Acute promyelocytic leukemia (APL) makes up approximately 10% of all cases of acute myeloid leukemia (AML) in adults and is considered an aggressive subtype of AML.1-3 APL is cytogenetically characterized by the t(15;17) chromosomal translocation, which produces a PML-RARA fusion gene.1 In the past, APL was fatal and had a high incidence of hemorrhagic mortality.4 However, with the current treatment of APL at presentation, patients’ survival has improved significantly.5
The treatment planning of APL is primarily based on risk stratification of the disease.3 Regardless of risk stratification, a combination regimen of arsenic trioxide and all-trans retinoic acid is one of the preferred chemotherapy-sparing treatments for the management of low- and high-risk patients with APL.3
Arsenic trioxide has a black box warning for corrected QT (QTc) interval prolongation, with an up to 40% risk for a QTc interval of >500 milliseconds (msec), which could affect the continuation of treatment.6-8
QT prolongation can potentially lead to fatal torsades de pointes, a polymorphic type of ventricular arrhythmia that has been often observed in studies of treatment with arsenic trioxide.9-11 The exact mechanism of QTc interval prolongation by arsenic trioxide is not fully understood; however, an interaction between the potassium channel of the cardiomyocytes and arsenic trioxide could cause QTc interval prolongation.12
The general recommendations for the management of prolonged QTc interval include frequent electrocardiogram (EKG) monitoring, discontinuation of other QTc interval–prolonging drugs, and monitoring and replacement of serum potassium and magnesium.6,13
In addition, based on the study by Lo-Coco and colleagues that established arsenic trioxide as a part of the standard of care for APL, arsenic trioxide should be withheld for prolonged QTc interval readings (males: QTc interval >450 msec; females: QTc interval >460 msec), until the QTc interval is normalized.7 Per the treatment protocol, once the QTc interval was normalized, arsenic trioxide was resumed at a lower dose of 0.075 mg/kg (ie, 50% dose reduction) for 7 days; if there was no further QTc interval prolongation, the dose of arsenic trioxide was increased to 0.11 mg/kg (ie, 75% of full dose) for 7 days, and then to the full dose of 0.015, as long as there was no additional QTc interval prolongation.7
These holding parameters and dose reductions could result in an extended duration of therapy and a prolonged hospital stay, and could limit the total therapeutic dose of the drug administered.
Congenital long QT syndrome (LQTS) is a hereditary cardiac disease that is characterized by a prolongation of the QTc interval at baseline EKG, which could lead to life-threatening arrhythmias.14 The use of beta-blockers in LQTS has shown benefits in shortening the QTc interval and has been mentioned in small studies and in 1 meta-analysis study.15-20 However, to our knowledge, there are no studies of drug-induced QTc interval prolongation in patients with APL who were receiving arsenic trioxide treatment and beta-blockers.15-20
We present 3 case reports of the use of beta-blockers in patients with APL and prolonged QTc interval who are receiving an arsenic trioxide–based regimen.
Case 1
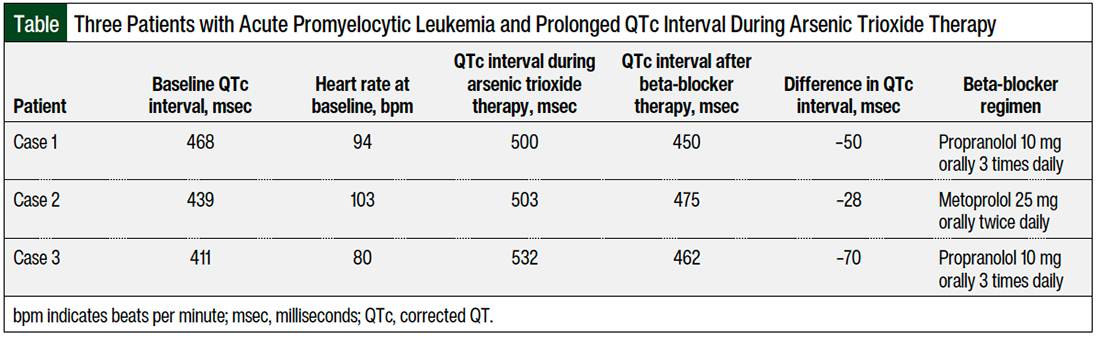
Patient 1 was a 35-year-old man with no significant medical history, who was admitted to the hospital with a diagnosis of low-risk APL (white blood cell count <10,000/mcL). The patient was initiated treatment with a regimen of all-trans retinoic acid and arsenic trioxide and had a baseline 12-lead EKG that showed a QTc per the Fridericia formula (QTcF) of 468 msec. On day 2, an EKG showed a prolonged QTcF to 500 msec, a heart rate of 94 bpm, and blood pressure of 119/82 mm Hg.
Because of the QTc interval prolongation, the arsenic trioxide regimen was held. On the same day, propranolol 20 mg orally 3 times daily was added off-label to manage the QTc interval prolongation. On day 3, the QTcF decreased to 450 msec, and treatment with arsenic trioxide was resumed after holding it for 1 day.
Propranolol treatment was continued until the completion of the arsenic trioxide regimen, and there was no further incidence of QTc interval prolongation.
Case 2
Patient 2 was a 46-year-old woman with a medical history of asthma and anxiety, who was admitted to the hospital with a diagnosis of low-risk APL. A regimen of all-trans retinoic acid and arsenic trioxide was planned to be started; however, her baseline EKG showed a QTcF of 506 msec, and her heart rate was 103 bpm. Hence, all-trans retinoic acid was started alone, without arsenic trioxide. Other QTc interval–prolonging drugs were eliminated on admission. On day 5, an EKG showed a QTcF of 439 msec and the patient was started treatment with arsenic trioxide.
Two days later, the QTcF was prolonged to 506 msec and arsenic trioxide was held. Subsequently, metoprolol 12.5 mg orally twice daily was initiated to slow the QTc interval. On day 9, the QTcF decreased to 461 msec, and treatment with arsenic trioxide was resumed. However, on the next day, the QTc interval prolonged again, to 503 msec, and the metoprolol dose was increased to 25 mg orally twice daily.
The QTcF slowed to 475 msec on the next day, and treatment with arsenic trioxide was restarted. The therapy was not interrupted again after the increased dose of metoprolol, until the patient’s APL was in remission.
Case 3
Patient 3 was a 54-year-old man with a medical history of hypertension, hypothyroidism, and diabetes mellitus, who was admitted to the hospital with a diagnosis of low-risk APL. A regimen of all-trans retinoic acid and arsenic trioxide was started after a baseline EKG showed a QTcF of 411 msec. On days 17 and 18 after starting the therapy, EKGs showed prolonged QTcFs of 510 msec and 532 msec, respectively.
Arsenic trioxide therapy was held on day 17, and citalopram was held to reduce other risk factors that could prolong the QTc interval. Subsequently, propranolol was added to shorten the QTc interval. A dose of 10 mg orally 3 times daily of propranolol was given on day 18. On the next day, an EKG showed a QTcF of 462 msec, and treatment with arsenic trioxide was resumed.
On day 22, the patient was discharged with all-trans retinoic acid and propranolol, with a dose reduction to 10 mg orally twice daily and a plan to taper off the arsenic trioxide treatment slowly, over 2 weeks.
Discussion
All 3 patients were given a treatment protocol of potassium and magnesium to maintain a potassium level of >4 mEq/L and a magnesium level of >2 mg/dL. In addition, the 3 patients were screened for medications known to prolong QTc interval, and their treatments were switched or discontinued accordingly, as described in case 3.
After the normalization of the QTc interval, the patients’ EKGs were monitored weekly, or based on the physician preference. During discharge, all 3 patients were counseled and advised on how to taper off and discontinue the beta-blockers slowly, over 2 weeks.
Using a PubMed search with the key words “beta-blocker,” “QTc changes,” “arsenic trioxide,” and “QTc prolongation,” we found 62 publications with these terms between 1977 and 2021. To our knowledge, based on our database search, this is the first documentation of beta-blocker use for shortening the QTc interval in patients with drug-induced QTc interval prolongation.
The majority of articles that mentioned the use of a beta-blocker to shorten the QTc interval were in patients with congenital LQTS who received a beta-blocker as a first-line therapy of choice.15-20 Of note, the mechanism of action of arsenic trioxide–induced QTc interval prolongation may be similar to that of hereditary LQTS, especially the LQT1 genotype.
In hereditary LQTS, the mechanism of QT prolongation is through the increase in depolarization of calcium currents or a decrease in the repolarization of potassium currents.14 The LQT1 genotype has a mutation in the KCNQ1 gene that encodes specifically for the potassium channel.14 Conversely, the proposed mechanism of action of arsenic trioxide–induced QTc interval prolongation is that calcium currents are increased, which reduces the surface expression of the cardiac potassium channel, leading to QT prolongation and torsades de pointes.21
The mechanism of action by which beta-blockers affect the QTc interval is not completely understood. However, these agents have varying properties, including differences in selectivity, lipophilicity, and sodium channel–blocking capacity.14,16 In LQTS, the efficacy of beta-blockers is variable across LQTS genotypes, and the protective effect is the highest with LQT1 genotype and is absent in LQT3 genotype.20 This could explain why beta-blockers may be effective in arsenic trioxide–induced QTc interval prolongation, because the mechanism of action is similar to LQT1. In addition, various factors, such as age, sex, and QTc intervals, may influence the effectiveness of beta-blockers.20
Propranolol is a nonselective beta-blocker; it has a better lipophilicity and the highest blocking effect on the sodium channels compared with other drugs in that class.14-16 Propranolol, followed by nadolol, are the most effective treatment options for LQTS, which is attributed to the nonselectivity of propranolol and nadolol for beta 1 or 2 receptors, compared with metoprolol, which is selective for beta 1.16 This could explain why our patient in case 2 was not fully managed with metoprolol to shorten the QTc interval (Table). Another explanation could be that metoprolol tends to require higher doses than standard dosing to control QTc interval, as was shown in the study by Abu-Zeitone and colleagues.19 We later increased the dose of metoprolol in case report 2 up to 25 mg twice daily.
A case report published by Zeitjian and colleagues discussed a patient with APL who received treatment with arsenic trioxide and had ventricular tachycardia that was independent of QT prolongation.22 Arsenic trioxide treatment was not discontinued, because the patient did not have a prolonged QTc interval, and the researchers decided to initiate nadolol 40 mg daily as an off-label treatment for ventricular tachycardia. The episodes of ventricular tachycardia ceased after starting nadolol, and the patient was able to complete the course of arsenic trioxide.22 In our 3 case reports, the QTc interval shortening effect by a beta-blocker was similar to that reported by Zeitjian and colleagues, but their case varied notably from our 3 cases in that their patient did not have a prolonged QTc interval.22
QTc interval prolongation is a well-known side effect of arsenic trioxide and can occur in up to 40% of patients.12 In our 3 cases, the patients had noncongenital prolonged QTc interval induced by the use of an arsenic trioxide–containing regimen, and none of these patients had cardiac disease. However, 2 of our 3 patients had prolonged QTc interval at baseline from unknown reasons. In case 2, the patient was a woman, which may be a contributing factor for a prolonged QTc interval at baseline. The addition of a beta-blocker to our patients’ regimen until the completion of a hospital course helped to maintain the QTc intervals at <500 msec, and thus allowed for uninterrupted arsenic trioxide therapy.
In general, the best practice is to avoid adding more medications to a patient’s therapy, because of the risks associated with polypharmacy. However, the uniqueness of the application here is that arsenic trioxide therapy is not lifelong; thus, adding a beta-blocker will only be temporary and will have minimal side effects.
Using this strategy could potentially improve patients’ outcomes, by allowing the on-time administration of arsenic trioxide therapy through the maintenance of normal QTc intervals. However, because of the small number of cases presented here, it is impossible to determine if the QTc interval reduction was truly the result of the beta-blocker initiation or of holding arsenic trioxide therapy.
Conclusion
To our knowledge, this is the first case series about the use of beta-blocker therapy to shorten the QTc interval in patients with cancer who are receiving an arsenic trioxide–containing regimen and to resume an arsenic trioxide treatment regimen successfully after starting beta-blocker therapy. The limited number of cases presented here hinders generalizability regarding the use of beta-blocker therapy to shorten the QTc interval or the assessment of causality between beta-blocker use and the QTc interval shortening effect. Future prospective studies are needed to determine a potential causality between beta-blocker therapy and shortening the QTc interval for drug-induced QTc interval prolongation.
Author Disclosure Statement
Dr Alsuhebany, Dr Larriva, and Dr Gowin have no conflicts of interest to report. Dr McBride is a Consultant to Coherus Biosciences, Pfizer, Sandoz, MorphoSys, and Bristol Myers Squibb, and is on the Speaker’s Bureau of Coherus Biosciences.
References
- Chen Z, Wang ZY, Chen SJ. Acute promyelocytic leukemia: cellular and molecular basis of differentiation and apoptosis. Pharmacol Ther. 1997;76:141-149.
- Stone RM, Mayer RJ. The unique aspects of acute promyelocytic leukemia. J Clin Oncol. 1990;8:1913-1921.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Acute Myeloid Leukemia. Version 1.2022. December 2, 2021. www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed December 7, 2021.
- Coombs CC, Tavakkoli M, Tallman MS. Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood Cancer J. 2015;5:e304. doi: 10.1038/bcj.2015.25.
- Cingam SR, Koshy NV. Acute Promyelocytic Leukemia. Treasure Island, FL: StatPearls Publishing; Updated July 2, 2021. www.ncbi.nlm.nih.gov/books/NBK459352/. Accessed March 8, 2022.
- Barbey JT, Pezzullo JC, Soignet SL. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol. 2003;21:3609-3615.
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111-121.
- Trisenox (arsenic trioxide) injection, for intravenous use [prescribing information]. Teva Pharmaceuticals; October 2020. www.trisenox.com/globalassets/trisenoxhcp/trisenox-prescribing-information.pdf. Accessed March 8, 2022.
- Soignet SL, Frankel SR, Douer D, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852-3860.
- Goldsmith S, From AHL. Arsenic-induced atypical ventricular tachycardia. N Engl J Med. 1980;303:1096-1098.
- Chiang CE, Luk HN, Wang TM, Ding PYA. Prolongation of cardiac repolarization by arsenic trioxide. Blood. 2002;100:2249-2252.
- Porta-Sánchez A, Gilbert C, Spears D, et al. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: a systematic review. J Am Heart Assoc. 2017;6:e007724. doi: 10.1161/JAHA.117.007724.
- Roboz GJ, Ritchie EK, Carlin RF, et al. Prevalence, management, and clinical consequences of QT interval prolongation during treatment with arsenic trioxide. J Clin Oncol. 2014;32:3723-3728.
- Schwartz PJ, Crotti L, Insolia R. Long-QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5:868-877. Erratum in: Circ Arrhythm Electrophysiol. 2012;5:e119-e120.
- Ackerman MJ, Priori SG, Dubin AM, et al. Beta-blocker therapy for long QT syndrome and catecholaminergic polymorphic ventricular tachycardia: are all beta-blockers equivalent? Heart Rhythm. 2017;14:e41-e44.
- Chockalingam P, Crotti L, Girardengo G, et al. Not all beta-blockers are equal in the management of long QT syndrome types 1 and 2: higher recurrence of events under metoprolol. J Am Coll Cardiol. 2012;60:2092-2099.
- Bennett MT, Gula LJ, Klein GJ, et al. Effect of beta-blockers on QT dynamics in the long QT syndrome: measuring the benefit. Europace. 2014;16:1847-1851.
- Moss AJ, Zareba W, Hall WJ, et al. Effectiveness and limitations of β-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616-623.
- Abu-Zeitone A, Peterson DR, Polonsky B, et al. Efficacy of different beta-blockers in the treatment of long QT syndrome. J Am Coll Cardiol. 2014;64:1352-1358.
- Han L, Liu F, Li Q, et al. The efficacy of beta-blockers in patients with long QT syndrome 1–3 according to individuals’ gender, age, and QTc intervals: a network meta-analysis. Front Pharmacol. 2020;11:579525. doi: 10.3389/fphar.2020.579525.
- Ficker E, Kuryshev YA, Dennis AT, et al. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66:33-44.
- Zeitjian V, Moazez C, Arslan W, Saririan M. QT independent ventricular tachycardia induced by arsenic trioxide. Case Rep Cardiol. 2019;2019:9870283. doi: 10.1155/2019/9870283.