Background: In 2011, the first anticytotoxic T-lymphocyte antigen (CTLA)-4 therapy ipilimumab was approved by the US Food and Drug Administration for the treatment of patients with unresectable or metastatic melanoma based on a demonstrated overall survival benefit with this agent in phase 3 clinical trials.
Objectives: To review ipilimumab’s novel mechanism of action, efficacy, and toxicities, and to provide a practical overview of the use of this drug for the management of patients with melanoma.
Discussion: Ipilimumab is unique in its response patterns, toxicities, and activity in subgroups of patients with melanoma. This drug produces responses that differ from the conventional responses that are observed with cytotoxic agents. Therefore, new immune-related response criteria have been proposed to prevent immature withdrawal of therapy with ipilimumab. Across ipilimumab studies, the most frequently affected organs were the gastrointestinal tract and skin; less frequently seen were hepatic, endocrine, and neurologic events. Ipilimumab’s immune related adverse events may be severe and long-lasting, but these are reversible with prompt recognition and early treatment.
Conclusion: The first-in-class anti–CTLA-4 therapy to be approved, ipilimumab has demonstrated efficacy and overall survival benefit in patients with unresectable or metastatic melanoma, but toxicity is a concern. Ipilimumab is currently available as a monotherapy, but current research is expected to help shape the melanoma treatment landscape by establishing the most effective combination regimens, without compromising tolerability.
The outlook for patients with advanced or metastatic melanoma is poor; historical benchmark data from a recent meta-analysis encompassing more than 2000 patients with advanced or metastatic melanoma indicate that only 25% of patients are still alive at 1 year.1 There has been little change in the standard of care for metastatic melanoma for several decades. Dacarbazine, which was approved by the US Food and Drug Administration (FDA) in 1975, remains the only chemotherapy approved for use in this setting. However, single-agent dacarbazine achieved overall response rates of between 5% and 10% in recent phase 3 trials of patients with melanoma, with very few complete responses and no impact on overall survival (OS).2,3 Although not approved by the FDA for this indication, other chemotherapies, including temozolomide, taxanes, and platinum agents, are in clinical use in this setting, with response rates of up to 26%.4-11
Interleukin (IL)-2 was approved by the FDA in 1998 for the treatment of patients with metastatic melanoma. Although durable complete and partial responses are possible, its routine clinical use has been limited for this patient population by low response rates (approximately 16% with high-dose IL-2), lack of consistent improvement on OS, and risk of significant toxicity,12 necessitating that patients receive therapy in the intensive care unit of facilities specializing in the administration of IL-2.
In 2011, 2 new medications were approved by the FDA for patients with advanced melanoma. The first of these, ipilimumab, is a recombinant, human monoclonal antibody (immunoglobulin G1 subtype) that augments antitumor T-cell responses by binding to and neutralizing the cytotoxic T-lymphocyte antigen (CTLA)-4 immune repressor cell-surface receptor on T-cells. Ipilimumab has demonstrated improved OS in 2 phase 3 studies: as a single agent in previously treated patients and in combination with dacarbazine in treatment-naïve patients with unresectable stage III or stage IV melanoma.2,13 In previously treated patients, ipilimumab alone or in combination with the glycoprotein (gp)100 peptide vaccine reduced the risk of death by 34% and 32%, respectively, compared with the gp100 vaccine alone.13 In treatment-naïve patients, the combination of ipilimumab with dacarbazine reduced the risk of death by 28% compared with dacarbazine alone.2
The second therapy approved in 2011 is vemurafenib, a BRAF inhibitor that has antitumor activity in melanoma tumors with a BRAF V600E mutation. Approximately 40% to 60% of melanomas carry this mutation.3 In treatment-naïve patients with metastatic melanoma harboring the BRAF mutation, vemurafenib demonstrated improved 6-month OS compared with dacarbazine (84% vs 64%, respectively) and dramatically higher response rates (48% vs 5%, respectively).3 Although interim 6-month survival data for vemurafenib are encouraging, resistance already has been documented14-20; longer follow-up times will confirm the durability of the initial responses. In contrast, ipilimumab data have a longer follow-up time and show that for some patients, long-term survival can be achieved—on the order of 2 to 3 years for phase 3 studies and up to 4 years for phase 2 studies reported thus far.21,22
Although little information is available regarding whether vemurafenib and ipilimumab use is exclusive to each other, these are 2 very distinct therapies, with different mechanisms of action and nonoverlapping toxicity profiles. As such, the combination of these 2 agents is being evaluated in a phase 1/2 trial in patients with BRAF V600 mutation metastatic melanoma.23 Started in November 2011, the study aims to recruit 50 patients with the primary end point of the phase 1 and phase 2 portions being safety and OS, respectively. This review will focus on ipilimumab, its unique toxicities, and the management of these toxicities.
CTLA-4 as a Therapeutic Target
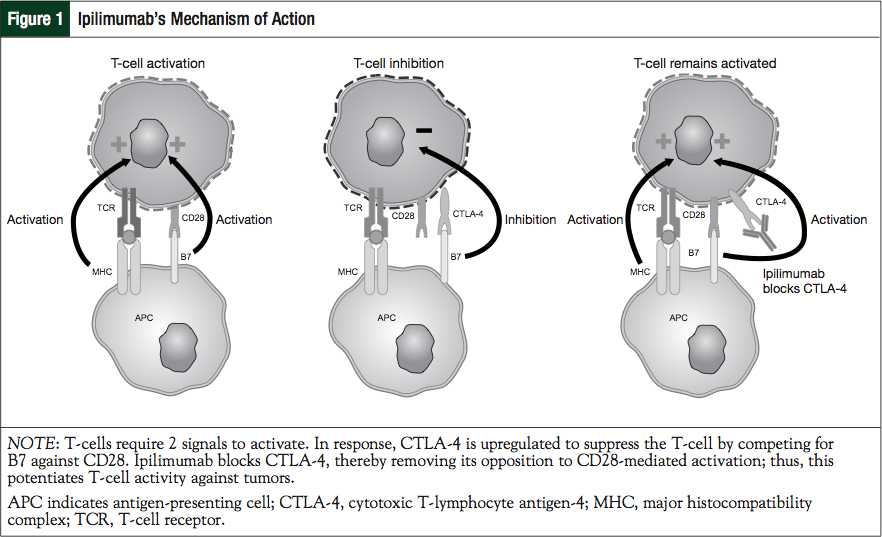
The recognition of CTLA-4’s critical role in immune downregulation led to drug development aimed at targeting this pathway.24 Two signals are required for the activation of a T-cell response. The first occurs when the T-cell binds the antigen presented by the antigen-presenting cell (APC) that is bound to the major histocompatibility complex. At the same time, CD28 interacts with the B7 molecule on the same APC, resulting in CTLA-4 upregulation. CTLA-4 competitively inhibits the binding of B7 to CD28 and therefore acts as a “brake” on the nascent immune response. The importance of this system is dramatically demonstrated in mice devoid of functional CTLA-4, who develop massive CD28-dependent expansion of autoreactive T-cells and die within 3 to 4 weeks as a result of rampant lymphoproliferation and lymphadenopathy. Ipilimumab blocks the CTLA-4 and, therefore, potentiates antitumor T-cell responses (Figure 1). Early studies in mice and primates, and later in humans, demonstrate that ipilimumab competitively binds to CTLA-4 more efficiently (an approximate 100-fold greater affinity) than native B7 while preserving CD28 signaling.25-30
Ipilimumab has demonstrated efficacy and safety in 2 randomized, multicenter, phase 3 trials. In the first study (MDX010-20), 676 previously treated patients with unresectable stage III or IV melanoma were randomized to receive ipilimumab alone (N = 137), ipilimumab in combination with the experimental vaccine gp100 (N = 403), or gp100 alone (N = 136).13 Patients were also required to be human leukocyte antigen (HLA)-A*0201–positive, a requirement based on the mechanism of action of the gp100 vaccine.13
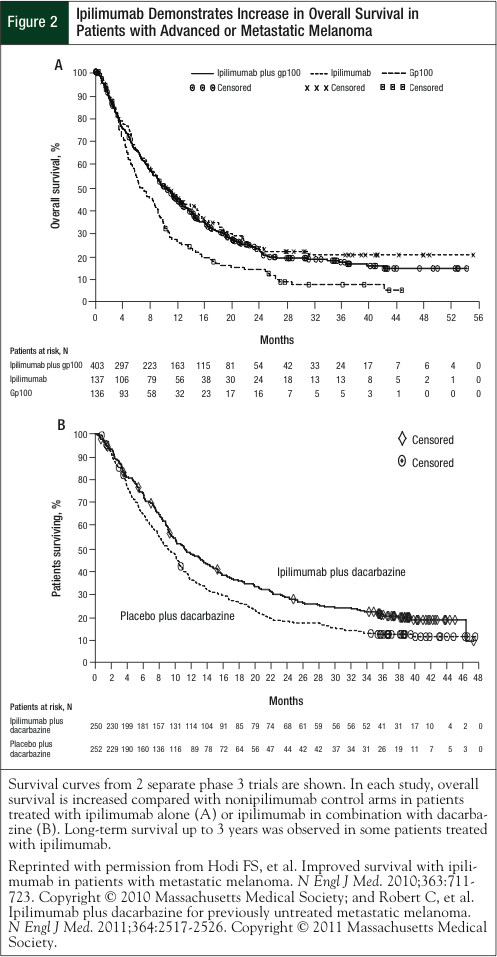
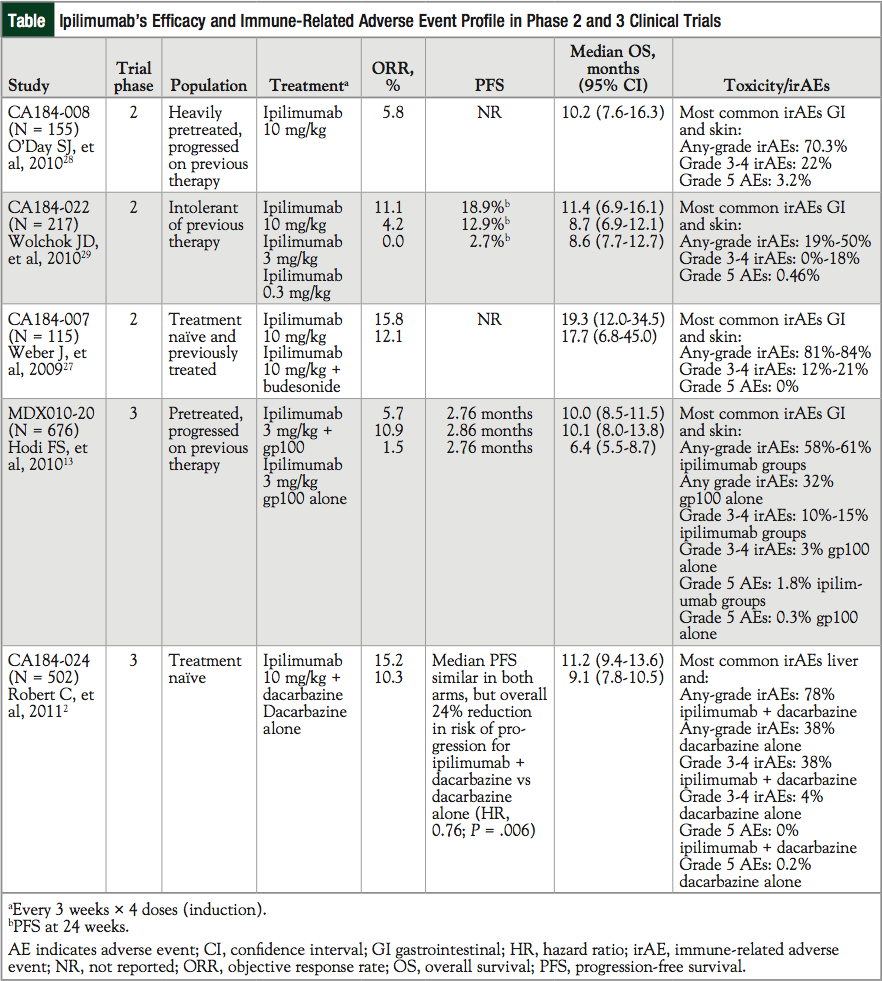
Ipilimumab 3 mg/kg, alone or in combination with gp100, significantly improved median OS versus gp100 alone (10.1 months, 10.0 months, and 6.4 months, respectively).13 No difference in OS was detected between the ipilimumab groups (Figure 2, Table).2,13,27-29 Of note, patients in this study had poor prognosis: more than 70% of patients had visceral metastases and more than 36% had elevated lactate dehydrogenase (LDH) levels, both of which are associated with reduced survival.13 These findings led to the FDA approval of 4 doses of ipilimumab, given at 3 mg/kg on an induction schedule of every 3 weeks for patients with unresectable or metastatic melanoma.
The second study—CA184-024—differed in ipilimumab dose, schedule, and subsequent toxicity profile.2 In this study, 502 treatment-naïve patients with unresectable stage III or stage IV melanoma received either ipilimumab in combination with dacarbazine (N = 250) or dacarbazine alone (N = 252). Ipilimumab was administered at a dose of 10 mg/kg on an induction of every 3 weeks for 4 cycles and included a maintenance phase. Patients with a response or stable disease to ipilimumab were randomized to ipilimumab or to placebo every 12 weeks as maintenance therapy.
Adding ipilimumab to dacarbazine significantly improved OS versus dacarbazine alone (11.2 months vs 9.1 months, respectively; Figure 2, Table).
This trial reported some differences in grade 3 or grade 4 adverse events (AEs) compared with other ipilimumab studies. Gastrointestinal (GI) complications (including GI perforations) and endocrinopathies, which are consistently part of ipilimumab’s toxicity profile, were lower in this trial, and no GI perforations were seen. Although not previously seen, hepatic toxicity was increased, possibly as a result of the higher dose of ipilimumab that was utilized (10 mg/kg) or because of the combination with dacarbazine and its associated hepatotoxicity.2,31 With the exception of hepatic toxicity, no new safety concerns were observed.2
The current scope of ipilimumab use is not restricted to specific patient populations within those with advanced melanoma. There is no HLA requirement,32 and ipilimumab has demonstrated safety and efficacy in both treatment-naïve and previously treated patients (Table).
Ipilimumab is showing interesting activity in patients with melanoma who have brain metastases, distinguishing it from other immunotherapies. This is one of the most difficult-to-treat patient subsets, because the median OS is only approximately 4 months after the diagnosis of brain metastases.33 The MDX010-20 study allowed the inclusion of patients with stable brain metastasis, and subsequent analysis of this subgroup demonstrates disease control in these patients, including responses and stable disease.13 The immune-related AE (irAE) profile in these patients did not differ from those treated patients without brain metastases.34 In addition, patients with advanced melanoma and stable asymptomatic brain metastases who entered the ipilimumab expanded-access program showed prolonged survival and durable responses without an increase in central nervous system (CNS)-related toxicities or unique toxicities.35
Furthermore, recent results from a prospective phase 2 study of patients with melanoma and symptomatic brain metastases showed that prolonged survival and durable responses can occur in some patients with brain metastases. The 1- and 2-year OS rates for patients with asymp- tomatic metastases were 31% and 26%, respectively, whereas respective rates for symptomatic patients were 19% and 10%, respectively. No new safety concerns or increases in CNS-related events were reported.36 A retrospective analysis of a phase 2 trial, which included patients with stable brain metastases, revealed a median OS of 14 months.37 In contrast, previous reports of this patient population who had undergone surgery, radiation, chemotherapy, or a combination resulted in a median OS of 9 months.38 Together, the data are encouraging and are the first to demonstrate the potential for immunotherapy to be effective in patients with melanoma who have brain metastases.
Although the underlying basis of ipilimumab activity in brain metastases is unknown, it is possible that this agent is able to cross the blood-brain barrier, which may be leaky as a result of the tumor, or that ipilimumab-activated T-cells are able to breach the barrier to exert antitumor effects against brain metastases. Steroid treatment is an additional and unknown confounding factor in the decision to administer ipilimumab to patients with brain metastases, because systemic steroids may theoretically blunt an immune response.
Ipilimumab also has similar activity regardless of patient characteristics, such as BRAF mutation status, and is independent of negative prognostic factors, such as advanced-stage disease (M1c), advanced age (>60 years), male sex, or elevated baseline LDH levels.39,40 In at least 1 reported case, a patient with melanoma and brain metastases experienced complete remission after being treated with ipilimumab.41 So far, ipilimumab appears active in a wide cross section of patients with melanoma, albeit with few complete responses.
Patterns of Response
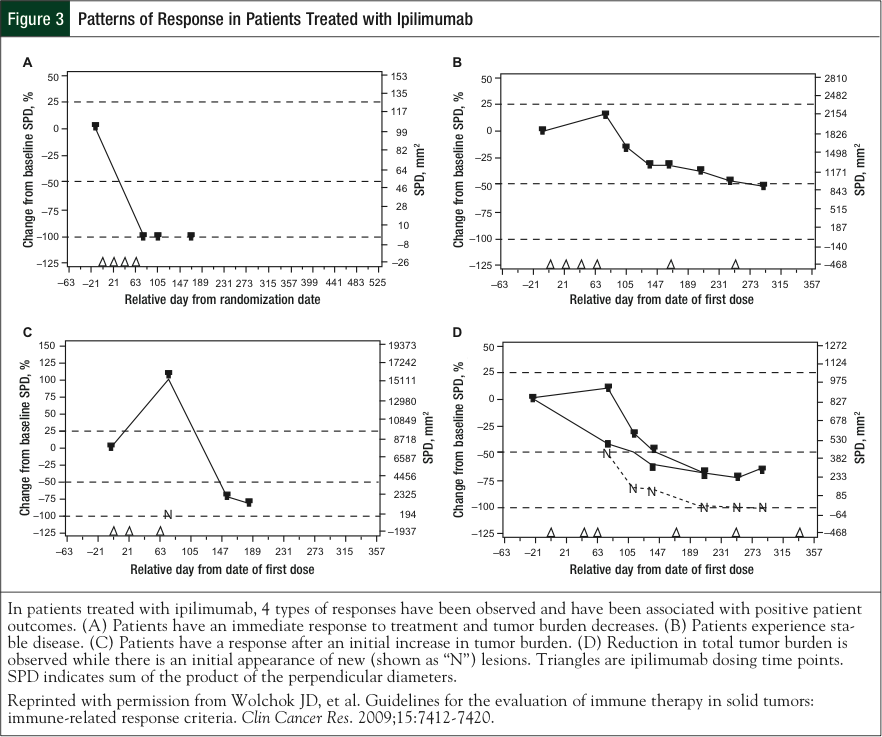
The pattern of clinical responses observed across ipilimumab studies can resemble both that of conventional cytotoxic agents and immunotherapies (Figure 3).2,13,42 An example of the former includes rapid decline of baseline lesions without evidence of new lesions after treatment. Stable disease, which in some cases may be followed by a slow and steady decline in tumor burden, is also observed in response to ipilimu- mab.42 In a more typical immune-like antitumor response, patients who receive ipilimumab have a slow evolution to long, durable, stable disease with responses lasting months or even years.21,29
Two novel “mixed-response” patterns have been observed during the clinical development of ipilimumab.42 In one, response to ipilimumab therapy is seen after an initial increase in tumor burden. This flare-like type of response is thought to be associated with the inflammatory infiltration of T-cells into the tumor, thereby expanding its size and mimicking progressive disease. In another pattern, the reduction in total tumor burden occurs during or after the appearance of new lesions. This could be because after ipilimumab-mediated activation of the immune system, an effective antitumor immune response takes time to develop, whereas tumor growth proceeds unabated. Both the traditional and the new response patterns are associated with favorable survival.42
It has become increasingly evident that standard response criteria developed for conventional cytotoxic therapy do not adequately capture the full range of antitumor immune responses. New response criteria for the evaluation of immune therapy have been proposed, the so-called immune-related response criteria (irRC).42 The use of irRC to capture additional responses to immunotherapeutic agents has been recommended to prevent premature withdrawal of therapy.43,44
Based on irRC, progressive disease is defined by at least a 25% increase in tumor burden compared with the nadir at 2 consecutive time points separated by at least 4 weeks. Complete response is a disappearance of all lesions, a partial response is at least a 50% decrease in tumor burden compared with baseline, and stable disease is all responses not meeting the criteria for a complete or partial response in the absence of progressive disease.
Adverse Events Associated with Ipilimumab
The most frequent AEs associated with ipilimumab are irAEs, which is most likely a result of the agent’s mechanism of action. A recent pooled analysis of 14 phase 1 to 3 studies at various doses of ipilimumab found that 64.2% of patients enrolled in ipilimumab clinical trials experienced an irAE of any grade, the majority of which were low grade (grade 1 or 2).45 Although irAEs can be potentially life-threatening, death occurred in <1% of patients.45 Of note, in the most recent phase 3 study, there were no deaths reported related to the study drug.2 The confounder in this trial was that the addition of dacarbazine may alter lymphocyte subsets during ipilimumab therapy, thereby modulating the toxicity profile.2 The lack of fatalities in this study2 also may reflect a growing familiarity of the irAEs and earlier recognition of toxicities, which are leading to earlier corrective therapy.
Across studies of ipilimumab, the most frequently affected organs were the GI tract and the skin (any grade, 31%-46% and 47%-68%, respectively; grade 3 or 4, 8%-23% and 0%-4%, respectively); less frequent were hepatic (any grade, 3%-9%; grade 3 or 4, 3%-7%), endocrine (any grade, 4%-6%; grade 3 or 4, 1%-5%), and neurologic events (any grade, <1%).45,46 Of note, increased liver function test values were observed more frequently than expected when ipilimumab was combined with dacarbazine.2
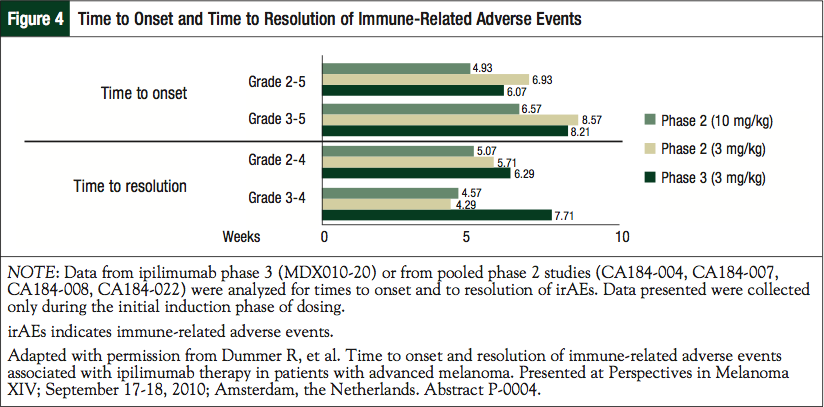
In addition, irAEs can have a rapid onset and are usually observed during the first 12 weeks of therapy, although a minority of events has occurred weeks or months after receiving the last dose of ipilimumab. In a pooled analysis of data from phase 2 and 3 studies with the approved dose of ipilimumab (3 mg/kg), median time to onset of grade 2 to 5 irAEs was 6 to 7 weeks.47,48 Overall, the times to onset and to resolution of irAEs are similar for the 3-mg/kg approved dose of ipilimumab and the investigational higher dose of 10 mg/kg (Figure 4).47 The onset is approximately 5 to 6 weeks, with resolution of between 4 and 8 weeks.47
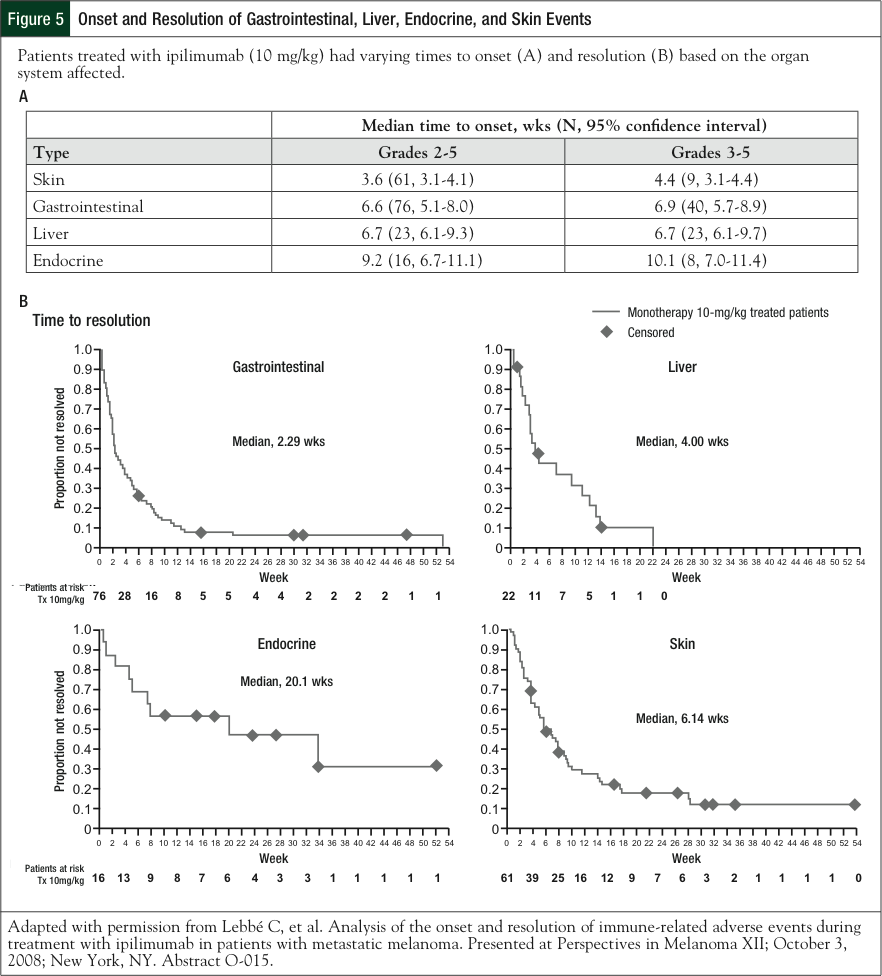
However, depending on the organ system affected, these rates can vary (based on the 10-mg/kg dose; Figure 5).48 Dermatologic irAEs are typically the earliest to occur, sometimes after only 1 or 2 doses of ipilimumab, whereas endocrine events appear within a median of 11 to 19 weeks after initiation of ipilimumab. The time to resolution also varies by organ system: skin, GI, and liver events resolve within a few weeks, whereas endocrine events take approximately 20 weeks to resolve and, in some cases, are irreversible (Figure 5).48 During the course of the clinical development of ipilimumab, a standard set of guidelines was developed to provide advice on the management of irAEs.
In the MDX001-20 study, 14 deaths (2.1%) were attributed to the study drug and 7 were related to irAEs.13 This demonstrates that irAEs can be life-threatening, and it highlights that vigilance is needed in monitoring patients and in educating them on the signs and symptoms and the need for timely reporting of possible irAEs. Reinforcement of the importance of early detection and prompt reporting may reduce serious and sometimes fatal events. Unless an alternative etiology can be identified, any sign or symptom of immune-mediated events should be considered drug-related and the appropriate treatment should be initiated.
Management of irAEs
At baseline and at each follow-up visit, patients should be assessed for the signs and symptoms of irAEs. Most low-grade (grade 1-2) events can be managed symptomatically.45,46 Patients exhibiting possible GI events should be assessed for changes in bowel habits and for the following signs and symptoms: diarrhea, abdominal pain, blood or mucus in stool with or without fever, peritoneal signs consistent with bowel perforation, and ileus.49 Low-grade events can be managed symptomatically (eg, dietary modifications and loperamide) while the etiology is being investigated.
Should symptomatic management not be effective after 1 week, prednisone (or an equivalent) should be initiated at 0.5 mg/kg daily or oral budesonide should be administered.49,50 The administration of prednisone 1-2 mg/kg or an equivalent is appropriate in patients with ≥7 stools daily over baseline, peritoneal signs consistent with bowel perforation, ileus, or patients with a fever. Early endoscopy should be considered if the diagnosis is uncertain. Of note, prophylactic steroid use has not proved effective in reducing the rate of grade ≥2 diarrhea.27,51 Beware that the use of opioids to manage abdominal pain may mask the signs of bowel perforation.45 Ipilimumab should be withheld for moderate GI adverse reactions until improvement to mild severity or complete resolution.
Ipilimumab should be permanently discontinued in patients with severe GI adverse reactions. In patients with persistent GI symptoms that do not resolve with systemic steroid treatment, administration of an alternative immunosuppressive therapy (ie, infliximab) should be considered. Of note, rapid tapering of steroids (<1 month) in severe cases can result in enterocolitis. Recognizing the importance of early detection, prompt reporting, and the appropriate management of diarrhea is important. Ipilimumab can result in severe inflammation of the GI tract,49 and routine oncologic diarrhea management may not include ruling out inflammatory etiology or the use of systemic corticosteroids as a treatment modality.
With regard to skin irAEs, patients should be evaluated for the signs and symptoms of pruritus, vitiligo, or rash. Mild-to-moderate events can be treated symptomatically, and topical moisturizers and oatmeal baths may help relieve such cases. Topical and/or systemic corticosteroids may be required to manage symptoms in moderate-to-severe cases. Ipilimumab should be withheld in patients with moderate-to-severe signs and symptoms, and it should be permanently discontinued in patients with Stevens-Johnson syndrome, toxic epidermal necrolysis, or rash that is complicated by full-thickness dermal ulceration or necrotic, bullous, or hemorrhagic manifestations.49
Liver function tests should be conducted on patients who are receiving ipilimumab at baseline and before each infusion or more frequently if warranted, especially because elevations of liver function test results may occur in the absence of clinical symptoms. Patients should also be monitored for any signs of autoimmune hepatitis. Ipilimumab should be withheld in patients with moderate aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevations of >2.5 times but ≤5 times upper limit of normal (ULN), or moderate total bilirubin elevation of >1.5 times but ≤3 times ULN. Because the rate of rise is not predictable, short interval testing or even daily monitoring may be helpful. Ipilimumab should be permanently discontinued for severe AST or ALT elevations of >5 times ULN, or total bilirubin elevations of >3 times ULN. Corticosteroid therapy may be appropriate for ≥grade 3 hepatotoxicity.49 Mycophenolate treatment has been administered in patients who have persistent, severe hepatitis, despite treatment with high-dose corticosteroids.46,52 Tacrolimus (target trough, 5-20 ng/mL) could also be considered for severe cases.53
Endocrinopathies are probably the most difficult irAEs to diagnose, because many of the signs and symptoms are protean and nonspecific. These symptoms include fatigue, headache, changes in mental status, abdominal pain, unusual bowel habits, and hypotension. The prescribing guidelines recommend that appropriate blood analysis be carried out to evaluate the function of the thyroid, adrenal, and pituitary glands before each ipilimumab dose52; however, the rate of functional change is such that our practice is to formally assess less frequently or when prompted by symptoms. Adrenal insufficiency is rare but may occur quietly and may manifest as adrenal crisis.54 Hypopituitarism and hypothyroidism have also been observed.13 Hypophysitis can give rise to bitemporal visual field loss, which is confirmed by magnetic resonance imaging of the brain with pituitary.49,54 Patients with ipilimumab-associated hypophysitis have been successfully treated with high-dose dexamethasone, but they may also require long-term physiologic doses of hydrocortisone.55 Ipilimumab should be withheld when moderate reactions or any symptomatic endocrinopathy occur, until complete resolution or until stability with hormone replacement therapy is reached. Long-term hormone replacement therapy may be necessary in some patients.
Other irAEs that can occur include neurologic events, such as sensory and motor neuropathy, and patients should be encouraged to report any signs of muscle weakness or sensory alternations. New-onset or worsening symptoms may require permanent discontinuation of ipilimumab. Rarely, Guillain-Barré syndrome, myasthenia gravis, and severe sensory alterations have occurred. Ocular events are also rare, and corticosteroid eyedrops should be administered to patients who develop uveitis, iritis, or episcleritis.49 Immune-related thrombocytopenia (grade 4) has recently been reported in a 57-year-old man who was receiving ipilimumab,56 highlighting the value of monitoring full blood counts during therapy.
General Safety Information
A high level of suspicion and recognition of the clinical presentation, coupled with judicious interpretation of the diagnostic blood panels, are important in the management of irAEs. Although irAEs can be severe in some patients, overall studies indicate that they are manageable, and most resolve in a reasonable amount of time if identified early and appropriate treatment is administered.47,48 The guidelines advise that on improvement to ≤grade 1 for all irAEs, corticosteroid tapering should be initiated and continue to be tapered over at least 1 month.45,46 It is also recommended that ipilimumab be discontinued in patients who have failed to complete the full treatment course within 16 weeks from the administration of the first dose. In addition, ipilimumab should be permanently discontinued in patients who are unable to have their corticosteroid dose reduced to prednisone 7.5 mg or an equivalent daily.
Conclusion
Ipilimumab, the first-in-class anti–CTLA-4 therapy to be approved by the FDA, has demonstrated efficacy and OS benefit in patients with unresectable or metastatic melanoma. The majority of patients experience an irAE, but these are usually low grade and manageable; the focus is on early recognition and vigilance with appropriate monitoring and the use of established irAE management guidelines. Low-grade AEs are typically managed symptomatically, although higher-grade AEs can be severe and life-threatening. The times to onset and to the resolution of irAEs are relatively consistent across the approved 3-mg/kg dose and the higher 10-mg/kg dose, but they vary for each organ system. Ipilimumab 3 mg/kg is currently available as a monotherapy, but ongoing research will help to shape the melanoma treatment landscape by establishing the most effective combination regimens, without compromising tolerability.
Acknowledgment
The authors take full responsibility for the content of this publication and confirm that it reflects their viewpoints and medical expertise. The authors also wish to acknowledge StemScientific, funded by Bristol-Myers Squibb, for providing writing and editorial support. Neither Bristol-Myers Squibb nor StemScientific influenced the content of the manuscript, nor did the authors receive financial compensation for authoring the manuscript.
Author Disclosure Statement
Dr Wong is on the Physician Advisory Board of Bristol-Myers Squibb and Genentech. Dr Jarkowski reported no conflicts of interest.
References
1. Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527-534.
2. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
3. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
4. Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745-2751.
5. Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22:1118-1125.
6. Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158-166.
7. Legha SS, Ring S, Papadopoulos N, et al. A phase II trial of taxol in metastatic melanoma. Cancer. 1990;65:2478-2481.
8. Einzig AI, Hochster H, Wiernik PH, et al. A phase II study of taxol in patients with malignant melanoma. Invest New Drugs. 1991;9:59-64.
9. Hodi FS, Soiffer RJ, Clark J, Finkelstein DM, Haluska FG. Phase II study of pacli- taxel and carboplatin for malignant melanoma. Am J Clin Oncol. 2002;25:283-286.
10. Rao RD, Holtan SG, Ingle JN, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006;106:375-382.
11. Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823-2830.
12. Kirkwood JM, Tarhini AA, Panelli MC, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26:3445-3455.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
14. Wagle N, Emery C, Berger MF, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29:3085-3096.
15. Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435.
16. Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71:2750-2760.
17. Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF–targeted melanoma cells. Cancer Res. 2010;70:6670-6681.
18. Shi H, Moriceau G, Kong X, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 2012;3:724.
19. Seghers AC, Wilgenhof S, Lebbé C, Neyns B. Successful rechallenge in two patients with BRAF-V600-mutant melanoma who experienced previous progression during treatment with a selective BRAF inhibitor. Melanoma Res. 2012;22:466-472.
20. Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500-504.
21. Haanen JB, Hodi FS, O’Day SJ, et al. Ipilimumab improves overall survival in patients with previously treated, advanced melanoma: long-term survival results from phase III trial. Ann Oncol. 2010;21(suppl 8):viii402. Abstract 1327P.
22. Wolchok JD, Weber JS, Maio M, et al. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II trials. Presented at: Perspectives in Melanoma XV; September 16, 2011; New York, NY. Abstract P-20.
23. ClinicalTrials.gov. Ph I/II Ipilimumab Vemurafenib Combo. ClinicalTrials.gov Identifier NCT01400451. http://clinicaltrials.gov/ct2/show/NCT01400451. Accessed October 27, 2011.
24. Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533-546.
25. Keler T, Halk E, Vitale L, et al. Activity and safety of CTLA-4 blockade combined with vaccines in cynomolgus macaques. J Immunol. 2003;171:6251-6259.
26. Weber JS, O’Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950-5956.
27. Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598.
28. O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717.
29. Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164.
30. Hersh EM, O’Day SJ, Powderly J, et al. A phase II multicenter study of ipilimu-mab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs. 2011;29:489-498.
31. Marsh JC. Hepatic vascular toxicity of dacarbazine (DTIC): not a rare complication. Hepatology. 1989;9:790-792.
32. Wolchok JD, Weber JS, Hamid O, et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun. 2010;10:9.
33. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117:1687-1696.
34. Lebbé C, McDermott DF, Robert C, et al. Ipilimumab improves survival in previously treated, advanced melanoma patients with poor prognostic factors: subgroup analyses from a phase III trial. Ann Oncol. 2010;21(suppl 8):viii401. Abstract 13240.
35. Heller K, Pavlick AC, Hodi FS, et al. Safety and survival analysis of ipilimu-mab therapy in patients with stable asymptomatic brain metastases. J Clin Oncol. 2011;29(suppl 15):Abstract 8581.
36. Margolin K, Hodi FS, McDermott DF, et al. Safety and efficacy of ipilimu-
mab-treated patients with melanoma and brain metastases. Eur J Cancer. 2011;47(suppl 1):S654. Abstract 9306.
37. Weber JS, Amin A, Minor D, et al. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 2011;21:530-534.
38. Eigentler TK, Figl A, Krex D, et al. Number of metastases, serum lactate dehydrogenase level, and type of treatment are prognostic factors in patients with brain metastases of malignant melanoma. Cancer. 2011;117:1697-1703.
39. Wolchok JD, de Pril V, Linette G, et al. Efficacy of ipilimumab 10 mg/kg in advanced melanoma patients (pts) with good and poor prognostic factors. J Clin Oncol. 2009;27(15 suppl):Abstract 9036.
40. Shahabi V, Whitney G, Hamid O, et al. Assessment of association between BRAF-V600E mutation status in melanomas and clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:733-737.
41. Schartz NEC, Farges C, Madelaine I, et al. Complete regression of a previously untreated melanoma brain metastasis with melanoma. Melanoma Res. 2010;20:247-250.
42. Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412-7420.
43. Pennock GK, Waterfield W, Wolchok JD. Patient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: how different are these from conventional treatment responses? Am J Clin Oncol. 2012;35:606-611.
44. O’Regan KN, Jagannathan JP, Ramaiya N, et al. Radiologic aspects of immune-
related tumor response criteria and patterns of immune-related adverse events in patients undergoing ipilimumab therapy. AJR Am J Roentgenol. 2011;197:W241-W246.
45. Ibrahim R, Berman D, de Pril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29(suppl): Abstract 8583.
46. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
47. Dummer R, Maio M, Hamid O, et al. Time to onset and resolution of immune-related adverse events associated with ipilimumab therapy in patients with advanced melanoma. Presented at Perspectives in Melanoma XIV; September 17-18, 2010; Amsterdam, the Netherlands. Abstract P-0004.
48. Lebbé C, O’Day SJ, Sileni VC, et al. Analysis of the onset and resolution of immune-related adverse events during treatment with ipilimumab in patients with metastatic melanoma. Presented at Perspectives in Melanoma XII; October 3, 2008; New York, NY. Abstract O-015.
49. Ipilimumab US prescribing information: risk evaluation and mitigation strategy (REMS). www.hcp.yervoy.com/pdf/rems-management-guide.pdf. Accessed September 12, 2011.
50. O’Day S, Weber JS, Wolchok JD, et al. Effectiveness of treatment guidance on diarrhea and colitis across ipilimumab studies. J Clin Oncol. 2011;29(suppl):Abstract 8554.
51. Grob JJ, Hamid O, Wolchok J, et al. Antitumor responses to ipilimumab in advanced melanoma are not affected by systemic corticosteroids used to manage immune-related adverse events (irAEs). Presented at Joint ECCO 15-34th ESMO Multidisciplinary Congress; September 22, 2009; Berlin, Germany. Abstract P-9312.
52. Ipilimumab package insert. Princeton, NJ: Bristol-Myers Squibb; March 2011. http://packageinserts.bms.com/pi/pi_yervoy.pdf. Accessed September 12, 2011.
53. Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864-872.
54. Dillard T, Yedinak CG, Alumkal J, et al. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29-38.
55. Blansfield JA, Beck KE, Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593-598.
56. Ahmad S, Lewis M, Corrie P, et al. Ipilimumab-induced thrombocytopenia in a patient with metastatic melanoma. J Oncol Pharm Pract. 2012;18:287-292.