a large phase 3 clinical trial is not possible. Thus, the benefit of midostaurin in advanced systemic mastocytosis was analyzed by a pooled analysis of 89 evaluable patients with a known date of diagnosis from the D2201 and the A2213 studies14,15 and comparing them with 42 matched controls from a registry of patients with advanced systemic mastocytosis who did not receive midostaurin and were diagnosed in a similar time frame as patients in the D2201 and A2213 studies.25 In that study, midostaurin reduced the risk for death by 50% compared with the controls.25
A French research group completed a similar analysis.26 This prospective survey compared 28 patients (25 with advanced systemic mastocytosis, 1 with mast-cell sarcoma, and 2 with progressive smoldering systemic mastocytosis) who received midostaurin versus 44 controls who were matched for age at diagnosis and WHO-defined subtype of mastocytosis.26 The ORR in the midostaurin group was 71%. Patients in the control group had more than twice the risk for death as patients in the midostaurin group (hazard ratio, 2.20; 95% CI, 1.08-4.47; P = .02). In patients receiving midostaurin, the most common adverse events were nausea or vomiting (89%), lymphocytopenia (61%), and photosensitivity (25%).26
These studies demonstrate that single-agent midostaurin is an effective treatment option for patients with advanced systemic mastocytosis and a viable alternative to traditional treatment with cladribine and interferon alfa.14,15,25,26
The results of these studies have led to the FDA approval of midostaurin for advanced systemic mastocytosis in 2017. Thus, treatment with midostaurin should be considered in all patients with this diagnosis in whom systemic treatment is necessary.
Safety and Tolerability of Midostaurin
Midostaurin has demonstrated acceptable tolerability at a variety of doses,2,5,7-16 although the rates of discontinuation are high (72%-92%; Table 2).8,14,15 When comparing the toxicity profile of midostaurin when added to cytarabine and daunorubicin induction therapy with that of placebo added to that regimen, only anemia (93% vs 88%; P = .03) and rash or desquamation (14% vs 8%; P = .008) were significantly more frequent in the midostaurin group.5
GI adverse events were the most common nonhematologic adverse events throughout midostaurin clinical trials.2,5,7-16 In patients with AML, the incidence of grade 1 or 2 nausea or vomiting was approximately 60% in the single-agent trials.2,7 The incidence of grade 3 or 4 nausea or vomiting in clinical trials of midostaurin in combination with standard-of-care therapies was higher than with standard-of-care therapy alone.5,8-12,16 In combination trials, the rate of diarrhea was higher than in single-agent studies, with an incidence rate of ≤75% for all grades and ≤16% for grade 3 or 4 diarrhea.5,8,10,12 In patients with advanced systemic mastocytosis who received midostaurin for longer periods (median duration of treatment, 3 months in the RATIFY trial vs 11 months in the D2201 trial), nausea and vomiting were again the most common nonhematologic adverse events (79%-88% and 66%-69%, respectively).5,14,15 In addition, when surveyed about the severity of 32 symptoms, patients reported an improvement in all but 2 symptoms—nausea and vomiting—while receiving midostaurin therapy.14
Hematologic adverse events were frequently observed in all clinical trials of midostaurin5,7-16; however, in the setting of MDS and AML, it is difficult to determine whether the source of the toxicity is the treatment or the disease itself. In patients with AML, the rates of grade ≥3 anemia, neutropenia, and thrombocytopenia (43%-93%, 73%-96%, and 73%-97%, respectively) were high when midostaurin was combined with chemotherapy or with hypomethylating agents.5,8,10,12
In patients with advanced systemic mastocytosis, the rates of grade ≥3 anemia, neutropenia, and thrombocytopenia were on the lower side (12%-41%, 8%-24%, and 8%-29%, respectively) when midostaurin was administered as a single agent.14,15 Nevertheless, the addition of midostaurin to standard induction and consolidation chemotherapy in patients with AML significantly increased grade ≥3 anemia compared with the addition of placebo (93% vs 88%; P = .03).5
Pulmonary adverse events were rare overall. Stone and colleagues described 3 deaths caused by pulmonary factors: progressive leukocytosis, progressive pulmonary failure, and infectious pneumonia that led to progressive pulmonary failure.2 Grade ≥3 infectious pneumonia was reported in other clinical trials as well, and was the cause of death in 3 patients.7,9,10 No clinical trial showed lung damage related to midostaurin use alone, but cases of grade ≥3 pneumonitis or pulmonary infiltrates were reported in the RATIFY study (8% in each arm), and 3 patients in the midostaurin arm died of pneumonitis.5,27 Thus, when administering midostaurin in combination with induction and consolidation chemotherapy, physicians are advised to monitor patients for the signs and symptoms of pulmonary toxicity.3
Cardiac adverse events were not common, but were reported in several studies of midostaurin. Stone and colleagues reported 1 instance of grade 3 tachycardia and 1 case of grade 4 elevated cardiac troponin,2 and Fischer and colleagues reported 1 death that resulted from cardiac failure.7 Cardiac adverse events were more common in patients who received midostaurin in combination with hypomethylating agents, particularly in those who had preexisting cardiac dysfunction or risk factors.9,12 Strati and colleagues reported an 11% incidence of grade 3 or 4 ejection fraction reduction; 50% of these patients had received previous anthracycline therapy, and 50% had significant cardiac risk factors.12 Treatment with midostaurin in combination with decitabine resulted in cardiac dose-limiting toxicities.9 Prolonged corrected QT (QTc) interval has also been reported in patients receiving midostaurin treatment (as discussed in further detail later).3,9,14
Hepatic and renal dysfunction were typically mild during clinical trials with midostaurin. Grade 3 or 4 renal and hepatic adverse events were less common than grade 1 or 2 events; Stone and colleagues reported the incidence of grade ≥3 hyperbilirubinemia and transaminitis as 7% and 13%, respectively, in patients who received midostaurin, but neither was significantly different from the incidences in the placebo arm.5 Strati and colleagues reported 2 cases of acute renal failure.12 Renal and hepatic dysfunction were among the exclusion criteria for many studies, which may not mirror typical patient populations in practice.
Ongoing or Pending Studies of Other Combinations
HSCT remains a goal for all patients with AML and FLT3 mutation, and an important outstanding question is the role that midostaurin plays after transplant.28 The phase 2 randomized RADIUS clinical trial evaluated the efficacy and safety of midostaurin added to standard of care as post–allogeneic HSCT maintenance therapy in patients with FLT3-ITD mutation.13 The results from that study13 are expected to answer many questions regarding the safety and tolerability of midostaurin in this fragile patient population. Any potential benefit needs to be carefully weighed against possible posttransplant complications and medication toxicities.
Among 60 patients randomized (30 to the midostaurin arm, 30 to the standard-of-care–only arm) to the exploratory RADIUS study, 30 patients completed 12 cycles of therapy (16 patients with midostaurin).13 The estimated relapse-free survival rate at 18 months after allogeneic HSCT was 89% with midostaurin and 76% with standard of care alone, which was a 54% relative reduction in the risk for relapse. In a correlative analysis that grouped patients by FLT3 inhibitory levels (above or below the median inhibition observed on day 1 of study cycle 3), achieving FLT3 inhibitory levels that were above the median was associated with improved relapse-free survival and overall survival.13
The safety results from the RADIUS study suggest that adding midostaurin to standard of care after transplant is feasible and does not change the rates of graft-versus-host disease.13 However, grade 1 or 2 GI events were higher in the midostaurin arm than in the standard-of-care arm.13 Midostaurin is also being investigated in combination with hypomethylating agents and in patients with impaired hepatic function.29
The treatment of patients older than 65 years and those with relapsed or refractory AML remains a significant challenge.30 Studies that incorporate well-designed combinations of midostaurin and an epigenetic agent that can be given in an outpatient setting are particularly desirable.
Challenges for Clinicians
Medication interactions, overlapping adverse reactions, and a financial burden can all affect treatment decisions and complicate patient care. Midostaurin is primarily metabolized by CYP3A4.3 If midostaurin is used concomitantly with CYP3A4 inhibitors or inducers, its concentrations can be affected, leading to additional toxicity with the former or to reduced efficacy with the latter. The concomitant use of midostaurin with CYP3A4 inhibitors is not contraindicated, but when used together, it is recommended that patients be watched closely because of the increased risk for adverse events, especially during the first week of therapy in patients with advanced systemic mastocytosis and during the first week of each chemotherapy cycle in patients with AML. The co-administration of midostaurin with strong CYP3A4 inducers should be avoided.3
An ongoing phase 1 clinical trial evaluating midostaurin treatment in patients with impaired liver function may provide further insight into the management of adverse events, midostaurin’s effects on pharmacokinetics, and the strategies for dosing in patients who are receiving concurrent medications that affect the metabolism of midostaurin.29
In some clinical trials of midostaurin, providers were cautioned against prescribing any medications that interfered with CYP3A4 because of an increased risk for pulmonary toxicity.5,31 However, a recent subgroup analysis of the RATIFY study comparing matched patients from the 2 study arms showed that patients who had concentrations of midostaurin above the median had a shorter time to a first grade 3 or 4 clinically notable adverse event than those with concentrations below the median (36 days vs 49 days; P = .0119) but no notable increase in midostaurin-related adverse events. In patients receiving midostaurin, higher dose intensities were associated with improved outcomes.32
As noted earlier, cases of QTc prolongation have been reported with midostaurin in patients with advanced systemic mastocytosis and rarely in patients with AML.3,9,14 This may lead to challenges for patients who are receiving concomitant medications with midostaurin or those who have underlying cardiac conditions.3 Nausea is the most common nonhematologic adverse event reported with midostaurin (Table 2), and many antiemetics, including 5-hydroxytryptamine-3 (5-HT3) receptor antagonists, can affect cardiac conduction.33
In the RATIFY study, dose modifications were allowed in patients with a QTc of >470 ms.5 Electrolytes were corrected if the QTc was >450 ms.5 Treatment discontinuation as a result of QTc prolongation has been reported in patients with advanced systemic mastocytosis.3,14 A 5-HT3 receptor antagonist may be co-administered with midostaurin; however, if a patient is at high risk for cardiac events or is receiving other medications that prolong the QTc interval, providers may consider monitoring with electrocardiogram assessments and replacing electrolytes.
Finally, midostaurin may cause financial difficulties for patients who are trying to obtain insurance coverage for treatment in the outpatient setting if the medication requires a high copay or is not otherwise covered by insurance.34-36 Some options for financial assistance are available through the drug manufacturer, and certain foundations assist with high copay amounts.37 The complicated treatment schedule for AML can pose an obstacle to obtaining midostaurin in a timely manner for outpatient care. Patients may need additional assistance to ensure that they remain on schedule with their treatment.
Conclusion
Midostaurin is currently approved by the FDA for the treatment of patients with newly diagnosed AML and FLT3 mutation and for advanced systemic mastocytosis. The addition of midostaurin to standard-of-care chemotherapy has provided a new opportunity in the treatment of a disease that for many years had very few treatment advances. Midostaurin adds substantial benefit, with acceptable additional toxicity, in the setting of high-risk AML and in advanced systemic mastocytosis.
The full benefit of FLT3 inhibition should be further defined as the AML therapy landscape evolves. Because the management of adverse events and drug interactions is not completely defined, pharmacists and providers should apply their clinical skills to mitigate the risks associated with the use of midostaurin therapy. Patients should be continually counseled on the use of oral anticancer medications, with regular follow-up, to minimize the barriers to patient adherence to treatment and for the management of adverse events.
Acknowledgments
The authors thank JoAnna Anderson, PhD, and Katherine Mills-Lujan, PhD, CMPP, of ArticulateScience, LLC, for their medical editorial assistance for this manuscript. The authors made all decisions on all aspects of this article.
Funding Source
Funding for medical editorial assistance was provided to ArticulateScience, LLC, by Novartis Pharmaceuticals. The authors did not receive any funding and had full control of the content of this article.
Author Disclosure Statement
Dr Bivona and Dr Elsey have no conflicts of interest to report. Dr Williams has received research and consultation funding from Takeda Oncology and research funding from Tesaro.
References
- Fröhling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372-4380.
- Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54-60.
- Rydapt (midostaurin) capsules, for oral use [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals; June 2018.
- Fabbro D, Buchdunger E, Wood J, et al. Inhibitors of protein kinases: CGP 41251, a protein kinase inhibitor with potential as an anticancer agent. Pharmacol Ther. 1999;82:293-301.
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377:454-464.
- Propper DJ, McDonald AC, Man A, et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol. 2001;19:1485-1492.
- Fischer T, Stone RM, DeAngelo DJ, et al. Phase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28:4339-4345.
- Stone RM, Fischer T, Paquette R, et al. Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia. 2012;26:2061-2068.
- Williams CB, Kambhampati S, Fiskus W, et al. Preclinical and phase I results of decitabine in combination with midostaurin (PKC412) for newly diagnosed elderly or relapsed/refractory adult patients with acute myeloid leukemia. Pharmacotherapy. 2013;33:1341-1352.
- Cooper BW, Kindwall-Keller TL, Craig MD, et al. A phase I study of midostaurin and azacitidine in relapsed and elderly AML patients. Clin Lymphoma Myeloma Leuk. 2015;15:428-432.
- Gallogly MM, Tomlinson BK, Bunner P, et al. A phase II study of midostaurin and 5-azacitidine for elderly patients with acute myeloid leukemia. Blood. 2017;130(suppl 1):1332.
- Strati P, Kantarjian H, Ravandi F, et al. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am J Hematol. 2015;90:276-281.
- Maziarz RT, Fernandez H, Patnaik MM, et al. RADIUS: midostaurin (mido) plus standard of care (SOC) after allogeneic stem cell transplant (alloSCT) in patients (pts) with FLT3-internal tandem duplication (ITD)–mutated acute myeloid leukemia (AML). Biol Blood Marrow Transplant. 2019;25:S11-S12.
- Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2530-2541.
- DeAngelo DJ, George TI, Linder A, et al. Efficacy and safety of midostaurin in patients with advanced systemic mastocytosis: 10-year median follow-up of a phase II trial. Leukemia. 2018;32:470-478.
- Schlenk RF, Weber D, Fiedler W, et al. Midostaurin added to chemotherapy and continued single agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133:840-851.
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752-1759.
- Swords R, Freeman C, Giles F. Targeting the FMS-like tyrosine kinase 3 in acute myeloid leukemia. Leukemia. 2012;26:2176-2185.
- Abu-Duhier FM, Goodeve AC, Wilson GA, et al. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113:983-988.
- Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434-2439.
- Weisberg EL, Puissant A, Stone R, et al. Characterization of midostaurin as a dual inhibitor of FLT3 and SYK and potentiation of FLT3 inhibition against FLT3-ITD-driven leukemia harboring activated SYK kinase. Oncotarget. 2017;8:52026-52044.
- Jin G, Matsushita H, Asai S, et al. FLT3-ITD induces ara-C resistance in myeloid leukemic cells through the repression of the ENT1 expression. Biochem Biophys Res Commun. 2009;390:1001-1006.
- Kristensen T, Vestergaard H, Møller MB. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J Mol Diagn. 2011;13:180-188.
- Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366-2372.
- Reiter A, Kluin-Nelemans HC, George T, et al. Pooled survival analysis of midostaurin clinical study data (D2201+A2213) in patients with advanced systemic mastocytosis compared with historical controls. Haematologica. 2017;102(s2):321.
- Chandesris MO, Damaj G, Canioni D, et al. Midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2605-2607.
- Stone RM, Mandrekar S, Sandord BL, et al. The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose C consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18-60 with FLT3 mutations (muts): an international prospective randomized (rand) P-controlled double-blind trial (CALGB 10603/RATIFY [Alliance]). Blood. 2015;126:6.
- Pratz KW, Levis M. How I treat FLT3-mutated AML. Blood. 2017;129:565-571.
- ClinicalTrials.gov. PK and safety of midostaurin in subjects with impaired hepatic function and subjects with normal hepatic function. https://clinicaltrials.gov/ct2/show/NCT01429337. Accessed October 6, 2017.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424-447.
- Maziarz RT, Scott BL, Mohan SR, et al. A phase II, randomized trial of standard of care with or without midostaurin to prevent relapse following allogeneic stem cell transplantation in patients with FLT3-ITD mutated acute myeloid leukemia. J Clin Oncol. 2015;33(15_suppl):Abstract TPS7094.
- Ouatas T, Duval V, Sinclair K, Berkowitz N. Concomitant use of midostaurin with strong CYP3A4 inhibitors: an analysis from the Ratify trial. Blood. 2017;130(suppl 1):3814.
- Zuo P, Haberer LJ, Fang L, et al. Integration of modeling and simulation to support changes to ondansetron dosing following a randomized, double-blind, placebo-, and active-controlled thorough QT study. J Clin Pharmacol. 2014;54:1221-1229.
- Dusetzina SB, Winn AN, Abel GA, et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32:306-311.
- Shen C, Zhao B, Liu L, Shih YT. Adherence to tyrosine kinase inhibitors among Medicare Part D beneficiaries with chronic myeloid leukemia. Cancer. 2018;124:364-373.
- Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56-66.
- Novartis. Novartis Oncology Universal Co-pay Program. www.copay.novartisoncology.com. Accessed October 6, 2017.