Carboplatin is a platinum chemotherapeutic agent introduced in 1981 as an alternative to cisplatin. The 2 agents have similar antitumor activity and spectrum.1 Although carboplatin’s degree of activity depends heavily on the disease state, it is frequently used because of its documented activity against a broad range of tumors.2 Although not proved efficacious for some disease states, the advantage of carboplatin compared with cisplatin is a decrease in nonhematologic toxicities (eg, nephrotoxicity, ototoxicity, and peripheral neurotoxicity).1 The dose-limiting toxicity is bone marrow suppression, specifically thrombocytopenia.
Although carboplatin does not cause significant renal toxicity, pretreatment renal function is an important factor in the severity of carboplatin-induced thrombocytopenia.1 Approximately 70% of an administered carboplatin dose is eliminated in the urine, and the renal clearance is closely correlated with glomerular filtration rate (GFR).3,4 This suggests that the renal excretion of the drug is exclusively by glomerular filtration.1
The area under the concentration/time curve (AUC) for carboplatin is determined primarily by the pretreatment GFR. The AUC determines toxicity of carboplatin.1 Therefore, the formula used most often in clinical practice to dose carboplatin, the Calvert formula, takes into account the patient’s GFR and desired AUC.5 This formula—dose (mg) = AUC (mg/mL-1 min) × [GFR (mL/min) + 25 (mL/min)]—has proved to be a reliable tool to calculate the optimal dose of carboplatin in retrospective and prospective studies.5
Although the Calvert analysis used chromium 51-ethylenediaminetetra-acetic acid measurements because of its ability to directly measure GFR,1 prediction of GFR for use in the Calvert formula is often accomplished by estimating the creatinine clearance (CrCl) using the Cockcroft-Gault formula. The Cockcroft-Gault formula uses variables such as age, weight, sex, and serum creatinine (SCr). In clinical practice, patients may have low SCr values that are not always accurate representations of their renal function. For example, in elderly patients who have decreased muscle mass, the corresponding decreased SCr could be misinterpreted as better-than-actual renal function.6
There is concern about the accuracy of CrCl estimates when these low SCr values are used in the Cockcroft-Gault formula.7 Some institutions round low values up to 1 mg/dL to prevent overestimation of renal function.7 Controversy exists regarding how to properly calculate CrCl values, and therefore dose carboplatin by using the Calvert formula for patients with an SCr
The objective of this study was to determine the tolerability of carboplatin based on adverse events, including nausea, vomiting, and myelosuppression, for patients with SCr values
Methods
Study Patients
Patients receiving a carboplatin-containing chemotherapy regimen were identified through pharmacy chemotherapy records. Patients were included in the study if they initiated their first cycle of carboplatin-containing chemotherapy and received their first dose of carboplatin as an inpatient between January 1, 2004, and December 31, 2009. Additional inclusion criteria were SCr <1 mg/dL, carboplatin dose determined using the Calvert formula, CrCl calculated using the Cockcroft-Gault formula, and patients receiving 100% of the calculated dose.
Exclusion criteria were SCr ≥1 mg/dL, carboplatin dose determined using any method other than the Calvert formula, and CrCl calculated using any method other than the Cockcroft-Gault formula. Patients were excluded if they initiated and received their first dose of carboplatin-containing chemotherapy outside of the institution or in the outpatient setting but received subsequent doses as an inpatient during the study period. Those who initiated carboplatin therapy as an outpatient were excluded primarily as a result of the limited availability of outpatient medical records to accurately access tolerability.
Patients meeting the inclusion criteria were divided into 3 groups based on the practices observed at our institution. The first group included patients whose CrCl was calculated using the actual SCr value (ie, the actual SCr group). The remaining 2 groups included patients whose CrCl was calculated using a rounded-up SCr value: 1 group used a rounded SCr value of 1 mg/dL (ie, the SCr 1 group) and 1 group used a rounded SCr value of 0.8 mg/dL (ie, the SCr 0.8 group).
For all patients, the decision to use the actual SCr value or a rounded SCr value was made by the prescribing oncologist. This in turn dictated the placement of patients within the 3 study groups. Institutional Review Board approval was obtained before initiation of the study.
Data Collection and Analysis
This study was a retrospective chart review. Demographic data (ie, age, sex, weight, height, and diagnosis) were collected for all patients included in the study. Information regarding the chemotherapy regimen, antiemetic regimen, and use of granulocyte colony-stimulating factor (GCSF) was also collected.
The incidence of nausea within the first 24 hours of carboplatin administration as noted in the medical record was recorded. The number of times rescue antiemetics were administered to the patients and the number of emetic episodes charted were collected from pharmacy and medical charts to assess acute nausea and vomiting (within the first 24 hours of carboplatin administration).
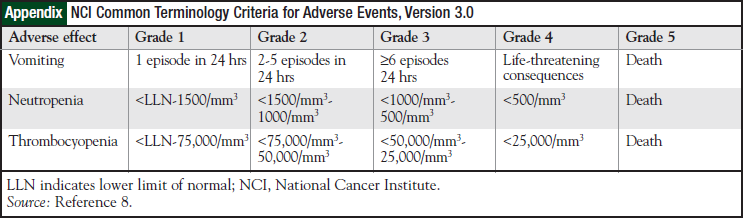
Laboratory records were reviewed for all patients, and nadir platelet and nadir neutrophil counts were recorded to assess bone marrow toxicity. The severities of bone marrow suppression and emesis were graded using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events, Version 3.0 (Appendix).8 The date of cycle 2 was collected to assess for delays in treatment.
End Points
The study end points were the incidence of nausea, frequency of rescue antiemetic use, and incidence and severity of emesis, thrombocytopenia, and neutropenia. Treatment delays between cycle 1 and cycle 2 of carboplatin were also evaluated.
Statistical Analysis
A 1-way analysis of variance was used for parametric data, including age, height, weight, body surface area, body mass index (BMI), CrCl, dose, platelet and neutrophil nadirs, and treatment delays. The Kruskall-Wallis test was used for nonparametric data and data that were not normally distributed (ie, SCr and AUC). The chi-square test was used for categorical data, including sex, scheduled antiemetics, GCSF use, diagnosis, chemotherapy regimen, rescue antiemetic use, and incidence and grade of nausea, emesis, thrombocytopenia, and neutropenia. A Tukey posttest was performed as needed (ie, only if P <.05). Differences were considered statistically significant if P <.05.
Results
Patients
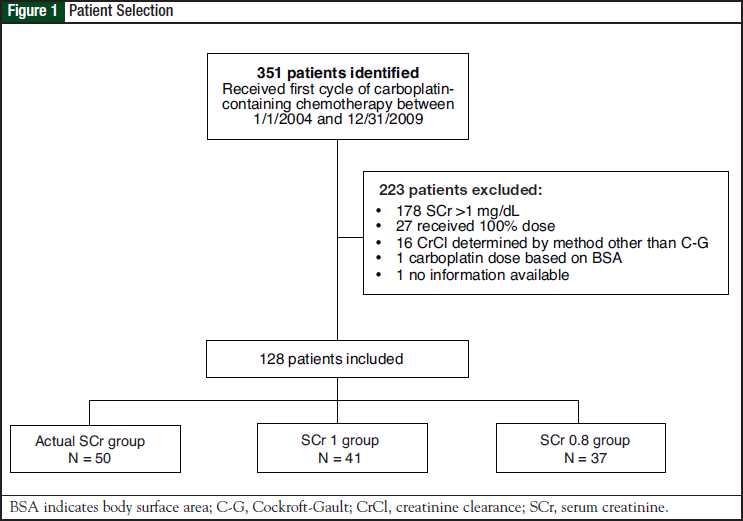
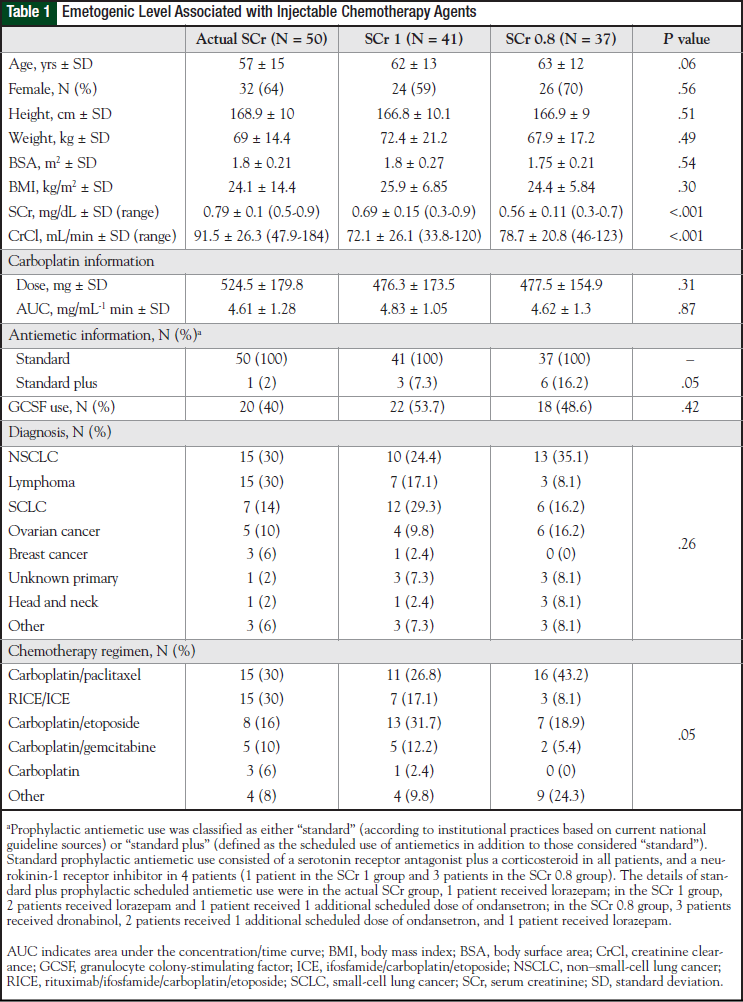
A total of 351 patients were identified who received their first cycle of carboplatin-containing chemotherapy as an inpatient between January 1, 2004, and December 31, 2009. Of these, 128 patients were included in the study and were divided into 3 groups: actual SCr group (N = 50), SCr 1 group (N = 41), and SCr 0.8 group (N = 37). The reasons for patient exclusion are shown in Figure 1. Baseline characteristics of the patient population are shown in Table 1.
Many demographic characteristics were similar between the groups, although some differences were observed. Diagnoses were numerically different among the groups, but this difference was not significant (P = .26). In addition, variation was noted with respect to chemotherapeutic regimens and the use of more than standard antiemetics between groups, differences that approached significance (P = .05 for each comparison).
Although baseline SCr significantly differed among the 3 groups (P <.001), and the calculated CrCl was significantly different between the actual SCr and SCr 1 groups and the actual SCr and SCr 0.8 groups (P <.001), carboplatin doses did not differ significantly (P = .31) among the 3 groups.
Nausea and Emesis
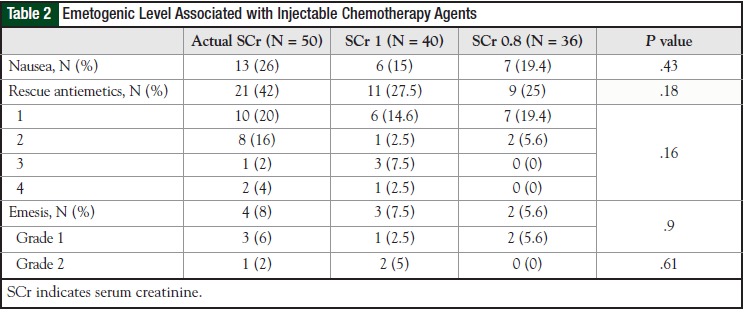
Results for nausea and emesis were available for all 50 patients in the actual SCr group, for 40 patients in the SCr 1 group, and for 36 patients in the SCr 0.8 group. The incidence results for nausea and emesis are summarized in Table 2. Nausea and emesis data were not available for 1 patient in the SCr 1 group and for 1 patient in the SCr 0.8 group. There was no significant difference among the 3 groups in nausea (P = .43) or in rescue antiemetic use (P = .18). Differences in emesis did not reach significance (P = .9). No patient in any group experienced grade 3 or 4 emesis.
Thrombocytopenia and Neutropenia
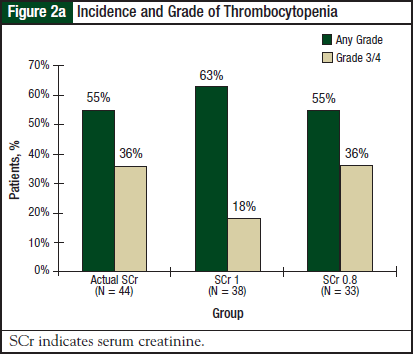
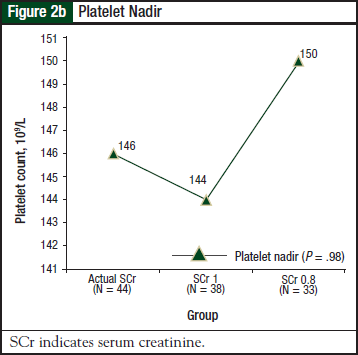
Results for thrombocytopenia and neutropenia were available for 44 patients in the actual SCr group, 38 patients in the SCr 1 group, and 33 patients in the SCr 0.8 group. Results for thrombocytopenia are shown in Figure 2a and Figure 2b.
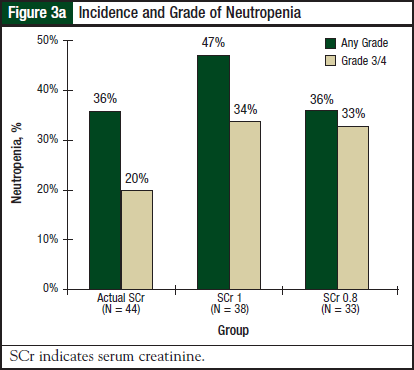
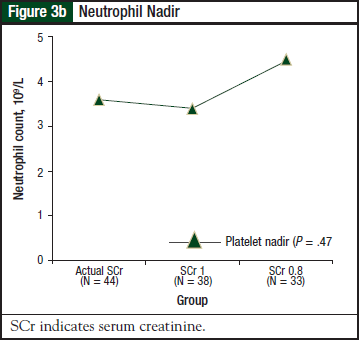
There was no significant difference in the incidence or severity of thrombocytopenia (P = .68) or platelet nadir (P = .98) among the 3 groups. Results for neutropenia are shown in Figure 3a and Figure 3b. There was no significant difference in the incidence or severity of neutropenia (P = .53) or neutrophil nadir (P = .47) among the 3 groups.
Treatment Delays
Results for treatment delays were available for 33 patients in the actual SCr group, 25 patients in the SCr 1 group, and 23 patients in the SCr 0.8 group. The average number of days that cycle 2 of carboplatin was delayed was 2.2 days (± 4 days) in the actual SCr group, 4.8 days (± 8.9 days) in the SCr 1 group, and 4.2 days (± 7.1 days) in the SCr 0.8 group (P = .42).
Discussion
Our results indicate that the tolerability of carboplatin was unchanged when the dose was calculated using the actual SCr value versus the rounded SCr value. The prescriber’s decision to use actual SCr values rather than rounded values to calculate CrCl and subsequently dose carboplatin using the Calvert formula appeared to be largely influenced by the patient’s baseline SCr values. This is evident from the finding that patients with an SCr closer to 1 mg/dL were more likely to have CrCl calculated using their actual SCr value (average baseline SCr in the actual SCr group,
Despite significant differences in baseline SCr, the calculated CrCl did not differ between the SCr 1 and SCr 0.8 groups. The differences were significantly different between the actual SCr and SCr 1 groups (average CrCl, 91.5 mL/min and 72.1 mL/min, respectively; P <.001), and between the actual SCr and the SCr 0.8 groups (average CrCl, 91.5 mL/min and 78.7 mL/min, respectively; P <.05).
Although the CrCl varied slightly among the groups, these differences were not substantial enough to translate into significantly dissimilar carboplatin doses (average doses, 524.5 mg, 476.3 mg, and 477.5 mg for the actual SCr, SCr 1, and SCr 0.8 groups, respectively). This similarity in dose could account for the observed similarity in tolerability. Indeed, the data would indicate that for patients with an SCr of approximately 0.8 mg/dL to 1 mg/dL, despite small differences in calculated CrCl, the resultant carboplatin dose would not be expected to differ significantly, and therefore no increased toxicity would be anticipated.
Although it seems illogical that significant differences in SCr and CrCl would not result in significant differences in carboplatin doses when the AUC is similar between groups, this scenario can be explained. Both CrCl and AUC contribute to the carboplatin dose calculated by use of the Calvert formula; however, differences in AUC influence the carboplatin dose to a greater extent than CrCl, because the AUC is a multiplier whereas the CrCl is additive.
The difference in CrCl between the actual SCr and the SCr 1 groups is 26.9%, which decreases to 19.9% when the constant of 25 is added. Although not statistically different, the average AUC is 4.4% higher in the SCr 1 group than in the actual SCr group. These 2 factors tend to move the groups closer to each other and make the differences in carboplatin doses insignificant.
The same explanation can be applied to the observed carboplatin doses in the actual SCr and the SCr 0.8 groups. Interpretation and application of the study results are more difficult for patients with a baseline SCr of <0.8 mg/dL, although the approach at our institution of using a rounded SCr value of 0.8 mg/dL for calculating CrCl results in carboplatin doses that are well tolerated.
Limitations
There were several limitations to this study. The small sample size may have compromised the statistical power to detect meaningful differences between groups. The retrospective analysis posed limitations in the availability of medical records and documentation of tolerability information, causing a loss of some patients in the tolerability analysis. In addition, for the end point involving treatment delays in cycle 2 of carboplatin, although it was presumed that these delays were related to carboplatin toxicity, it was not feasible to determine the exact reasons for treatment delays.
The various diagnoses and chemotherapy regimens made for a very heterogeneous group of patients, which could be considered a limitation, because these factors may affect carboplatin tolerability in different ways. Although not significantly different, there were numeric differences in CSF use among the groups. Patients who received CSF would be expected to have higher neutrophil nadir counts. It is unknown how this affects the interpretation of the neutrophil tolerability results among the 3 groups. Furthermore, more patients in the SCr 0.8 group received additional scheduled antiemetics compared with the other 2 groups, which could confound the nausea and vomiting tolerability results.
The appropriate body weight to use for CrCl calculations using the Cockcroft-Gault formula in obese patients is a known area of controversy. Although not addressed in this study, the majority of patients had their doses calculated using actual body weight. An adjusted body weight was used in a small number of patients who were considered overweight or obese.
The overall study population could be described as normal in size, with an average weight of
A disadvantage of the Calvert formula is that the GFR must be assayed, involving inconvenient and invasive methods. Several studies have used CrCl in place of GFR in the Calvert formula, determined either by a 24-hour urine collection with the SCr or by another method.5
The formula that best estimates CrCl for dosing carboplatin is not clear. In our study, CrCl was estimated using the Cockcroft-Gault formula, which has been validated as a reliable estimation of CrCl in patients with cancer.9 Jelliffe and Chatelut formulas have been studied as well, with varying results.2,10-12
Recently, the US Food and Drug Administration (FDA) in conjunction with the NCI/Cancer Therapy Evaluation Program informed oncology practitioners about the potential for inadvertent carboplatin overdosing.13 This concern resulted from a change in the method of the SCr measurement by laboratories in the United States that was to be completed by the end of 2010. The new isotope dilution mass spectrometry method would appear to underestimate the SCr values compared with older methods, especially when the SCr is low.
To avoid toxicity, the FDA recommended to limit the GFR used in the Calvert formula to
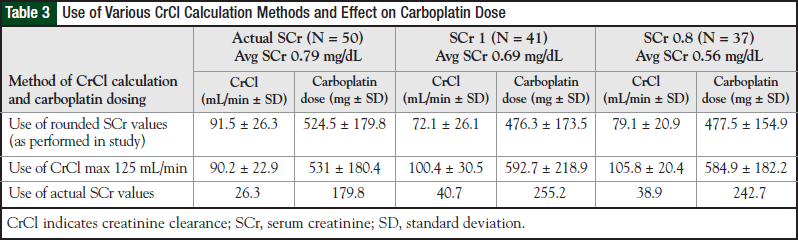
To investigate what effect the use of this strategy would have on the existing study data, a post hoc analysis was conducted. Compared with the doses administered to patients, application of a maximum CrCl of 125 mL/min would result in higher average doses with greater deviation in the SCr 1 and SCr 0.8 groups (approximately 24% and 22% higher, respectively).
To provide perspective, had the actual SCr values been used to calculate CrCl, carboplatin doses would be approximately 32% higher in the SCr 1 group and 34% higher in the SCr 0.8 group (approximately 6% and 10% higher, respectively, than the doses achieved by using a maximum CrCl of 125 mL/min). Details of this analysis are presented in Table 3. Although the doses achieved differ greatly, application of a maximum CrCl of 125 mL/min could represent an alternative to using a rounded SCr value for CrCl and carboplatin dose calculations.
Conclusions
Future studies will need to address the effect of rounded SCr values in patients with
This scenario, however, is not what is observed in practice at our institution, because baseline SCr was found to dictate carboplatin prescribing practices. Results of such studies would complement the results of this study and give prescribers a clearer answer on how to best estimate CrCl and dose carboplatin in these patients.
For prescribers who are questioning how to calculate CrCl in patients with low SCr, the results from this study suggest that for patients with a baseline SCr as low as 0.8 mg/dL, using the actual SCr will yield a similar carboplatin dose and will not result in decreased tolerability compared with rounding SCr to 1 mg/dL. Future studies are needed to clarify the appropriate dosing strategy for patients with a baseline SCr <0.8 mg/dL, as well as the effect of using rounded SCr values on carboplatin efficacy.
Acknowledgement
The authors would like to thank John M. Koerber, PharmD, for his assistance with the statistical analysis.
Author Disclosure Statement
Dr Mazloom and Dr Bestul have reported no conflicts of interest.
References
- Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748-1756.
- Donahue A, McCune JS, Faucette S, et al. Measured versus estimated glomerular filtration rate in the Calvert equation: influence on carboplatin dosing. Cancer Chemother Pharmacol. 2001;47:373-379.
- Harland SJ, Newell DR, Siddik ZH, et al. Pharmacokinetics of cis-diammine-1,1-cyclobutane dicarboxylate platinum(II) in patients with normal and impaired renal function. Cancer Res. 1984;44:1693-1697.
- Gaver RC, Colombo N, Green MD, et al. The disposition of carboplatin in ovarian cancer patients. Cancer Chemother Pharmacol. 1988;22:263-270.
- van Warmerdam LJ, Rodenhuis S, ten Bokkel Huinink WW, et al. The use of the Calvert formula to determine the optimal carboplatin dosage. J Cancer Res Clin Oncol. 1995;121:478-486.
- Charleson HA, Bailey RR, Stewart A. Quick prediction of creatinine clearance without the necessity of urine collection. N Z Med J. 1980;92:425-426.
- Dooley MJ, Singh S, Rischin D. Rounding of low serum creatinine levels and consequent impact on accuracy of bedside estimates of renal function in cancer patients. Br J Cancer. 2004;90:991-995.
- National Cancer Institute. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 3.0. August 9, 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf#search=.” Accessed February 15, 2012.
- Robinson BA, Frampton CM, Colls BM, et al. Comparison of methods of assessment of renal function in patients with cancer treated with cisplatin, carboplatin or methotrexate. Aust N Z J Med. 1990;20:657-662.
- Okamoto H, Nagatomo A, Kunitoh H, et al. Prediction of carboplatin clearance calculated by patient characteristics or 24-hour creatinine clearance: a comparison of the performance of three formulae. Cancer Chemother Pharmacol. 1998;42:307-312.
- van Warmerdam LJ, Rodenhuis S, ten Bokkel Huinink WW, et al. Evaluation of formulas using the serum creatinine level to calculate the optimal dosage of carboplatin. Cancer Chemother Pharmacol. 1996;47:266-270.
- Nagao S, Fujiwara K, Imafuku N, et al. Difference of carboplatin clearance estimated by the Cockcroft-Gault, Jelliffe, Modified-Jelliffe, Wright or Chatelut formula. Gynecol Oncol. 2005;99:327-333.
- US Food and Drug Administration Center for Drug Evaluation and Research. About FDA. Carboplatin dosing. Updated October 8, 2010. www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm228974.htm. Accessed July 7, 2011.