Paclitaxel is a taxane-derived antineoplastic agent that is often used for US Food and Drug Administration (FDA)-approved indications such as breast cancer, AIDS-related Kaposi sarcoma, ovarian cancer, and non–small-cell lung cancer (NSCLC).1 It is also widely used for a number of off-label indications, and it works by promoting the assembly of microtubules, further enhancing the inhibition of cellular replication. It is administered as an intravenous (IV) solution, and its dosage and duration of treatment vary based on the indication.1 Patients with high-risk breast cancer in the preoperative or the adjuvant setting can receive either dose-dense paclitaxel (ie, 175 mg/m2 every 2 weeks for 4 cycles) or weekly paclitaxel (ie, 80 mg/m2 weekly for 12 weeks). In breast cancer, paclitaxel can be administered after the use of an anthracycline, in combination with cyclophosphamide.2
Paclitaxel is highly effective against breast cancer; however, it is associated with several treatment-limiting adverse effects. Common paclitaxel-related side effects are alopecia, hypersensitivity reactions, gastrointestinal events, myalgia or arthralgia, neuropathies, and hematologic toxicities.1 Rarely, paclitaxel has been associated with pulmonary injury, including acute diffuse interstitial pneumonitis, subacute diffuse interstitial pneumonia, pulmonary opacities with peripheral eosinophilia, lung fibrosis, pulmonary embolism, pleural effusion, acute noncardiogenic pulmonary edema, and respiratory failure.1,3
The most common pulmonary adverse event with paclitaxel is interstitial pneumonitis. Several published reports describe pulmonary toxicities in patients with NSCLC or with hematologic malignancies; however, these can be seen in other cancers as well.4-6 The interstitial pneumonitis induced by taxanes are thought to represent an immune-mediated delayed hypersensitivity (class IV) reaction.7
Paclitaxel is a taxane-derived antineoplastic agent extracted from the yew tree.1 Because of its unique manufacturing with solvents, such as cremophor, the use of paclitaxel requires medications before its administration to prevent infusion-related reactions.6,7
Nab-paclitaxel was approved by the FDA in 2005 for metastatic breast cancer based on a phase 3 clinical trial that compared paclitaxel with nab-paclitaxel, showing that nab-paclitaxel was more effective than paclitaxel in the metastatic breast cancer setting.8 The FDA approved the use of nab-paclitaxel for patients with metastatic breast cancer at a dose of 260 mg/m2 IV over 30 minutes every 3 weeks.9 Nab-paclitaxel is also indicated for the treatment of patients with NSCLC, as well as for patients with adenocarcinoma of the pancreas, at differing dosing regimens.9 It has been theorized that the uptake of albumin by tumor cells may allow for improved intratumoral delivery of nab-paclitaxel.10,11 Indeed, a 2015 study comparing paclitaxel and nab-paclitaxel confirmed that nab-paclitaxel is more effective than paclitaxel in the metastatic breast cancer setting.12 In addition, higher doses of nab-paclitaxel can be administered over a shorter infusion time, leading to rapid distribution to peripheral compartments.9 Interstitial pneumonitis is less frequently associated with nab-paclitaxel than with paclitaxel, as is evidenced in paclitaxel-induced pneumonitis.1,9
To the best of our knowledge, no studies to date have looked at the use of nab-paclitaxel as an alternative treatment in patients with paclitaxel-induced pneumonitis. We report and discuss 3 patients with breast cancer who received initial treatment with paclitaxel and then were switched to nab-paclitaxel after being diagnosed with paclitaxel-induced pneumonitis. This case series was approved by our Institutional Review Board; it did not involve human subjects research.
Case 1
A 53-year-old female patient presented with bilateral breast masses that had increased in size progressively over approximately 4 years. The patient underwent bilateral mammograms and bilateral breast and axillary ultrasound, with biopsies of both breast lesions and fine-needle aspiration of axillary lymphadenopathy. A targeted ultrasound of both breasts showed irregular hypoechoic masses measuring 4.6 cm × 2.8 cm × 2.7 cm in the right breast and 5.4 cm × 4.1 cm × 2.4 cm in the left breast. The pathology confirmed stage IIB (T3, N0, M0), grade 2, estrogen receptor–positive (ER+), progesterone receptor–positive (PR+), HER2/neu-negative ductal carcinoma on the left breast and stage IIIB (T4b, N1a, M0), grade 3, ER+, PR+, HER2/neu-negative invasive ductal carcinoma of the right breast.
Genomic testing of both breast tissue lesions resulted in an Oncotype DX score of 20 in the right breast, indicating intermediate risk for recurrence; and a score of 33 in the left breast, indicating high risk for recurrence. The patient was treated with 4 cycles of neoadjuvant dose-dense doxorubicin and cyclophosphamide, followed by weekly paclitaxel. After the first weekly dose of paclitaxel (80 mg/m2), she complained of weakness and dyspnea on exertion. After evaluation, the decision was made to proceed with the second weekly dose of paclitaxel. Eight days after the administration of the second dose of paclitaxel, the patient presented to the emergency department with a fever of 102°F, chills, aches, a nonproductive cough, and malaise. Her absolute neutrophil count was 6.5 cells per mm3. She received treatment with cefepime and vancomycin. Her fever subsided, along with the chills, aches, and malaise. Despite antibiotic treatment, her cough remained.
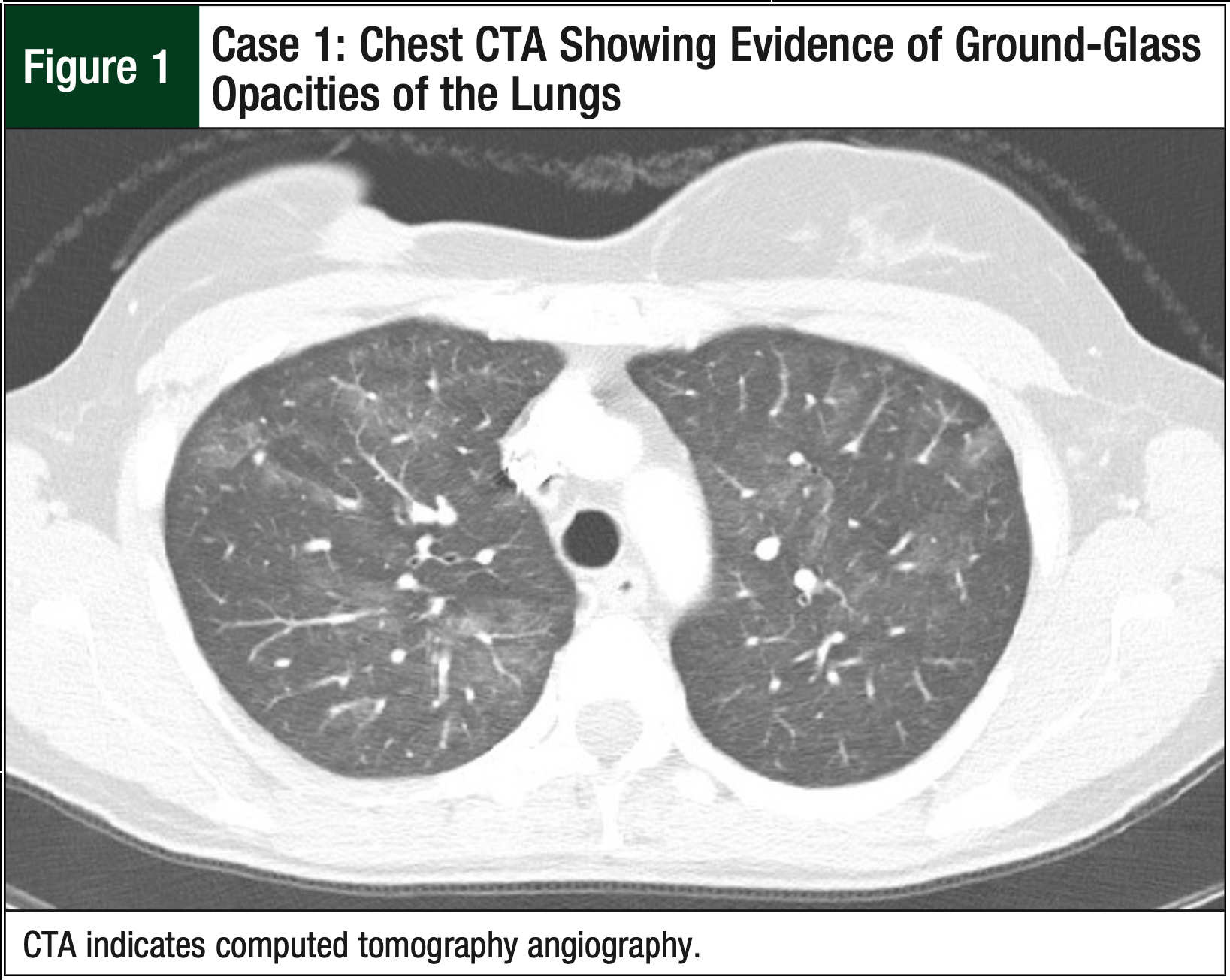
Shortly after discharge, the patient again reported having a nonproductive cough, intermittent fevers, dyspnea on exertion, and tachycardia. She underwent an urgent computed tomography angiography (CTA) of the chest to rule out a pulmonary embolism, which was negative. However, the CTA revealed diffuse patchy ground-glass opacities throughout her lungs, consistent with viral, hypersensitivity, or drug-induced pneumonitis (Figure 1). Given the course of events, the oncologist determined that the pneumonitis was the result of the administration of paclitaxel and decided to discontinue paclitaxel treatment.
After 4 weeks, her pneumonitis symptoms largely resolved, without the need for steroids. No further imaging was done to confirm the resolution of pneumonitis in this patient, because the oncologist did not deem it clinically necessary. The patient began receiving nab-paclitaxel to complete the neoadjuvant therapy. She received a total of 10 doses of nab-paclitaxel at a weekly IV dose of 100 mg/m2 without incidence or recurrence of pneumonitis. The patient completed chemotherapy, and a restaging positron emission tomography and computed tomography showed significant response in both breasts and axilla, with no evidence of metastatic disease.
Upon completion of chemotherapy, the patient underwent a bilateral mastectomy, sentinel lymph node biopsy, and axillary lymph node dissection of both breasts. The right and left breasts revealed evidence of reduction in tumor size and negative margins within the majority of lymph nodes. Subsequently, she received treatment with adjuvant radiation and endocrine therapy.
Case 2
A 58-year-old female patient had a routine mammogram after not doing so in 4 years. The mammogram showed a spiculated abnormality of the right upper outer quadrant. An ultrasound confirmed a 3.5- to 4-cm distortion and associated mass. A sentinel node biopsy of the right breast was completed, and the pathology analysis revealed stage IIIA (pT3, N1, M0) invasive carcinoma, ER+, PR+, HER2/neu-negative right breast cancer. Examination revealed 3 positive lymph nodes.
The oncologist decided to proceed with neoadjuvant chemotherapy before axillary dissection and radiation. Afterward, the patient received dose-dense doxorubicin and cyclophosphamide for 4 cycles, followed by dose-dense paclitaxel at a dose of 175 mg/m2. However, 11 days after the initial paclitaxel dose, the patient presented to an outside hospital with fever of 102°F, nasal congestion, significant shortness of breath, feelings of malaise, cough, tachycardia, and severe arthralgia. Her oxygen saturation at room air was 94%. She was given a 10-day course of levofloxacin, with little improvement in her symptoms.
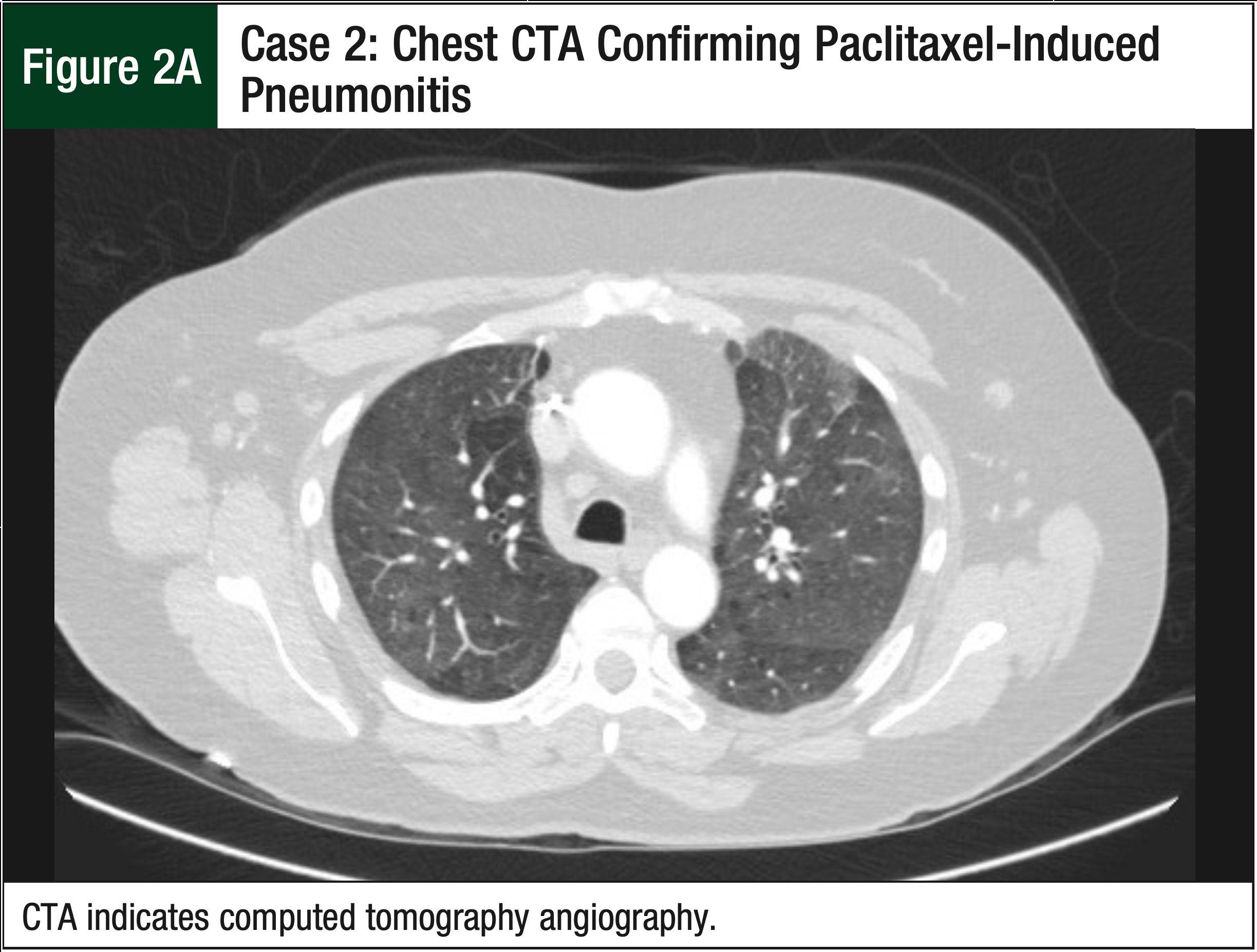
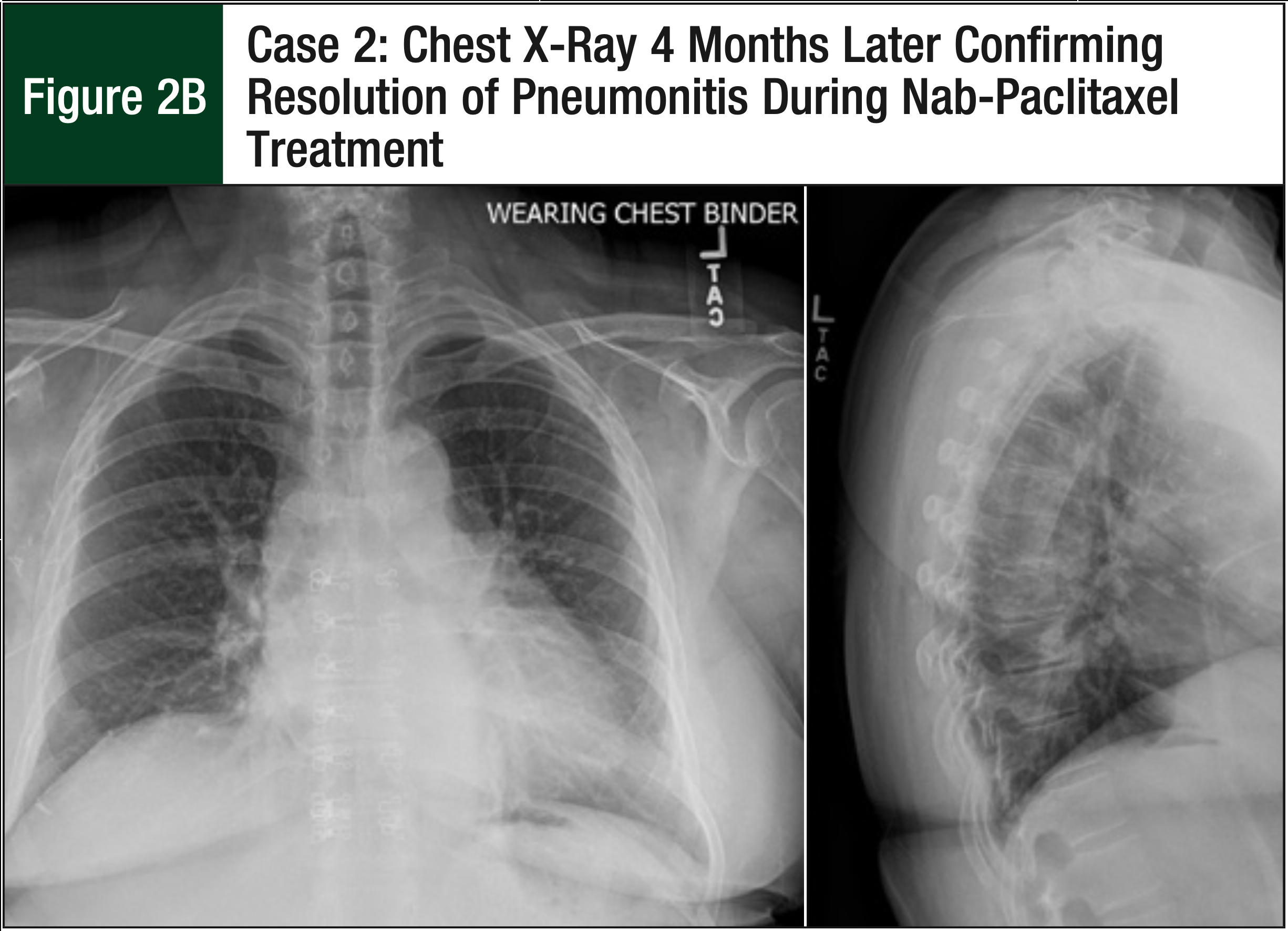
The patient underwent a CTA, which ruled out a pulmonary embolism, but the CTA did show new extensive and near-diffuse bilateral ground-glass opacities (Figure 2A). The pulmonologist was consulted and confirmed that the imaging was consistent with hypersensitivity pneumonitis or with cellular nonspecific interstitial pneumonia. The patient reported feeling better, with reduced symptoms 9 days after the CTA, despite not receiving any fluids or steroids. The oncologist decided not to proceed with a bronchoscopy or steroid therapy, because the patient’s symptoms continued to improve. A chest x-ray also confirmed the resolution of pneumonitis that was previously displayed on the chest CTA (Figure 2B).
Based on the timing of paclitaxel administration, paclitaxel was deemed the likely cause of the hypersensitivity pneumonitis. Paclitaxel was discontinued and was replaced with 9 weekly 100-mg/m2 doses of nab-paclitaxel. The patient tolerated the nab-paclitaxel regimen and no longer reported severe pneumonitis symptoms for the remainder of the chemotherapy course. She underwent planned axillary lymph node dissection and a right lumpectomy. Pathology analysis revealed an absence of invasive disease and a reduction of the number of positive lymph nodes. The patient subsequently underwent radiotherapy and endocrine therapy.
Case 3
A 53-year-old female patient presented with a complaint of intermittent left breast pain that occurred for the past few months. She underwent a bilateral diagnostic mammogram, left breast ultrasound, and breast magnetic resonance imaging, which indicated an enlarged mass measuring 2.5 cm × 3.8 cm × 2 cm in the left central midbreast. Initial staging revealed stage IIA (T2, N0, M0), grade 2, ER+, PR+, HER2/neunegative invasive ductal carcinoma. Neoadjuvant therapy with dose-dense doxorubicin and cyclophosphamide every 2 weeks for a total of 4 cycles was initiated. Afterward, the patient began treatment with a planned regimen of dose-dense paclitaxel at a dose of 175 mg/m2 every 2 weeks.
Eight days later, the patient reported a fever of 102°F, along with cold sweats at night, increased fatigue, and shortness of breath. Her white blood cell count and oxygen saturation were normal, at 10.4 cells per mm3, and 100%, respectively. The patient was hospitalized with a diagnosis of sepsis and unspecified upper respiratory tract infection, and her absolute neutrophil count was 5.1 cells per mm3. She was given adequate fluid resuscitation and cefepime, which resulted in resolution of the fever. Despite the administration of IV fluids and antibiotics, the patient continued to have persistent dyspnea on exertion, tachycardia, pleuritic chest pain, and a nonproductive cough.
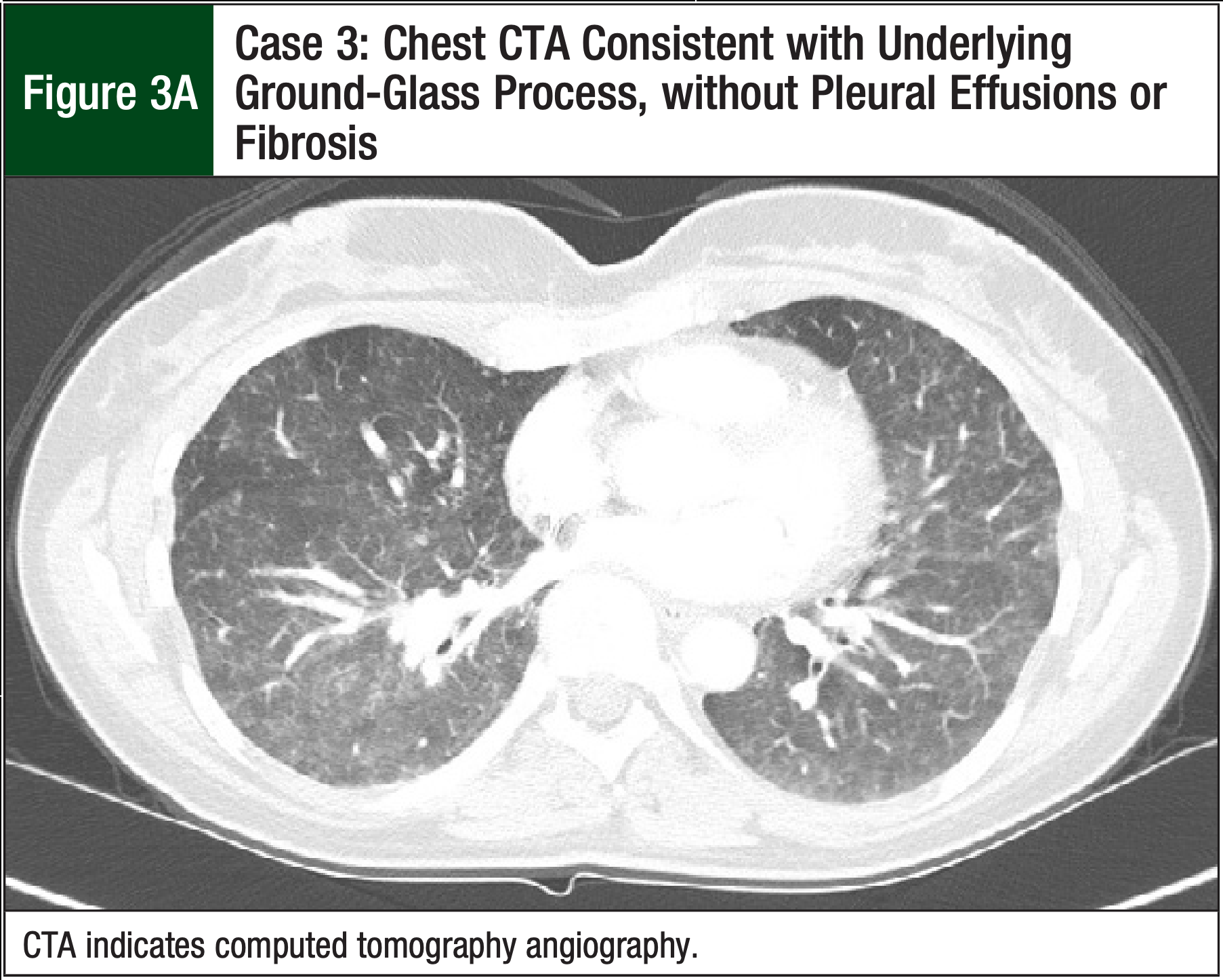
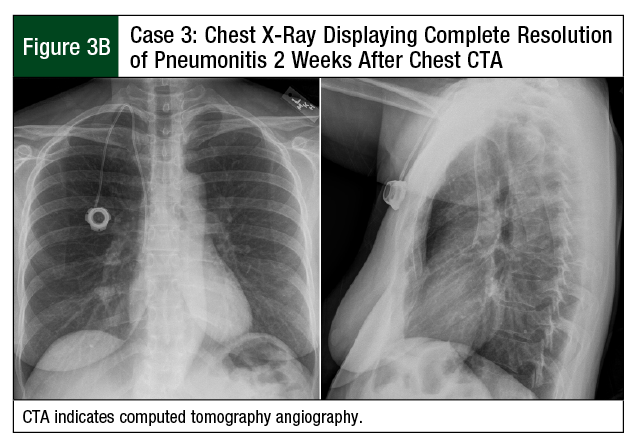
A CTA, which was performed to rule out a pulmonary embolism, was negative, but it revealed evidence of ground-glass opacities consistent with pneumonitis (Figure 3A). The patient received treatment with a high-dose prednisone taper (60 mg for 3 days, followed by 40 mg for 4 days) for presumed paclitaxel-mediated pneumonitis. Her shortness of breath improved after 7 days of therapy. A chest x-ray confirmed complete resolution of diffuse ground-glass opacity, with minimal residual scattered left-lung nodularity (Figure 3B).
The patient’s sixth cycle of chemotherapy was delayed by 1 week to allow recovery time from the previous chemotherapy cycle. Her oncologist decided to discontinue the paclitaxel treatment and switch her chemotherapy to nab-paclitaxel at a dose of 100 mg/m2 on the first week, with a subsequent reduction to 80 mg/m2 for 8 doses because of neuropathy.
Postchemotherapy, the patient underwent bilateral mastectomy with sentinel lymph node biopsy, followed by axillary lymph node dissection of the left breast. This showed evidence of a decrease in the size of the tumor and the grade after nab-paclitaxel treatment. The patient subsequently underwent adjuvant radiation therapy and endocrine therapy.
Discussion
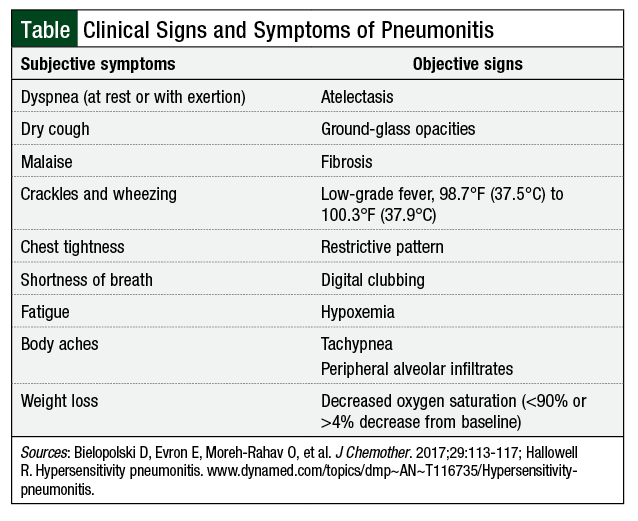
We describe 3 patients with breast cancer who received treatment in the neoadjuvant setting with dose-dense doxorubicin and cyclophosphamide, followed sequentially by paclitaxel. The patients were diagnosed based on the 7th edition of the American Joint Committee on Cancer’s Cancer Staging Manual TNM staging categories.13 Each of the 3 patients developed pneumonitis shortly after starting paclitaxel therapy. The frequency of pneumonitis signs and symptoms varied, but each patient reported signs and symptoms consistent with a clinical presentation of pneumonitis (Table).3,14
The symptoms of pneumonitis began in each patient between 8 and 11 days (mean, 9 days) after the initial or second paclitaxel doses. In all 3 cases, the patients received antimicrobial therapy due to concern for infection. However, the patients had a resolution of pneumonitis symptoms after discontinuation of paclitaxel and not with the administration of antibiotics. Although published reports show some association of mild pneumonitis with the use of nab-paclitaxel,15,16 none of the 3 patients presented in our cases had any further symptoms of pneumonitis when switched from paclitaxel to nab-paclitaxel. The 3 patients responded to chemotherapy with nab-paclitaxel, and the change in therapy did not disrupt their treatment regimen.
The resolution of pneumonitis symptoms without the use of steroids in our first and second cases was an interesting finding. Of note, none of our 3 patients had a history of underlying lung disease, including no evidence of reactive airway disease, but the second patient was a former smoker. This might have contributed to the rationale for the treatment team to consult a pulmonologist, which led to a more comprehensive pulmonary assessment in this case than in the other 2 cases. It is difficult to make an association with smoking and an increased risk for paclitaxel-induced pneumonitis based solely on our 3 cases.
It remains unclear whether the likelihood of paclitaxel-induced pneumonitis is caused by the strength of the dose or by the accumulation of the drug in the body. Because pneumonitis is a hypersensitivity reaction, it is likely caused by exposure to paclitaxel. In our case series, the first patient began to show signs of pneumonitis after the second dose of paclitaxel, whereas the patients in the second and third cases demonstrated symptoms of pneumonitis after the administration of the first dose of paclitaxel. This could indicate that pneumonitis from paclitaxel therapy is a dose-dependent adverse event: the first patient received a smaller dose of paclitaxel (80 mg/m2) than the second and third patients (175 mg/m2 each).
A study looking at the pharmacokinetic relationship of paclitaxel dosing to toxicity showed that pulsed low-dose paclitaxel chemoradiation was associated with lower adverse reactions, including pneumonitis.16 It is important to note that patients in that pharmacokinetic study only received paclitaxel at doses ranging from 15 to 25 mg/m2, received radiation therapy, and the study did not assess the adverse-event profile with higher doses of paclitaxel that is seen with breast cancer treatment regimens.17
Furthermore, 2 meta-analyses evaluating the use of paclitaxel-based chemotherapy regimens administered weekly compared with once-every-3-week dosing showed fewer adverse events associated with the weekly dosing regimen.18,19 However, those studies did not report the development of pneumonitis with either regimen.18,19
Interstitial pneumonitis induced by paclitaxel is thought to represent an immune-mediated delayed hypersensitivity reaction that was proved through a positive leukocyte migration test in 1 case report.20 The incidence of nab-paclitaxel–induced pneumonitis is lower than paclitaxel-induced pneumonitis, with the majority of these events being mild.15 This may lead to underreporting in other published clinical trials.15 Pharmacologists have developed an intriguing theory as to the reasoning why nab-paclitaxel leads to less severe pneumonitis. Serum albumin, the most ubiquitous protein in the body, can be found at higher levels in solid tumors because of insufficient lymphatic drainage, leading to accumulation of macromolecules (ie, albumin).21
Nab-paclitaxel is composed of an outer layer of albumin surrounding an inner core of paclitaxel. Each albumin nanoparticle is approximately 100 times smaller than a red blood cell. Albumin has the natural ability to promote the delivery of cytotoxic drugs into tumors by initiating albumin receptor, glycoprotein (gp60)-mediated transcytosis across endothelial cells, leading to accumulation of the drug in tumor cells by binding to secreted protein acidic and rich in cysteine.22-24 Albumin can thereby increase the local drug concentration of paclitaxel and enhance the ability of targeted tumor destruction, which may result in reduced paclitaxel toxicity and ultimately a decrease in the adverse event of pneumonitis.22-24
One advantage that has been associated with the use of nab-paclitaxel includes its use in patients with a history of paclitaxel hypersensitivity. Nab-paclitaxel is associated with fewer hypersensitivity reactions than paclitaxel, has a shorter infusion time, and does not require premedications (ie, dexamethasone, diphenhydramine, and histamine-2 receptor antagonists) before administration.9
Finally, the cost of nab-paclitaxel can be a barrier to its use. The average wholesale price of nab-paclitaxel is $1508.68 per dose, compared with the average wholesale price of paclitaxel, which ranges from $15.36 to $152.40 per dose.25 This could hinder the use of this medication in patients with limited financial resources and healthcare options. Nab-paclitaxel is not interchangeable with paclitaxel, which may disrupt the therapeutic response in some patients, although it did not appear to affect the efficacy in our cases. Although docetaxel is less costly than nab-paclitaxel, no studies or reports are available regarding the safety of using docetaxel after paclitaxel-induced pneumonitis.
Limitations
Our 3 case reports have several limitations. Cremophor, a frequently used formulation vehicle for paclitaxel, has been associated with symptoms similar to pneumonitis, including shortness of breath, hypersensitivity reactions, and anaphylaxis. The use of a leukocyte migration test has been shown to assist in differentiating the cause of pneumonitis between paclitaxel and cremophor.20 Although it is not standard practice, a leukocyte migration test was not performed on any of the patients in our cases to determine whether the reaction was caused by paclitaxel or by cremophor.
Furthermore, other potential nonchemotherapy-related causes of pneumonitis could not be excluded in these cases, because the diagnosis of pneumonitis was not confirmed by biopsy. A meta-analysis suggested that taxane-induced pneumonitis, in combination with gemcitabine, can be dependent on the type of cancer being treated.26 This may present a limitation to the 3 case reports discussed here, given that they included only patients who received treatment for breast cancer.
Conclusion
Pneumonitis is a potentially life-threatening complication of paclitaxel therapy and may warrant the initiation of a different chemotherapy regimen. In our 3 cases, the use of nab-paclitaxel was shown to be a possible alternative treatment for patients with breast cancer who had pneumonitis while using paclitaxel. The efficacy and safety profile displayed by nab-paclitaxel may warrant the use of this medication for patients with breast cancer who have paclitaxel-induced pneumonitis during treatment.
These 3 cases illustrate that paclitaxel can induce pneumonitis early in the treatment of patients with breast cancer. Clinicians should be aware of this possibility, considering that taxanes are one of the most effective classes of drugs for the treatment of breast cancer. It is reassuring that pneumonitis caused by paclitaxel can be resolved with the discontinuation of its use, and may not reactivate with the subsequent administration of nab-paclitaxel.
Acknowledgments
The authors wish to thank Kathleen Kemmer, MD, Oncologist, Department of Hematology and Medical Oncology, Oregon Health & Science University Knight Cancer Institute, Portland, OR, for her assistance with the manuscript.
Author Disclosure Statement
Dr Palumbo is on the Advisory Board of Oncology Reimbursement Management, and has received an honorarium from the Oregon Society of Health-System Pharmacists. Dr Njigha, Dr Vuky, and Dr Paxton have no conflicts of interest to report.
References
- Taxol (paclitaxel) injection [prescribing information]. Rockford, IL: Mylan Institutional; December 2018.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast Cancer. Version 1.2019. September 6, 2019. www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed September 9, 2019.
- Bielopolski D, Evron E, Moreh-Rahav O, et al. Paclitaxel-induced pneumonitis in patients with breast cancer: case series and review of the literature. J Chemother. 2017;29:113-117.
- Reckzeh B, Merte H, Pflüger KH, et al. Severe lymphocytopenia and interstitial pneumonia in patients treated with paclitaxel and simultaneous radiotherapy for non-small-cell lung cancer. J Clin Oncol. 1996;14:1071-1076.
- Ramanathan RK, Reddy VV, Holbert JM, Belani CP. Pulmonary infiltrates following administration of paclitaxel. Chest. 1996;110:289-292.
- Yasuda K, Igishi T, Kawasaki Y, et al. Phase II trial of weekly paclitaxel in previously untreated advanced non-small-cell lung cancer. Oncology. 2003;-65:224-228.
- Wang GS, Yang KY, Perng RP. Life-threatening hypersensitivity pneumonitis induced by docetaxel (taxotere). Br J Cancer. 2001;85:1247-1250.
- Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794-7803.
- Abraxane for injectable suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound) [prescribing information]. Summit, NJ: Celgene; August 2018.
- Xing P, Zhu Y, Shan L, et al. The role of weekly nanoparticle albumin bound paclitaxel monotherapy as second line or later treatment for advanced NSCLC in China. Oncotarget. 2017;8:87442-87454.
- Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl Oncol. 2009;2:59-64.
- Palumbo R, Sottotetti F, Trifirò G, et al. Nanoparticle albumin-bound pac-litaxel (nab-paclitaxel) as second-line chemotherapy in HER2-negative, taxane-pretreated metastatic breast cancer patients: prospective evaluation of activity, safety, and quality of life. Drug Des Devel Ther. 2015;9:2189-2199.
- Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010.
- Hallowell R. Hypersensitivity pneumonitis. Updated February 13, 2019. www.dynamed.com/topics/dmp~AN~T116735/Hypersensitivity-pneumonitis. Accessed June 30, 2019.
- Yasuda Y, Nomizo T, Ozasa H. Retrospective analysis of acute exacerbation of interstitial lung diseases with nanoparticle albumin-bound paclitaxel in patients with advanced lung cancer with preexisting interstitial lung disease. Mol Clin Oncol. 2017;7:677-680.
- Peddi PF, Cho M, Wang J, et al. Nab-paclitaxel monotherapy in refractory pancreatic adenocarcinoma. J Gastrointest Oncol. 2013;4:370-373.
- Chen Y, Pandya KJ, Feins R, et al. Toxicity profile and pharmacokinetic study of a phase I low-dose schedule-dependent radiosensitizing paclitaxel chemoradiation regimen for inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;71:407-413.
- Gao G, Chu H, Zha L, et al. A meta-analysis of paclitaxel-based chemotherapies administered once every week compared with once every 3 weeks first-line treatment of advanced non-small-cell lung cancer. Lung Cancer. 2012;76:380-386.
- Huang TC, Campbell TC. Comparison of weekly versus every 3 weeks pac---litaxel in the treatment of advanced solid tumors: a meta-analysis. Cancer Treat Rev. 2012;38:613-617.
- Fujimori K, Yokoyama A, Kurita Y, et al. Paclitaxel-induced cell-mediated hypersensitivity pneumonitis. Diagnosis using leukocyte migration test, bronchoalveolar lavage, and transbronchial lung biopsy. Oncology. 1998;55:340-344.
- Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin: more than just a serum protein. Front Physiol. 2014;5:299.
- Von Hoff DD, Ramanathan R, Borad M, et al. SPARC correlation with response to gemcitabine (G) plus nab-paclitaxel (nab-P) in patients with advanced metastatic pancreatic cancer: a phase I/II study. J Clin Oncol. 2009;27(15 suppl):4525.
- Schiemann BJ, Neil JR, Schiemann WP. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor--beta-signaling system. Mol Biol Cell. 2003;14:3977-3988.
- John TA, Vogel SM, Tiruppathi C, et al. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. 2003;284:L187-L196.
- Lexicomp. Clinical Drug Information. https://online.lexi.com/lco/action/login. [Requires log in]. Accessed May 18, 2018.
- Binder D, Hubner RH, Temmesfeld-Wollbruck B, et al. Pulmonary toxicity among cancer patients treated with a combination of docetaxel and gemcita-bine: a meta-analysis of clinical trials. Cancer Chemother Pharmacol. 2011;68:1575-1583.