The antineoplastic and antimetabolite fluorouracil has been used extensively to treat colon, breast, and other solid tumor cancers.1 Treatment regimens incorporating fluorouracil vary widely depending on patient and tumor characteristics, and may range from intermittent bolus doses with radiation therapy to multiday, continuous intravenous (IV) infusions as part of combination chemotherapy.
Continuous fluorouracil infusions are commonly administered via an ambulatory infusion pump, which allows the patient to receive therapy at home. According to the National Institutes of Health, approximately 275,000 patients in the United States receive fluorouracil annually as part of their chemotherapy regimen. Approximately 3% of these patients will have some degree of toxic reaction, and >1300 deaths occur in the United States each year because of fluorouracil exposure.2 Continuous infusion pumps can predispose patients to a fluorouracil overdose because of potential errors in programming, calculation errors, and device malfunctions, which have resulted in numerous cases where patients received higher than intended doses or rates of administration.3,4 Other potential causes of overexposure to fluorouracil include intrinsic deficiency in metabolism through dihydropyrimidine dehydrogenase gene mutations,5,6 and accidental administration of the oral prodrug capecitabine.5
Complications from overexposure can manifest during a period of days or weeks. Known side effects such as nausea, vomiting, diarrhea, and mucositis typically have an early onset, manifesting approximately 3 to 8 days after exposure.7 Low blood counts have a delayed onset, appearing approximately 9 to 14 days after exposure to fluorouracil. Although not common, delay in low white counts appearing up to 20 days later have been reported.1
In the past, patients who received an overdose of fluorouracil were managed solely with supportive care, including colony-stimulating factors and antibiotics.7 Despite these measures, fluorouracil overdose can result in serious toxicities, including death. The oral investigational drug uridine triacetate, which exerts its action by competing with fluorouracil for ribonucleic acid (RNA) integration, is the only antidote available for fluorouracil overdose. To date, approximately 140 cases of overdose have been treated with uridine triacetate, with a reported 96% survival rate. In addition, 50% of the patients treated with uridine triacetate were able to resume their chemotherapy treatment within 30 days of a fluorouracil overdose. Five deaths have been reported to date, but according to the manufacturer, these were not related to the antidote (R. Tremmel, PharmD, Clinical Safety Manager, Wellstat Therapeutics, e-mail communication, May 2014).
In addition to uridine triacetate, the administration of oral glutamine has been proposed to prevent intestinal changes that affect absorption, hence reducing fluorouracil-induced diarrhea.8 If the overdose rate is greater than 10 times the intended rate with a completed delivery of 50% of the intended total fluorouracil dose, supportive therapy with IV hydration, filgrastim, fluoroquinolones, and oral glutamine is recommended.9
To our knowledge, this case report is the first to extensively describe a patient’s clinical course, complications, and response to treatment following a fluorouracil overdose.
Case Report
A 60-year-old man with rectal carcinoma and liver metastases was admitted to the emergency department following a confirmed fluorouracil overdose.
The patient, who weighed 84.1 kg on admission, received his tenth cycle of IV chemotherapy earlier that day: bevacizumab 5 mg/kg (422 mg), oxaliplatin 85 mg/m2 (176 mg), leucovorin 400 mg/m2 (828 mg), and fluorouracil 1920 mg/m2 (3974 mg) infusion administered during the course of 46 hours.
An infusion pump malfunction resulted in the entire dose of fluorouracil and an additional 469 mg, which was contained within the overfill compartment, being administered to the patient in 20 minutes. The patient was immediately referred to his local emergency department and, within 90 minutes, transferred to a tertiary care center.
The patient was asymptomatic at presentation to the tertiary center, with stable vital signs and a benign electrocardiogram (ECG). Review of laboratory results indicated that the basic metabolic panel and liver function tests were within normal range. Phosphorus was depleted (1.5 mg/dL), and magnesium was on the low end of normal (1.5 mEq/L). The patient received electrolyte repletion, as well as glutamine 15 g orally every 12 hours, with the first dose given 9 hours after the overdose.
Uridine triacetate was obtained in accordance with the investigational protocol provided by Wellstat Therapeutics. The patient’s regimen of uridine triacetate 10 g orally every 6 hours for 20 doses was initiated 20 hours after the overdose. To ensure the absorption of the antidote amid chemotherapy-induced nausea and vomiting, the patient received ondansetron 8 mg intravenously prior to each dose of uridine triacetate. Filgrastim 5 mcg/kg (420 mcg) intravenously was also administered.
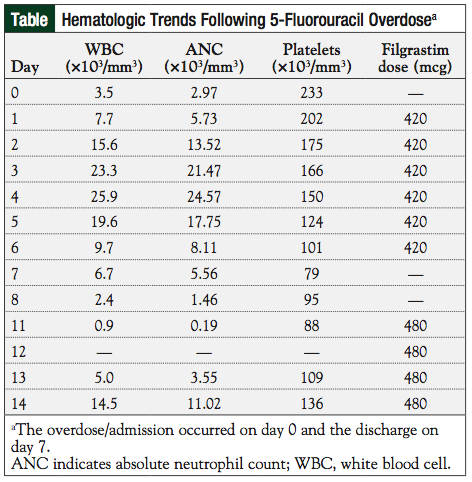
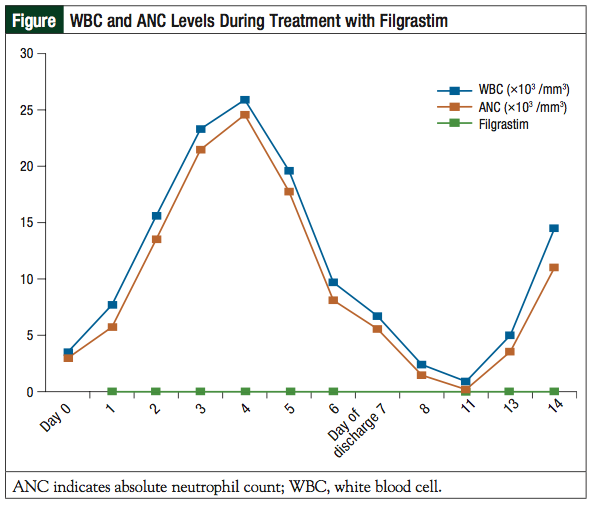
On day 1, the patient was admitted for observation. He continued to receive uridine triacetate per protocol. He also continued therapy with glutamine 15 g orally every 12 hours. On day 3, an ECG demonstrated 3 beats of nonsustained ventricular tachycardia (NSVT) and premature ventricular contractions (PVCs). On day 6, the patient had an episode of hypotension and intermittent, asymptomatic tachycardia, which resolved with the administration of IV fluids. On days 1 to 4, the patient had a moderate increase in loose ostomy output, nausea with 1 episode of vomiting, and mucositis with 1 ulceration on the inside of his cheek. He received loperamide 4 mg orally daily, clotrimazole troches 4 times daily, equal parts of antacid (Maalox), lidocaine, and diphenhydramine (magic mouthwash) 10 mL orally 3 times daily, filgrastim 420 mcg intravenously daily (total of 6 doses), and levofloxacin 500 mg orally daily, which was later increased to 750 mg. On day 4, his complete blood count (CBC) revealed that his white blood cells (WBCs) increased from 3.5 × 10³ cells/mm3 at presentation to 25.9 × 10³ cells/mm3, likely secondary to preemptive filgrastim administration.
The patient’s WBC subsequently fell to 6.7 × 10³ cells/mm3 at discharge on day 7, despite continued filgrastim administration (see the patient’s hematologic trends in the Table). The patient’s absolute neutrophil count (ANC) at discharge was 5.56 × 10³ cells/mm³. His platelets declined from 233 × 10³/mm³ at presentation to 79 × 10³/mm³ at discharge. No significant changes were observed in his basic metabolic panel, hemoglobin/hematocrit, or liver function tests.
The patient was discharged on day 7 with prescriptions for clotrimazole troches 4 times daily, magic mouthwash 10 mL 3 times daily, and loperamide 2 mg orally every 4 hours as needed for loose stool. He received 6 days of levofloxacin therapy as an inpatient and was to continue levofloxacin upon discharge, to be discontinued at the discretion of the patient’s outpatient oncologist. The patient declined further levofloxacin treatment postdischarge upon discussion with his provider.
At outpatient follow-up on day 11, the patient presented with grade 3 mucositis. His CBC revealed a WBC count of 0.9 × 10³ cells/mm3, an ANC of 0.19 × 10³ cells/mm3, and platelets of 88 × 103/mm3. Filgrastim was reinitiated at 480 mcg subcutaneously daily and continued through day 14, when his WBC count was 14.5 × 103 cells/mm3 and ANC was 11 × 10³ cells/mm3. Approximately 1 month following the overdose, chemotherapy was discussed with the patient, but he declined further treatment.
On day 28 he was described by his primary oncologist as having fully recovered, with no mucositis or gastrointestinal problems, and a stable CBC. The patient’s only residual side effect was alopecia.
Discussion
Infusion pump malfunctions and programming errors have led to numerous cases of fluorouracil overdose, often resulting in serious harm to the patients.
In one case reported to the Institute for Safe Medication Practices (ISMP) Canada, a 43-year-old woman with advanced nasopharyngeal carcinoma died of an accidental fluorouracil overdose because of a possible infusion pump error.3 Bamat and colleagues described 98 cases in which patients received uridine triacetate following fluorouracil overdose.5 Of these patients, 96 recovered fully after treatment with uridine triacetate. However, the number of cases related to pump malfunctions and programming errors was not defined, and the supportive measures used during treatment were not discussed. McEvilly and colleagues reported the case of a 55-year-old man whose 46-hour 5-fluorouracil infusion had been incorrectly programmed to be administered over 4 hours. Treatment with uridine triacetate was described with a brief mention of supportive measures, such as pegfilgrastim.10 However, the authors did not expand on the role of supportive treatment measures, which may include fluid and electrolyte repletion, and management of gastrointestinal and hematologic adverse effects.
The ISMP has identified many causal factors for fluorouracil overdoses relating to infusion pump errors, and provides safe practice recommendations for fluorouracil overdose prevention.3 Examples of causative factors include a lack of programming safeguards, miscalculations, faulty pharmacy label designs, failed independent double-check systems, lack of familiarity with protocol, and pump design contributions.3 To minimize the risk for error, the ISMP encourages the use of enhanced independent double check during infusion pump programming, using infusion pumps with safeguards, and reviewing the pump-dosing screen with the patient. The patient in our case report was transferred from an outside facility, which hindered our ability to identify the exact cause of the overdose other than what was reported. We would recommend evaluating the pump for possible malfunctions or other causative factors such as programming errors. Pumps that have malfunctioned should be reported to the manufacturer.
Understanding fluorouracil’s mechanism of action is essential to understanding the treatment of a fluorouracil overdose. Fluorouracil exhibits cytotoxic activity through disruption of the biologic processes of both RNA and deoxyribonucleic acid (DNA) thereby inhibiting normal function. Fluorouracil is metabolized into the active metabolites, fluorouridine triphosphate (FUTP), fluorodeoxyuridine triphosphate (FdUTP), and fluorodeoxyuridine monophosphate (FdUMP). FdUTP and FdUMP target DNA through disincorporation of FdUTP during DNA synthesis and the inhibition of thymidylate synthase. FUTP is responsible for the interruption of RNA transcription and function, resulting in apoptosis. Uridine is a naturally occurring pyrimidine nucleoside and is 1 of the 4 basic nucleosides responsible for the formation of RNA.11 It is part of the same biochemical pathway as FUTP, which makes it an effective antidote, reducing the effects of the active metabolite. Intrinsically, uridine is converted to uridine triphosphate (UTP), which then competitively inhibits the incorporation of FUTP into RNA. UTP inherently reduces the toxic effects of FUTP on the gastrointestinal mucosa and the bone marrow.7
Uridine triacetate has been granted orphan drug status by the US Food and Drug Administration for the treatment of fluorouracil overdose. Uridine triacetate is available through an orphan drug program from Wellstat Therapeutics.4,12 When overexposure to fluorouracil is suspected, the patient’s provider can contact Wellstat Therapeutics, who will facilitate procurement of the medication. The recommended adult dose of uridine triacetate is 10 g orally every 6 hours for a total of 20 doses, initiated within 3 to 96 hours following overexposure. The antidote does not require adjustment for weight, compromised renal or hepatic functions, or advanced age (>65 years). Uridine triacetate is available as orange-flavored packets, which can be mixed in applesauce to improve palatability. Because of anticipated nausea and vomiting associated with fluorouracil, Wellstat Therapeutics recommends preceding each dose of uridine triacetate with an antiemetic to ensure full absorption. If a patient vomits within 2 hours of uridine triacetate administration, a repeat dose of uridine triacetate 10 g is recommended within 15 minutes. The next dose is then given at the originally scheduled time. Our patient received 8 mg of ondansetron 20 minutes before each dose of uridine triacetate. Throughout his inpatient course, he experienced nausea with 1 episode of vomiting, which did not interfere with his antidote regimen. Because of recent concerns about ondansetron dose-dependent risk of arrhythmia,13 we would encourage using the minimum effective dose. Several medications may interfere with the absorption of uridine triacetate, including bismuth, sucralfate, and cholestyramine. Wellstat Therapeutics provides a list of medications that should be avoided during the treatment period.
In addition to uridine triacetate, our patient received glutamine, which is a nonessential amino acid that can be synthesized from glucose.14 Intrinsically, glutamine is part of the regulatory system for cell growth in the gastrointestinal tract. Oral glutamine is traditionally given as a nutritional supplement for the treatment of short bowel syndrome.15 In recent years, its use has expanded to critically ill patients on mechanical ventilation, burn patients, and patients with major elective surgery, trauma, or head and neck cancer, because of its effects on intestinal epithelium and gut integrity maintenance.16 The recommend oral dose is 30 g daily in divided doses; side effects may include nausea and vomiting.15 The use of glutamine to prevent chemotherapy-related mucositis and gastrointestinal side effects remains controversial. It is theorized that several cancer cells utilize glutamine for accelerated cell growth.14 While receiving oral glutamine 15 g twice daily following his fluorouracil overdose, our patient developed grade 3 mucositis.
In anticipation of neutropenia, the human granulocyte colony-stimulating factor filgrastim was initiated. With therapy, the patient’s WBC count and ANC initially increased, but began to drop on day 5, despite continued filgrastim administration. Filgrastim was not continued at discharge on day 7, and the patient presented to the outpatient clinic on day 11 with a WBC count of 0.9 × 10³ cells/mm3 and an ANC of 0.19 × 103 cells/mm3. Filgrastim was restarted on day 11 and continued through day 14, when neutropenia resolved (Figure). For future cases, we recommend continuing therapy with a human granulocyte colony-stimulating factor as appropriate, through the ANC nadir and to recovery, which may take up to 14 days.
Fluorouracil may cause diarrhea in up to 80% of patients.17 Our patient’s diarrhea resolved with the use of loperamide, an agent recommended by the American Society of Clinical Oncology guidelines. Loperamide doses as high as 4 mg at first onset, then 2 mg every 2 hours until the patient is diarrhea-free for 24 hours, have been recommended. The patient received a daily standing dose of loperamide because ostomy output was consistently loose. He had the option to receive additional loperamide, as needed, and required 1 additional dose during hospitalization. No adverse effects related to loperamide 4 mg daily were observed in our patient. The addition of antibiotics is recommended for patients with diarrhea for more than 24 hours despite treatment with loperamide.17 Hydration therapy consisting of dextrose 5% water and normal saline administered at a rate of 150 to 300 mL/hr was also administered to match the increase in the patient’s output and maintain total body burden.
Cardiotoxicity is a dose-dependent side effect of fluorouracil, occurring in 1.2% to 18% of cases.18 Cardiotoxic effects may include acute coronary syndrome, cardiomyopathy, vasospastic angina, coronary thrombosis and dissection, malignant arrhythmias, and cardiac death. Our patient had one instance of hypotension and intermittent tachycardia, which resolved with the administration of IV fluids. In addition, 3 beats of NSVT and PVCs were found on an ECG, but did not recur; hence no further intervention was pursued. Calcium channel blockers and nitrates, due to possible vasodilator effects, have been used to resolve fluorouracil-induced cardiotoxic effects.19,20 However, angiotensin-converting enzyme inhibitors have not been shown to be efficacious.21 We recommend continuous ECG monitoring and intervention with vasodilators if sustained cardiotoxic effects are observed. Close surveillance, repletion of electrolytes, and IV hydration may be helpful.
Additional considerations include avoiding medications that could reduce the clearance of fluorouracil. These include cimetidine, metronidazole, and thiazide diuretics. Because of fluorouracil’s strong inhibiting effect on cytochrome P450 2C9 (CYP2C9), we also advise using caution if the patient is receiving medications activated or metabolized by this enzyme (eg, phenytoin and clozapine).21
Conclusion
Fluorouracil overdose is a potentially life-threatening complication of therapy. Safeguards, as recommended by ISMP, should be used whenever possible to minimize the risks for dosing, programming, and pump delivery errors. The management of a fluorouracil overdose requires a comprehensive approach that extends beyond the initial hospitalization period. Uridine triacetate plays an important role in mitigating the potentially devastating toxicities of a fluorouracil overdose. Because of its orphan drug status, timely procurement and delivery of uridine triacetate is vital. Although antidote administration has improved rates of recovery, it is important for clinicians to continue to monitor the patient closely because of the prolonged/delayed side effects of fluorouracil. Myelosuppression, which may persist after the initial exposure period, may require supportive care with human granulocyte colony-stimulating factors and antibiotics for the prevention of infections. Close monitoring should be exercised during the critical period following a fluorouracil overdose and through the expected neutrophil nadir. Future opportunities include the development and implementation of treatment protocols that would help guide clinicians in providing care quickly, effectively, and consistently from presentation and through recovery.
Author Disclosure Statement
The authors reported no conflicts of interest.
Acknowledgments
Wellstat Therapeutics Corporation was the source for the investigational medication, uridine triacetate.
Drs Andreica, Rozov, and Tavares are Clinical Pharmacists, Department of Pharmacy, Massachusetts General Hospital, Boston, MA. Dr Pfeifer is Clinical Pharmacist, Children’s Hospital, Colorado, Aurora. Dr Shakurova is Clinical Informatics Pharmacist, Partners eCare, Boston, MA. Dr Ortiz is Oncologist, Wentworth-Douglass Hospital, Dover, NH.
References
1. Adrucil (fluorouracil) package insert. Irvine, CA: Gensia Sicor Pharmaceuticals, Inc; 1999.
2. National Institutes of Health. Public teleconference regarding licensing and collaborative research opportunities for: methods and compositions relating to detecting dihydropyrimidine dehydrogenase (DPD). Fed Regist. 2008;73:38233.
3. Institute for Safe Medication Practices. Fluorouracil error ends tragically, but application of lessons learned will save lives. www.ismp.org/newsletters/acutecare/articles/ 20070920.asp. Published September 20, 2007. Accessed February 4, 2015.
4. Bamat MK, Tremmel R, O’Neil JD, et al. Uridine triacetate: an orally administered, life-saving antidote for 5-FU overdose [ASCO abstract 9084]. J Clin Oncol. 2010; 28(suppl 15).
5. Bamat MK, Tremmel R, Helton J, et al. Clinical experience with uridine triacetate for 5-fluorouracil overexposure: an update. Ann Oncol. 2013;24(suppl 4):iv71.
6. Wellstat Therapeutics. Uridine triacetate as antidote for patients at excess risk of 5-FU toxicity due to overdosage or impaired elimination, In: ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine; 2014. www.clinicaltrials.gov/ct2/show/NCT01432301. NLM Identifier NCT01432301.
7. Fox A, Howland M. Vistonuridine®: a novel antidote for 5-fluorouracil. Toxicology letter. 2010;15. www.upstate.edu/poison/pdf/tox_newsletter/01_10toxnews.pdf. Accessed June 4, 2014.
8. Daniele B, Perrone F, Gallo C, et al. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: a double blind, placebo controlled, randomised trial. Gut. 2001;48:28-33.
9. Gill S. Management of 5-fluorouracil (5FU) infusion overdose at the BCCA (interim guidance). www.bccancer.bc.ca/NR/rdonlyres/46753FA3-46ED-4AD4-A450-40A1371E4BDD/49369/5FUInfusorOverdoseManagementGuideline_1Feb2011.pdf. Published February 1, 2011. Accessed February 20, 2015.
10. McEvilly M, Popelas C, Tremmel B. Use of uridine triacetate for the management of fluorouracil overdose. Am J Health Syst Pharm. 2011;68:1806-1809.
11. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330-338.
12. Wellstat Therapeutics. Uridine triacetate (formerly known as vistonuridine) and 5-FU overexposure. www.wellstattherapeutics.com/therapeutics/html/randd/compounds/ visto-5fu.html. Accessed February 20, 2014.
13. US Food and Drug Administration. FDA drug safety communication: new information regarding QT prolongation with ondansetron (Zofran). www.fda.gov/Drugs/DrugSafety/ucm310190.htm. Updated February 15, 2013. Accessed June 9, 2014.
14. Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427-433.
15. NutreStore (L-glutamine powder for oral solution) package insert. Torrance, CA: Emmaus Medical, Inc; 2008.
16. McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009;33:277-316.
17. Benson AB III, Ajani JA, Catalano RB, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004;22:2918-2926.
18. Sorrentino MF, Kim J, Foderaro AE, et al. 5-Fluorouracil induced cardiotoxicity: review of the literature. Cardiol J. 2012;19:453-458.
19. Farina A, Malafronte C, Valsecchi M, et al. Capecitabine-induced cardiotoxicity: when to suspect? how to manage? a case report. J Cardiovasc Med. 2009;10:722-726.
20. Sentürk T, Kanat O, Evrensel T, et al. Capecitabine-induced cardiotoxicity mimicking myocardial infarction. Neth Heart J. 2009;17:277-280.
21. Fluorouracil. Lexi-Comp Online [database online]. Hudson, OH: Lexi-Comp, Inc. June 9, 2014.