Symptom Overview
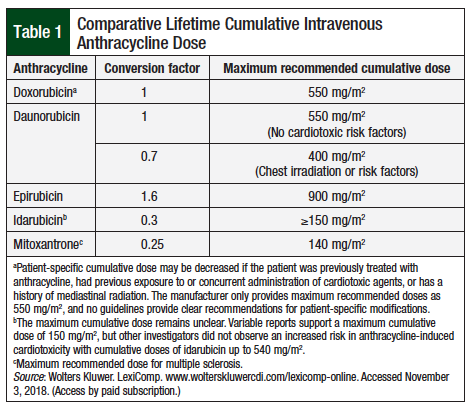
Anthracyclines, such as doxorubicin or epirubicin, have been used for decades for the treatment of a variety of cancers, including breast cancer, sarcoma, lymphoma, and leukemia.1 Type I cardiotoxicity is a known irreversible complication of anthracycline use and is associated with cumulative doses (Table 1).
It is estimated that 3% to 5%, 7% to 26%, and 18% to 48% of patients with cumulative doxorubicin doses of 400 mg/m2, 550 mg/m2, and 700 mg/m2, respectively, will develop doxorubicin-induced heart failure (HF).2
Cardiotoxicity is categorized as early or late depending upon whether HF is observed within 1 year (early) from completion of anthracycline therapy or after a minimum of 1 year (late).3
Common manifestations of cardiotoxicity include overt HF and left-ventricular (LV) dysfunction, as defined by LV ejection fraction (LVEF) decline of at least 5% to less than 55% with symptoms, or LVEF decline of at least 10% to less than 55% without symptoms.2
Anthracycline-induced cardiotoxicity is associated with many risk factors, such as age (young and elderly), female sex, obesity, history of mediastinal radiation, underlying cardiovascular disease, use of concomitant cardiotoxic medications, cumulative dose of anthracycline, intravenous bolus anthracycline administration increased time since anthracycline exposure, and increase in troponin or natriuretic peptides during and after anthracycline administration.2,4
The optimal method for preventing or managing anthracycline cardiotoxicity remains unknown. This symptom management overview seeks to highlight strategies for the management of anthracycline-induced cardiotoxicity.
Etiology
Previously, the primary mechanism of anthracycline-induced cardiotoxicity was attributed to the generation of reactive oxygen species (ROS) resulting in direct damage to cardiomyocytes. Recent literature suggests that the inhibition of topoisomerase 2-beta (topo 2B) is the primary mediator of anthracycline cardiotoxicity.1,5
Topo 2B inhibition results in alterations to antioxidant gene transcription and excess ROS production, reduction in mitochondrial biogenesis and regulation of mitochondrial ROS production, and activation of p53 and apoptotic pathways. Although the exact mechanism of anthracycline-induced cardiotoxicity is likely multifactorial, the cardioprotective effects in mice with topo 2B deletion emphasize the key role of topo 2B inhibition in promoting anthracycline cardiotoxicity.
Monitoring
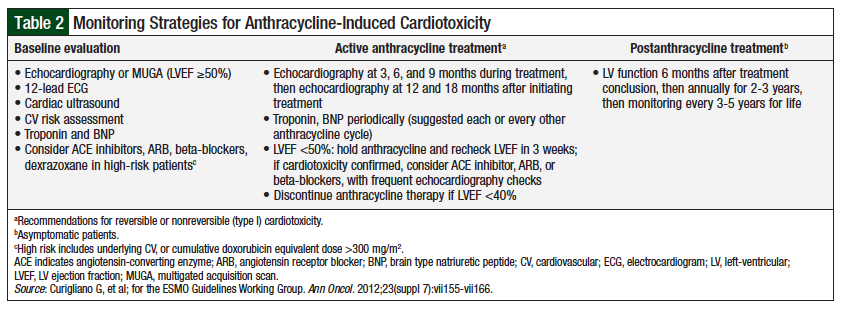
There is no consensus on how to best monitor anthracycline-induced cardiotoxicity.2 However, the European Society for Medical Oncology (ESMO) guidelines suggest monitoring strategies as shown in Table 2.
Evaluation of LVEF is a significant component to monitoring anthracycline-induced cardiotoxicity. A baseline LVEF of ≥50% is necessary before the initiation of therapy. LVEF decline to less than 50% requires holding anthracycline therapy and reevaluation after 3 weeks, and an LVEF decline to less than 40% requires discontinuation of anthracycline therapy.2 In addition, the ESMO guidelines include the option of monitoring biomarkers.2
No consensus exists on the benefit of biomarkers in predicting anthracycline-induced cardiotoxicity; however, some evidence is available to support the predictive value of troponin, brain natriuretic peptide (BNP), and N-terminal pro-BNP (NT-proBNP).4 Troponin elevation in the early phase of treatment has been observed to be predictive of LV dysfunction and associated with higher rates of cardiac events (early and late).4,6 The predictive value of NT-proBNP for LVEF is less clear, but persistent elevations at 72 hours postchemotherapy have been observed, resulting in worsening systolic and diastolic function over 12 months.4,7
Primary Prevention
Multiple approaches are used in the primary prevention of anthracycline-induced cardiotoxicity. Widely accepted strategies include limiting the cumulative anthracycline dose, using liposomal formulations, administering anthracycline agents via continuous infusion (ie, over 6-96 hours), and administering dexrazoxane in conjunction with anthracycline treatment.1,8 Dexrazoxane can reduce anthracycline toxicity, thanks to dexrazoxane’s ability to chelate iron and, more important, bind and alter the conformation of topo 2B, thereby preventing anthracycline from binding to topo 2B.
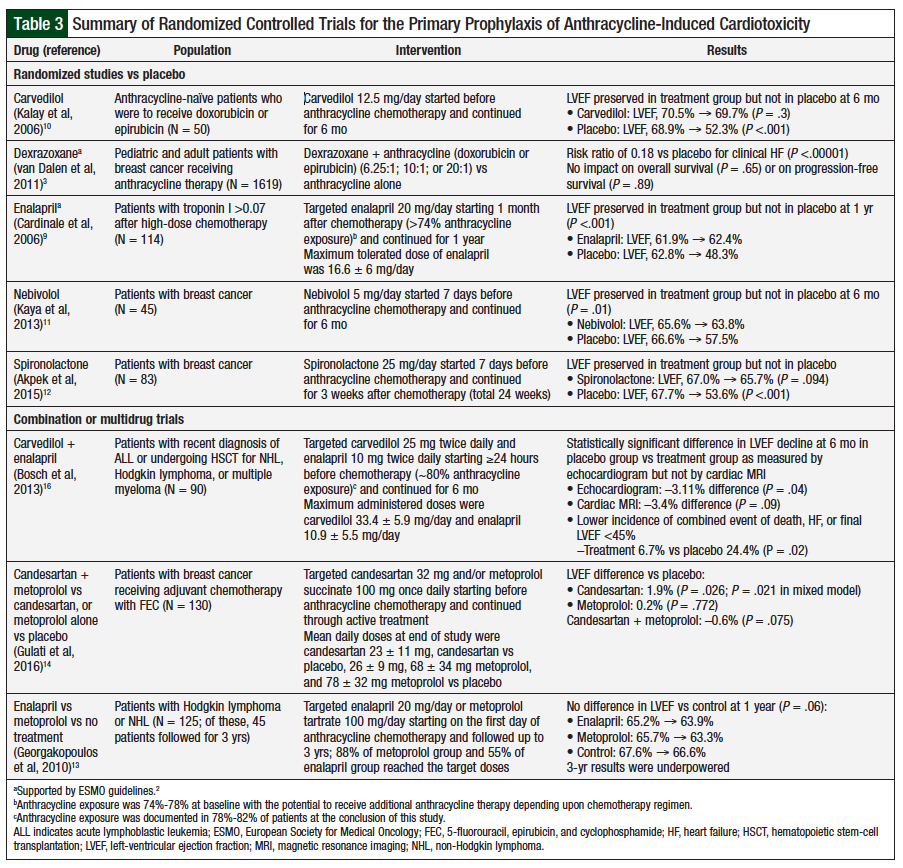
Other antioxidants or iron-chelating agents have not demonstrated efficacy in reducing anthracycline-induced cardiotoxicity. In addition to prophylaxis with dexrazoxane, the only other pharmacologic intervention supported by the ESMO guidelines is the initiation of enalapril with troponin I elevations of more than 0.07 after anthracycline therapy (Table 3).2,9 Enalapril should be continued for at least 1 year after the start of therapy for LVEF preservation.9
The investigation of additional agents for prophylaxis has led to several randomized controlled trials with angiotensin-converting enzyme inhibitors, beta-blockers, and aldosterone antagonists (Table 3). Carvedilol, nebivolol, and spironolactone have demonstrated benefits versus placebo at 6 months in preserving LVEF when initiated before anthracycline therapy.10-12 Metoprolol alone, or in combination with candesartan, has not provided protection against anthracycline-induced cardiotoxicity.13,14
Prophylaxis with candesartan has shown mixed effects, with statistically significant but unclear clinically relevant benefits, as monotherapy.14 Excluding the use of enalapril after troponin elevation with anthracycline therapy, enalapril has questionable efficacy, with randomized controlled trials demonstrating no benefit or statistically significant results with echocardiograms,13,15,16 but not with cardiac magnetic resonance imaging.16
Although some of these medications have demonstrated initial prophylactic benefits, the small patient populations and short trial durations limit any conclusions regarding long-term efficacy in preventing anthracycline-induced cardiotoxicity.
Treatment of Left-Ventricular Dysfunction
After LV dysfunction has been identified, treatment should be started promptly with medications recommended in the American College of Cardiology Foundation/American Heart Association guidelines.15 Patients with LVEF of 45% or less who started HF treatment with enalapril and carvedilol more than 6 months after anthracycline therapy were unable to fully recover their LVEF function.15
Conclusion
Despite decades of anthracycline use, there is no consensus on the long-term prevention and management of anthracycline-induced cardiotoxicity. Dexrazoxane is the only agent approved by the US Food and Drug Administration for the prevention of doxorubicin-induced cardiotoxicity and is supported by a large amount of evidence. However, dexrazoxane is only used in specific patient populations. Enalapril also affords short-term benefits, but the long-term efficacy remains unknown. Additional agents, such as carvedilol, nebivolol, and spironolactone, have demonstrated early benefit in the primary prevention setting, but additional clinical trials are needed to ascertain their clinical benefits.
Author Disclosure Statement
Dr Blake, Dr Diaz, and Dr Bubalo have no conflicts of interest to report; Dr Palumbo has received honoraria from Oncology Reimbursement Management and Oregon Society of Health-System Pharmacists.
References
- Vejpongsa P, Yeh ETH. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938-945.
- Curigliano G, Cardinale D, Suter T, et al; for the ESMO Guidelines Working Group. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012; 23(suppl 7):vii155-vii166.
- van Dalen EC, Caron HN, Dickinson HO, Kremer LCM. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. June 2011:CD003917.
- Jahangir E, Shah S, Shum K, et al. Risk assessment and management of anthracycline and HER2 receptor inhibitor-induced cardiomyopathy. South Med J. 2015;108:71-78.
- Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639-1642.
- Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36:517-522.
- Sandri MT, Salvatici M, Cardinale D, et al. N-terminal pro-B-type natriuretic peptide after high-dose chemotherapy: a marker predictive of cardiac dysfunction? Clin Chem. 2005;51:1405-1410.
- Conway A, McCarthy AL, Lawrence P, Clark RA. The prevention, detection and management of cancer treatment-induced cardiotoxicity: a meta-review. BMC Cancer. 2015;15:366.
- Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy–induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474-2481.
- Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258-2262.
- Kaya MG, Ozkan M, Gunebakmaz O, et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol. 2013;167:2306-2310.
- Akpek M, Ozdogru I, Sahin O, et al. Protective effects of spironolactone against anthracycline‐induced cardiomyopathy. Eur J Heart Fail. 2015;17:81-89.
- Georgakopoulos P, Roussou P, Matsakas E, et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin‐treated lymphoma patients: a prospective, parallel‐group, randomized, controlled study with 36‐month follow‐up. Am J Hematol. 2010;85:894-896.
- Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671-1680.
- Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213-220.
- Bosch X, Esteve J, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular dysfunction in patients with malignant hemopathies: the OVERCOME trial. J Card Fail. 2013;61:2355-2362.