Pharmacists play a vital role in ensuring the safe administration of medications. The US Food and Drug Administration (FDA) has issued a boxed warning for anti-CD20 monoclonal antibodies concerning the risk for reactivation of hepatitis B virus (HBV) infection resulting in fulminant hepatitis, hepatic failure, or death with the administration of these medications.1-4 HBV is a life-threatening infection that affects approximately 240 million people globally.5 Furthermore, approximately 780,000 people die from complications associated with this illness annually.5 For these reasons, all patients who receive rituximab, ofatumumab, or obinutuzumab should be screened for HBV infection via serologic markers, including hepatitis B surface antigen (HBsAg) and total hepatitis B core antibody (anti-HBc) to determine HBV infection status before the administration of anti-CD20 monoclonal antibodies.6,7
Approximately 59% to 80% of patients who are HBsAg-positive (ie, those with acute or chronic HBV infection) and receive the R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone/prednisolone) regimen or similar immunosuppressive regimens that include anti-CD20 monoclonal antibody experience reactivation of HBV infection if no viral prophylaxis is used.6,8 The rate of patients who are HBsAg-negative but are total anti–HBc-positive as a result of resolved HBV infection and experience infection reactivation is between 9% and 41.5%.6,9 Antiviral therapy should be considered for patients who screen positive for HBV exposure to prevent viral reactivation and the associated consequences.6,10
The purpose of this study was to compare the rate of HBV screening within 1 year before administration of the first dose of anti-CD20 monoclonal antibodies before and after the implementation of pharmacist-driven educational and technologic intervention at Augusta University Medical Center, GA. The FDA added a boxed warning to the prescribing information of anti-CD20 monoclonal antibodies in September 2013.3,4 Therefore, this study was performed to ensure that this institution was following safe practices associated with the administration of these drugs.
This study is part of the institution’s Medication Use Evaluation and Improvement Program and is not considered a human subjects research by the Augusta University Medical Center Institutional Review Board.
Methods
From September to October 2014, hematology/oncology pharmacists at Augusta University Medical Center were formally educated on the warning regarding the risk for HBV reactivation with the administration of anti-CD20 monoclonal antibodies as noted in the prescribing information in addition to education on the recommended screening tests. Furthermore, information technology pharmacists added a pop-up alert to the pharmacy verification software, which prompted pharmacists to confirm correct HBV screening before verifying the first dose of an anti-CD20 monoclonal antibody for administration. If the verifying pharmacist determined that the HBV screening was incomplete, the pharmacist was to then contact the provider and recommend the appropriate laboratory tests.
The educational intervention included a lecture and an associated slideshow presented by a PGY-2 hematology/oncology pharmacy resident. Pharmacists at all staffing locations, including any pharmacist involved in the dispensing of inpatient or outpatient anti-CD20 monoclonal antibodies, received the educational intervention. No required knowledge assessment was used
to assess the pharmacists’ understanding of the newly presented information. To assess the impact of the educational intervention and the pop-up alert, a retrospective chart review was performed on all patients who received a first dose of rituximab, ofatumumab, or obinutuzumab.
Charts were reviewed to determine whether HBsAg and total anti-HBc levels were drawn within 1 year before the first dose. Although the FDA recommends screening all patients for HBV before the initiation of anti-CD20 monoclonal antibodies, the FDA does not recommend a time period in which this screening should be done. We chose a time frame of 1 year before administration of the first dose as the screening window, because it was determined to be a reasonable time frame during which a clinician would consider a laboratory value relevant.
HBV screening test results were performed in the laboratory at Augusta University Medical Center and were reported in the electronic health record by the laboratory. The data were compared between 2 cohorts: a preintervention group consisting of patients who received the first dose between December 1, 2013, and August 31, 2014, and a postintervention group consisting of patients who received the first dose between November 1, 2014, and July 1, 2015. Patients receiving second or later doses of anti-CD20 monoclonal antibodies were excluded.
A power calculation was not performed, because the number of patients who received these drugs at this institution was limited and could not be changed. Because the FDA-mandated warning about the risk for HBV with monoclonal antibodies was issued in September 2013,4 the study was limited in the time span that could be covered.
The primary objective of this study was the rate of correct screening for HBV before and after the implementation of pharmacist-driven educational and technologic changes. The secondary objective was to evaluate the rate of HBV among patients at the Augusta University Medical Center who received anti-CD20 monoclonal antibodies. Chi-square and Fisher’s exact tests were performed to evaluate screening rates between the 2 cohorts as appropriate.
Results
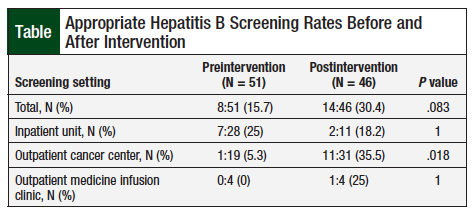
A total of 97 patients were assessed for the use of screening for HBV infection before administration of the first dose of anti-CD20 monoclonal antibodies. The study included 51 patients who received their first dose preintervention, and 46 patients who received the first dose postintervention. Appropriate HBV screening was performed in 15.7% (N = 8) of patients in the preintervention group and 30.4% (N = 14) of patients in the postintervention group (P = .083; Table).
More specifically, the rate of screening improved in the outpatient cancer center and in the outpatient medicine infusion clinics after the pharmacist intervention. The rate of screening in the outpatient cancer center increased from 5.3% before intervention to 35.5% after intervention. The rate in the noncancer outpatient medicine infusion clinic increased from 0% before intervention to 25% after intervention (Table). The correct screening rate on the inpatient units decreased from 25% to 18.2% before and after the intervention, respectively. Approximately 47% of the patients received anti-CD20 monoclonal antibodies at this medical center for the treatment of non-Hodgkin lymphoma (Figure).
The results revealed that 22 of the total 97 patients were appropriately screened for HBV before receiving anti-CD20 monoclonal antibodies. Of these patients, 20 were HBV-negative and 2 patients had total anti-HBc–positive and HBsAg-negative results, which likely indicate a resolved HBV infection, with the potential for reactivation.
Discussion
After the pharmacist-driven educational and technologic intervention, the incidence of appropriate screening for HBV in patients receiving the initial dose of anti-CD20 monoclonal antibodies at Augusta University Medical Center nearly doubled, from 15.7% to 30.4%. However, the improvement in correct screening rates was lower than anticipated; therefore, there is much room for improvement.
The overall correct HBV screening rate increased as a result of large improvements in the outpatient setting. Screening rates increased by approximately 30% in the outpatient cancer center and by 25% in the outpatient medicine infusion center. Although screening improved in both outpatient clinics, the difference in overall screening was not statistically significant between the preintervention and postintervention groups. The 25% increase in appropriate screening rates within the outpatient cancer center was found to be statistically significant (0% vs 25%, respectively; P = .0184). However, there were only 4 patients in each group.
Conversely, the rate of correct screening in the inpatient unit decreased by approximately 7%. Only 11 patients received monoclonal antibodies in the inpatient units after the intervention, and only 2 of those patients were screened correctly. In the entire postintervention group, 29 patients were tested for HBV status with HBsAg and HBV core immunoglobulin (Ig)M antibody instead of total anti-HBc. The presence of HBV core IgM antibody indicates acute infection, but it is not present in patients with resolved or chronic infection. Testing for total anti-HBc (which includes IgM and IgG) or HBV core IgG antibodies is important to detect patients with resolved or chronic infection.
The finding that a large percentage of patients were tested for HBV core IgM and not for total anti-HBc indicates that there was likely a misunderstanding regarding the total anti-HBc test, and that the pop-up alert for pharmacists should be more specific. To further improve screening rates, more efforts will need to be aimed at educating pharmacists about the details of the required laboratory tests. Other suggestions for improvement include using a multidisciplinary approach to review the pharmacy pop-up alert as well as creating an order set that includes all of the correct laboratory screening tests.
Finally, the postintervention group included patients who received the first dose of a monoclonal antibody immediately after November 1, 2014. Educational and technologic changes were made shortly before this, in September and October 2014. This quick turnaround between the intervention and the postintervention cohorts’ cut-off period might have contributed to a lower screening rate than expected, because the education and changes may have needed more time to take effect than this study allowed.
There are no set guidelines on how far in advance HBV screening should be done before administration of the first dose of anti-CD20 monoclonal antibodies. In our retrospective chart review, HBV screening was considered appropriate if performed within 1 year before the initial dose, because it was determined that this was a clinically reasonable time frame. Screening was only considered appropriate if it was performed within the 1 year before administration of anti-CD20 monoclonal antibodies; therefore, some patients might have been considered “not screened” when they were in fact screened more than 1 year before receiving the first dose. In addition, it is unclear whether patients who restart using anti-CD20 monoclonal antibodies should be rescreened.
Pharmacists should document any initiative taken to perform HBV screening in the electronic health record, especially if the primary care team does not accept this screening recommendation. Such documentation could potentially increase correct screening rates and promote awareness among healthcare providers of the warning regarding the risk for HBV associated with anti-CD20 monoclonal antibodies. This documentation can allow pharmacists to further identify specific barriers, if any, to this safe medication practice. Pharmacists should educate other pharmacists and providers who are prescribing anti-CD20 monoclonal antibodies on the FDA warning related to these drugs in detail.
The initiation of an institution-specific protocol authorizing pharmacists to order HBV laboratory panels before the initiation of anti-CD20 monoclonal antibodies could also be considered. Such a protocol could include steps for additional HBV workup in patients who screen positive for HBV, as well as steps for appropriate prophylaxis. These additional strategies to increase the rate of HBV screening before the administration of the first dose of anti-CD20 monoclonal antibodies should be addressed at Augusta University Medical Center.
Limitations
This study has several limitations. The preintervention group included more inpatients than the postintervention group. This imbalance could possibly explain why the overall difference did not reach significance even though the screening rate improved in 2 of the 3 areas. In addition, the study’s small sample size could explain why significance was not achieved, because a power calculation was not performed.
To better assess the screening strategy for HBV before anti-CD20 monoclonal antibody administration, in addition to the results of HBV screening, there should be a designated location in the electronic health record for pharmacists to document whether HBV screening was performed and if any interventions were made.
This lack of pharmacist documentation limited our ability to understand why screening may not have been performed in the above cases. There is a place for HBV status to be recorded in the electronic health record, but there was not a designated place for the pharmacist to record whether he or she checked for HBV status, and whether any intervention was performed, such as contacting the prescriber.
Other limitations associated with a retrospective study design exist, including missing data for laboratory tests done outside of the medical center.
Conclusion
With the administration of drugs that are associated with serious or life-threatening risks, especially those with FDA-mandated boxed warnings in the drug’s prescribing information, pharmacists play a vital, ongoing role in improving medication safety. Pharmacists can play a key role in identifying patients who are at risk for HBV reactivation caused by anti-CD20 monoclonal antibody therapy, and can promote appropriate prophylactic strategies. Pharmacist education and process changes should be performed regularly to promote optimal effects, and additional education may be warranted.
Author Disclosure Statement
Dr Hodgson, Dr Shah, and Dr Clark have no conflicts of interest to report.
References
- Rituxan (rituximab) prescribing information. South San Francisco, CA: Genentech, Inc; 2014. www.gene.com/download/pdf/rituxan_prescribing.pdf. Accessed March 6, 2016.
- Arzerra (ofatumumab) prescribing information. Research Triangle Park, NC: GlaxoSmithKline; 2009. www.accessdata.fda.gov/drugsatfda_docs/label/2009/125326lbl.pdf. Accessed March 6, 2016.
- Gazyva (obinutuzumab) prescribing information. South San Francisco, CA: Genentech, Inc; 2016. www.gene.com/download/pdf/gazyva_prescribing.pdf. Accessed March 6, 2016.
- US Food and Drug Administration. FDA drug safety communication: boxed warning and new recommendations to decrease risk of hepatitis B reactivation with the immune-suppressing and anti-cancer drugs Arzerra (ofatumumab) and Rituxan (rituximab). September 25, 2013. www.fda.gov/drugs/drugsafety/ucm366406.htm. Accessed March 6, 2016.
- Sundaram V, Kowdley K. Management of chronic hepatitis B infection. BMJ. 2015;351:h4263.
- Kusumoto S, Tobinai K. Screening for and management of hepatitis B virus reactivation in patients treated with anti-B-cell therapy. Hematology Am Soc Hematol Educ Program. 2014;2014:576-583.
- Hwang JP, Somerfield MR, Alston-Johnson DE, et al. Hepatitis B virus screening for patients with cancer before therapy: American Society of Clinical Oncology provisional clinical opinion update.
J Clin Oncol. 2015;33:2212-2220. - Yang F, Zhu HL, He C, et al. Effect of antiviral prophylaxis strategy for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma patients with hepatitis B virus infection: a retrospective cohort study. Indian J Hematol Blood Transfus. 2014;30:97-104.
- Seto WK, Chan TS, Hwang YY, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014;32:3736-3743.
- Villadolid J, LaPlant KD, Markham MJ, et al. Hepatitis B reactivation and rituximab in the oncology practice. Oncologist. 2010;15:1113-1121.