Acute graft-versus-host disease (GVHD) is an inflammatory process that primarily affects the gastrointestinal tract, liver, and skin. The pathophysiology involves activated donor T-cells that promote cytokine release and downstream tissue injury, leading to the physical manifestations of acute GVHD, including diarrhea, nausea, rash, and jaundice.1 Acute GVHD is one of the leading causes of mortality in patients undergoing allogeneic hematopoietic stem-cell transplant (HSCT), and contributes significantly to posttransplant morbidity.2
Calcineurin inhibitors, such as tacrolimus, are the mainstay of pharmacologic prophylaxis for GVHD. By inhibiting the transcription of interleukin-2, calcineurin inhibitors prevent the activation and proliferation of T-cells.3,4 The result is lower rates of GVHD when tacrolimus prophylaxis is used as monotherapy or in combination with other immunosuppressants.5-7
Target tacrolimus concentrations have been established to reduce the incidence of GVHD after allogeneic HSCT, while limiting toxicity, and they can vary by the conditioning regimen. Many myeloablative and reduced-intensity conditioning regimens target tacrolimus serum levels of 10 ng/mL to 15 ng/mL, nonmyeloablative regimens target 10 ng/mL to 20 ng/mL, and haploidentical regimens target 5 ng/mL to 15 ng/mL, although this can change based on institution preference.8,9 Tacrolimus concentrations of more than 12 ng/mL have been associated with reduced risk for acute GVHD, particularly in the first few weeks after transplant.8,9 Patients who do not maintain target serum concentrations are at an increased risk for GVHD.10
Therefore, continuous infusion of intravenous (IV) tacrolimus is often administered in the peritransplant setting to maintain therapeutic concentrations in patients undergoing HSCT.8,11 Continuous infusion of tacrolimus shows stable drug exposure, does not rely on the patient’s ability to take oral medications, bypasses first-pass metabolism in the liver, and is more readily adjustable than oral tacrolimus based on random serum concentrations, by immediate adjustment to infusion rate.4
Genetic polymorphisms, drug interactions, and renal and hepatic function are among the many variables that affect tacrolimus concentrations.11-16 Compared with IV tacrolimus, the oral formulation of the drug is more susceptible to drug interactions from intestinal and hepatic cytochrome (CY) P450 enzyme metabolism.16 Although many risk factors affect tacrolimus serum concentrations, standardized, evidence-based recommendations for dose adjustments to compensate for these effects are lacking.
When converting patients from IV to oral formulation, one challenge is to determine the appropriate conversion factor, given the variability in reported tacrolimus bioavailability.11,17,18 Supratherapeutic concentrations of tacrolimus increase the incidence of toxicity, whereas subtherapeutic concentrations may lead to acute GVHD, thereby increasing morbidity and mortality in patients undergoing allogeneic HSCT.
This study aimed to identify factors affecting tacrolimus levels after conversion from IV to oral form after allogeneic HSCT, to identify the impact of nontherapeutic tacrolimus levels on acute GVHD outcomes, and to determine optimal conversion factors based on patient-specific characteristics.
Methods
This retrospective chart review identified patients undergoing allogeneic HSCT between February 2014 and February 2017 at the University of Kansas Health System. All patients who received IV tacrolimus, were aged >18 years, received an allogeneic HSCT for a hematologic malignancy, and were admitted by day 0 of HSCT were eligible to be included in the study. Patients were also included if the tacrolimus serum concentration drawn before conversion to oral dosing was nontherapeutic. Patients who had undergone >1 allogeneic HSCT were eligible to be included in the study, but they were only included 1 time, using data from their first transplant if able, or from their second transplant if the first transplant met the exclusion criteria.
Patients were excluded if they were aged <18 years, received IV tacrolimus outside the peritransplant period, had tacrolimus therapy interrupted for >24 hours, received an allogeneic HSCT for an underlying disease other than hematologic malignancy, or received only oral tacrolimus as part of an outpatient transplant. The University of Kansas Health System Institutional Review Board reviewed and approved the study protocol.
The primary objective of the study was to determine the proportion of patients who attained a therapeutic tacrolimus level within 72 hours after conversion from IV to oral formulation. The secondary objectives were the time (in days) to target tacrolimus levels after conversion from IV to oral tacrolimus, and the effect of age, race, change in renal function, total bilirubin, albumin, antifungal agent, total parenteral nutrition use, presence of diarrhea at conversion, mucositis at time of conversion, and time to reaching target tacrolimus serum concentrations. Additional objectives of interest included the incidence of grade II to grade IV acute GVHD in patients with subtherapeutic versus therapeutic tacrolimus levels, and the mean IV to oral conversion factor that produces therapeutic serum concentrations after transplant. Conversion factor was defined as the number needed to multiply the total daily IV tacrolimus dose to obtain total daily oral dose.
Standardized institutional protocols were followed for tacrolimus dose adjustments based on serum concentrations, renal function, and antifungal drug coverage. Our standard conversion factors from IV to oral tacrolimus based on antifungal drug coverage included the following: 2 for fluconazole, 4 for micafungin, and 1.5 each for posaconazole and for voriconazole. In addition, for patients with serum creatinine >2.0 mg/dL or >2 times the patient’s baseline, tacrolimus was held for at least 12 hours and then resumed at a 50% dose reduction.
Tacrolimus serum concentrations could be drawn at any time for patients who received IV tacrolimus and were drawn as troughs for patients who received oral tacrolimus 10 hours to 12 hours after the last dose and before administering the next oral dose. The first tacrolimus trough was drawn between 48 hours and 72 hours after conversion from IV to oral tacrolimus to ensure that the trough was evaluating steady-state concentration.
Dose adjustments by 25% to 50% based on clinical judgment and rounded to tablet size were made for tacrolimus serum concentrations outside the target range. For patients who received myeloablative or reduced-intensity conditioning regimens from a matched sibling or from a matched unrelated donor, the target tacrolimus concentrations were defined as 10 ng/mL to 15 ng/mL on day 0 through day 30, and 5 ng/mL to 15 ng/mL on day 31 through day 100. For patients who received nonmyeloablative conditioning regimens (nonhaploidentical), target tacrolimus concentrations were defined as 10 ng/mL to 20 ng/mL day 0 through day 30, then 5 ng/mL to 20 ng/mL on day 31 or later. For patients who received haploidentical transplants (regardless of conditioning regimen intensity), the target tacrolimus concentrations were defined as 5 ng/mL to 15 ng/mL on day 7 through day 180.
For tacrolimus levels to be considered therapeutic, the patient had to be within the target tacrolimus range on 2 consecutive laboratory draws at least 24 hours apart. The first trough within range was considered the date of therapeutic tacrolimus. Falsely elevated levels, defined as a tacrolimus level of 150% or more than a repeated level drawn within 24 hours, were excluded from the study. Mucositis was assessed by the Glucksberg grading scale.19
Data collected for all patients included age, sex, ideal body weight, race, diagnosis, transplant date, cell source, conditioning regimen, degree of human leukocyte antigen (HLA)-match, donor relation to the patient, type of antifungal drug coverage, concomitant CYP3A4 inhibitors/inducers, date of IV to oral tacrolimus conversion, tacrolimus doses and serum concentrations, serum creatinine, total bilirubin, albumin, use of total parenteral nutrition, presence of diarrhea and mucositis, and presence and grade of acute GVHD.
Continuous variables were described by the median and range. Categorical variables were described by frequency and percentages, and chi-square test was used to compare those variables. Logistic regression was performed to identify risk factors for increased time to reaching target tacrolimus levels and for acute GVHD. A multivariate linear regression model was built with the factors that were significant at 0.10 in univariate logistic regression analysis. A P value of <.05 was considered significant. The SPSS 25.0 statistical software package (SPSS, Inc; Chicago, IL, USA) was used for statistical analysis.
Results
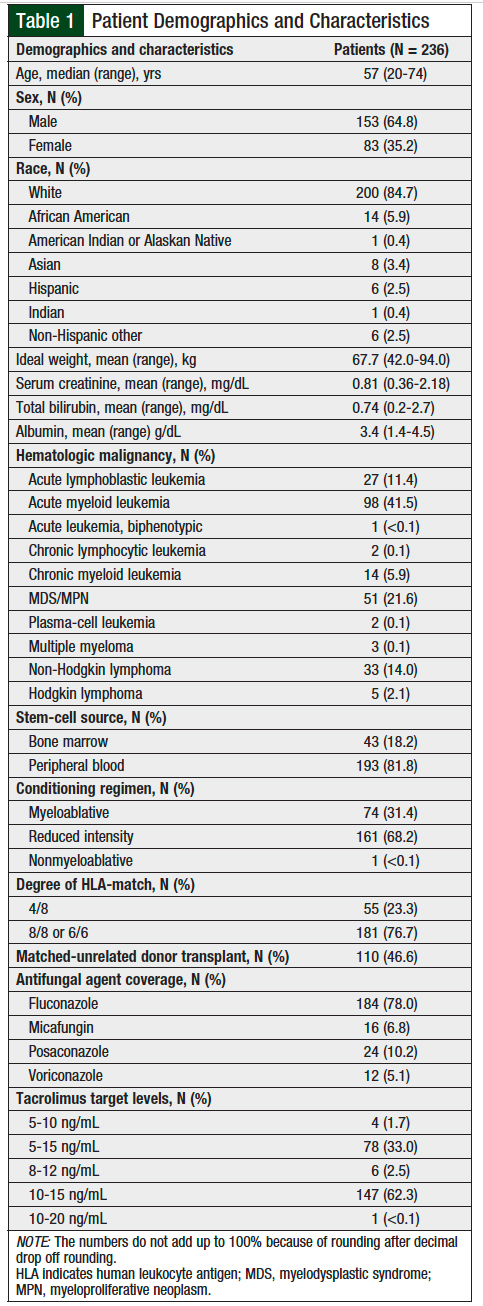
A total of 236 patients were included in the final analysis; 3 patients were excluded because their tacrolimus therapy was interrupted for >24 hours. Patient demographics are summarized in Table 1. The patients’ median age was 57 years. The majority of patients were male (64.8%) and white (84.7%). Acute myeloid leukemia was the most common (41.5%) underlying hematologic malignancy. The majority of patients received peripheral blood stem cells (81.8%), a reduced-intensity conditioning regimen (68.2%), and cells from an 8/8 HLA-matched donor (76.7%).
The most common antifungal agent used during transplant was fluconazole (78%; Table 2). No strong CYP3A4 inhibitors or inducers were given concomitantly to patients while receiving tacrolimus, other than the antifungal agents discussed.
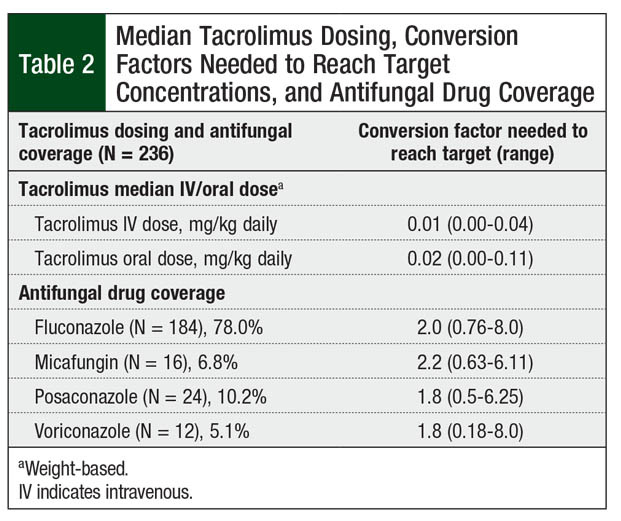
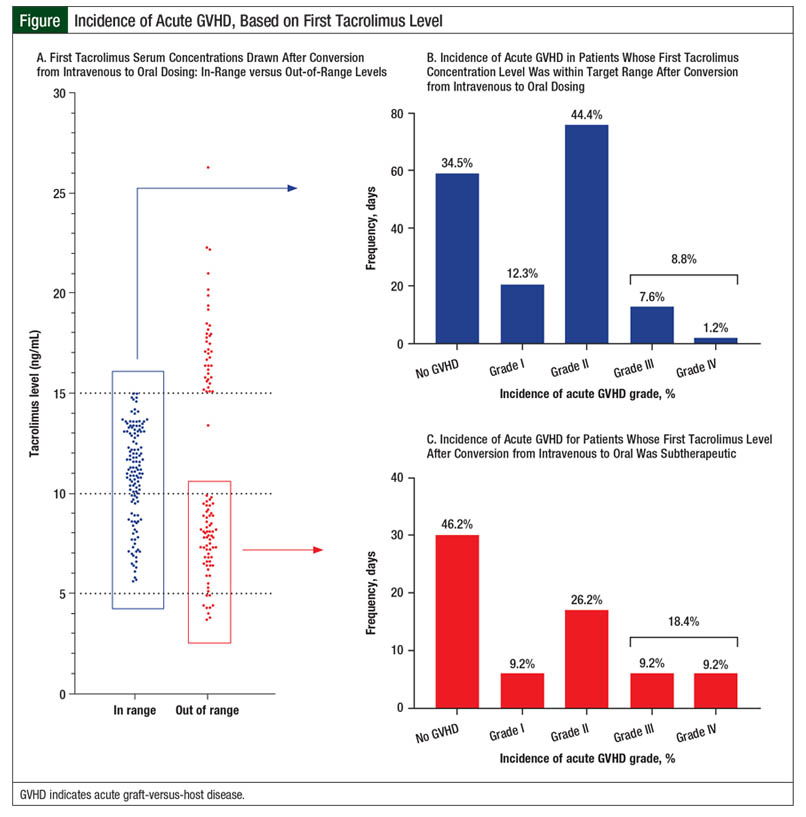
Overall, 67 (28.4%) patients had an initial tacrolimus subtherapeutic level after conversion from IV to oral tacrolimus, 30 (12.7%) patients had supratherapeutic levels on initial testing, and 139 (58.9%) patients had levels within the target range (Figure). However, only 63 (26.7%) patients with an initial level within the target range maintained the tacrolimus target serum concentrations on their next draw. These patients achieved their target levels within 3 days of conversion from IV to oral tacrolimus. The median tacrolimus oral dose that produced target serum concentrations varied by concomitant azole therapy (Table 2). Patients had to have a median of 7 days to achieve target serum concentrations after conversion from IV to oral tacrolimus.
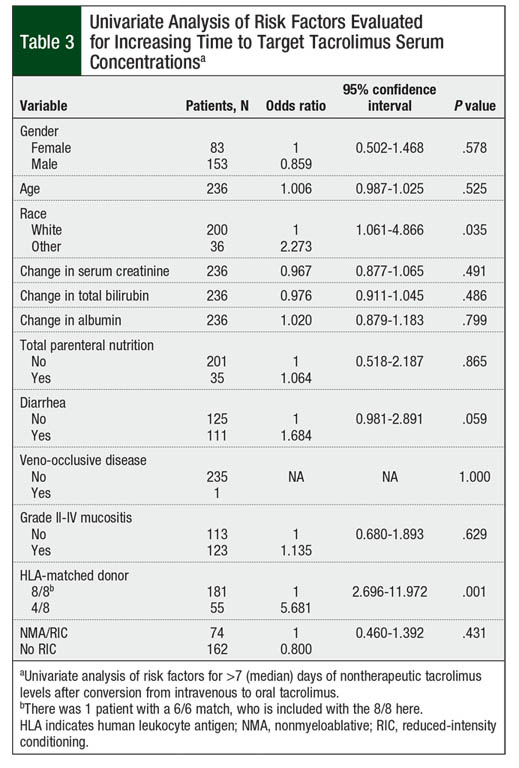
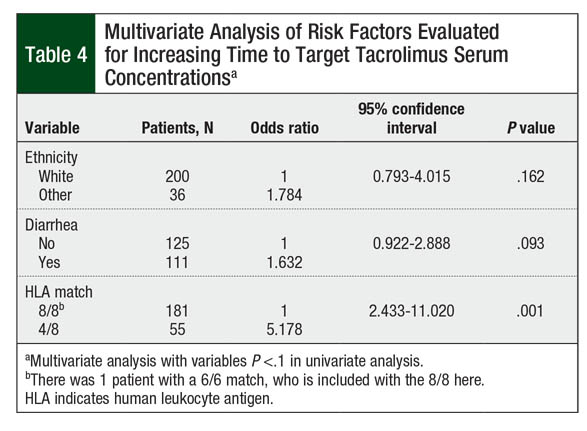
On univariate analysis, race, diarrhea, and HLA-matched donor significantly affected the time to therapeutic tacrolimus levels (Table 3). Nonwhite patients, patients with diarrhea at time of conversion, and those undergoing haploidentical transplant were more likely to have a longer time than the median of 7 days to achieve the target tacrolimus serum concentrations (P = .035, P = .059, and P = .001, respectively). Gender, age, changes in hepatic or renal function, albumin, total parenteral nutrition use during the peritransplant period, presence of veno-occlusive disease, grade II to grade IV mucositis, and intensity of conditioning regimen were not statistically significant in univariate analysis (P >.10; Table 3).
On multivariate analysis, however, only patients with 4/8 HLA-matched donor were likely to require more than the median of 7 days to achieve target tacrolimus serum concentrations (P = .001; Table 4). The presence of diarrhea at time of IV-to-oral conversion numerically increased the time to target tacrolimus concentrations, although this did not reach statistical significance (P = .093). Race did not significantly affect the time to target tacrolimus concentrations (P = .162; Table 4).
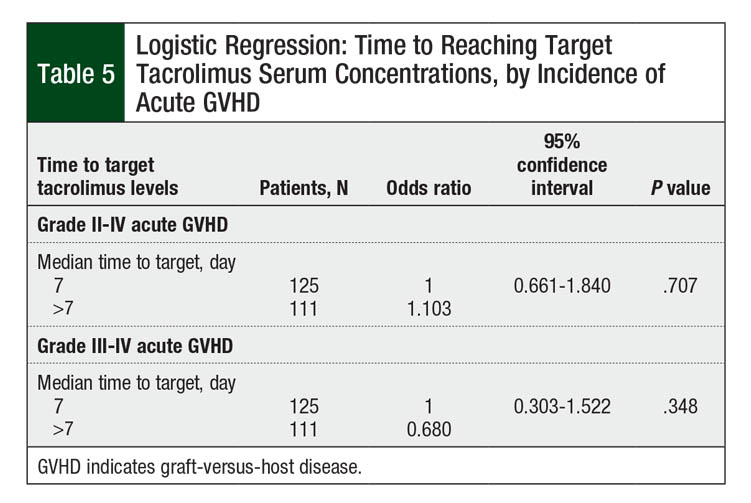
The incidence of grade II to grade IV acute GVHD in our patient population was 50.8% (N = 120). The rate of grade III or IV acute GVHD was 11.4% (N = 27). Table 5 shows the number of patients who achieved target tacrolimus serum levels by a median of day 7 or later. Longer time to target concentrations beyond the median of 7 days did not increase the risk for grade II to grade IV acute GVHD (P = .707; Table 5). The incidence of grade III to grade IV acute GVHD was also not affected by increased time to target tacrolimus serum concentrations after conversion (P = .348; Table 5).
Discussion
To our knowledge, this is the first study that identifies the risk factors for nontherapeutic tacrolimus levels in adults after HSCT. Although 139 (58.9%) patients were within target tacrolimus serum concentrations on the initial serum drawn after conversion from IV to oral dosing, they did not maintain therapeutic levels by the second draw. Only 63 (26.7%) of these 139 patients remained at therapeutic levels on the first 2 draws after conversion. This resulted in a median number of 7 days to achieve therapeutic tacrolimus concentrations, which is consistent with findings in the literature.20
A potential contributing factor is the time of discharge for patients after switching from IV to oral tacrolimus. Ideally, patients would be switched to oral tacrolimus at least 24 hours before discharge to ensure tolerability; however, this may not always coincide with engraftment. In addition, the timing of administration of oral tacrolimus after discontinuation of the IV form is not well established in the literature, resulting in practice inconsistencies.
Conversion factors based on antifungal agent use were similar to current recommendations.12,16 The conversion factor for fluconazole remained at 2.0. The conversion factor for posaconazole and for voriconazole was 1.8 based on our data, which is slightly higher than our standard of 1.5. The use of micafungin required a median conversion factor of 2.2, which is lower than the standard of 4. This lower value may reflect the increased bioavailability of tacrolimus in patients undergoing HSCT compared with healthy individuals.21
In a univariate analysis, patients from races other than white demonstrated longer time to target tacrolimus concentrations, but this was not the case in a multivariate analysis. Specific variations in the CYP3A5 genotype can alter the metabolism of tacrolimus.13,14 Specifically, CYP3A5*3 decreases the metabolism of tacrolimus, resulting in higher concentrations, with similar starting doses.14 The frequency of this variation differs in various races. Whites and Asians are shown to have increased frequency of CYP3A5*3, but they were included in different groups in our analysis.14 We were unable to determine the frequency of this variant in our population, because of a lack of pharmacogenomic analysis for more accurate representation. This might have contributed to the lack of statistical difference found among patients’ races.
The presence of diarrhea at the time of conversion from IV to oral tacrolimus showed a trend toward increased time to target levels, although it was not statistically significant in multivariate analysis. Diarrhea is a possible surrogate marker for severe mucositis, separate from documentation in the medical record. Patients with mucositis may have a continually healing gastrointestinal lining that does not absorb oral tacrolimus as well after conversion, requiring higher doses.
The presence of diarrhea itself suggests poor absorption of oral medications because of increased gut transit time. Patients with diarrhea receiving fluconazole at the time of conversion had a median conversion factor of 2 to achieve target tacrolimus serum concentrations. The pharmacokinetic variability in patients with diarrhea may put them at an increased risk for acute GVHD from nontherapeutic tacrolimus concentrations. Therefore, patients with diarrhea may benefit from continuing IV tacrolimus until the diarrhea has resolved, to decrease the pharmacokinetic variability and the risk for subtherapeutic concentrations.
Patients with 4/8 HLA-matched donor had significantly longer times to target tacrolimus levels compared with patients with full 8/8 (or 6/6) HLA-matched donor. This is unexpected, considering the wider range for tacrolimus serum concentrations of 5 ng/mL to 15 ng/mL. A potential cause for these variable tacrolimus levels could be an interaction with posttransplant cyclophosphamide given for haploidentical transplants. These 2 medications are substrates of hepatic enzyme CYP3A4.4,12,22,23
Studies with cyclophosphamide and CYP3A4 inhibitors have shown various effects on metabolism; however, tacrolimus is proved to have increased serum concentrations with CYP3A4 inhibitors, although it has not been studied with cyclophosphamide directly.22,23 The concurrent exposure to cyclophosphamide and tacrolimus could cause CYP3A4-substrate competition and alter the serum concentrations of tacrolimus. This effect would be greatest shortly after cyclophosphamide administration, considering its short half-life even at high doses.24
However, cyclophosphamide has been shown to cause moderate hepatic impairment for up to 20 days after administration, which could vary the metabolism of tacrolimus long after administration.25 Although hepatic impairment may be part of the explanation, change in total bilirubin did not have a significant effect on the time to therapeutic tacrolimus levels. This unexpected finding requires more investigation to identify all potential causes to prevent significant time outside the target tacrolimus concentrations for patients undergoing haploidentical transplant.
The incidence of grade II to grade IV acute GVHD in our study was similar to previously reported data, at 50.8%.1 However, our data suggest that this incidence is not related to immunosuppression conversion (Table 5 and Figure). A possible explanation for this lack of correlation is the amount of time patients received IV tacrolimus, specifically if 2 weeks or longer.8,9 This may confound the effects of the time to therapeutic serum concentrations on acute GVHD incidence, because the first 2 weeks after transplant are the most crucial for the prevention of GVHD.9
Based on our study results, we have amended our institutional practice to improve the transition from IV to oral tacrolimus administration. We have established standard procedures to discontinue IV tacrolimus at 9:00 pm, the time of administration of the first oral dose of tacrolimus. We are currently reviewing the efficacy of this strategy on subsequent tacrolimus levels and hope to publish the results at a future date.
Limitations
Our analysis has some limitations. As a retrospective chart review at a single institution, this may limit the generalizability of the findings.
In addition, the study included unidentified confounding factors, such as how long after transplant patients were converted to oral tacrolimus, the timing of patient discharge, and changes in antifungal coverage before achieving therapeutic tacrolimus serum concentration. After discharge, patients may receive their tacrolimus and other medications at more variable times of the day compared with standardized administration times, and timing of food intake and administration may be more variable, affecting absorption.
Furthermore, we did not have access to pharmacogenomics analyses to determine inherent differences in metabolism of tacrolimus among patients.
Finally, our institution did not have a standard timing of oral tacrolimus administration after discontinuation of continuous-infusion IV tacrolimus.
Conclusions
Our findings showed that the majority of our patients were not within the target range after the initial conversion from IV to oral tacrolimus. The patients required a median of 7 days to reach the target concentrations, which was lengthened by the presence of diarrhea at time of conversion or by haploidentical donor cells. However, increased time to target levels after conversion did not increase the incidence of acute GVHD. Considering these findings, we have established a standardized timing for IV tacrolimus discontinuation and oral tacrolimus administration. The effectiveness of an optimized conversion factor from IV to oral tacrolimus on achieving therapeutic serum concentrations and this effect on the incidence of acute GVHD should be further studied in prospective trials.
Acknowledgments
We thank Neil Dunavin, MD, MHS, for help with preparation of the figures.
Author Disclosure Statement
Dr Rockey has received honoraria from Herm Therapeutics. Dr McGuirk is on the Speaker Bureau of Kite Pharma and Novartis and has received research funding from Novartis, Fresenius Biotec, Astellas Pharma, Bellicum Pharmaceuticals, Gamida Cell, and Pluristem. Dr Konrardy, Dr Hosmer, Dr Mahmoudjafari, Dr Grauer, Mr Henry, and Dr Shune have no conflicts of interest to report.
References
- Zeiser R, Blazar BR. Acute graft-versus-host disease — biologic process, prevention, and therapy. N Engl J Med. 2017;377:2167-2179.
- D’Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2019. www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx. Accessed August 2, 2019.
- Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood. 2014;124:363-373.
- Prograf (tacrolimus) capsules/injection/granules for oral use/for intravenous use/for oral suspension [prescribing information]. Northbrook, IL: Astellas Pharma; June 2019.
- Elgarten CW, Arnold DE, Bunin NJ, Seif AE. Outcomes of matched sibling donor bone marrow transplantation in children using single-agent calcineurin inhibitors as prophylaxis for graft versus host disease. Pediatr Blood Cancer. 2018;65:e26726. doi.org/10.1002/pbc.26726.
- Gao L, Liu J, Zhang Y, et al. Low incidence of acute graft-versus-host disease with short-term tacrolimus in haploidentical hematopoietic stem cell transplantation. Leuk Res. 2017;57:27-36.
- Törlén J, Ringdén O, Garming-Legert K, et al. A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation. Haematologica. 2016;101:1417-1425.
- Ram R, Storer B, Mielcarek M, et al. Association between calcineurin inhibitor blood concentrations and outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:414-422.
- Ganetsky A, Shah A, Miano TA, et al. Higher tacrolimus concentrations early after transplant reduce the risk of acute GvHD in reduced-intensity allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:568-572.
- Moiseev IS, Burmina EA, Muslimov AR, et al. Pharmacokinetic comparison of cyclosporin A and tacrolimus in graft-versus-host disease prophylaxis. Ann Hematol. 2017;96:935-942.
- Jacobson P, Ng J, Ratanatharathorn V, et al. Factors affecting the pharmacokinetics of tacrolimus (FK506) in hematopoietic cell transplant (HCT) patients. Bone Marrow Transplant. 2001;28:753-758.
- Leather HL. Drug interactions in the hematopoietic stem cell transplant (HSCT) recipient: what every transplanter needs to know. Bone Marrow Transplant. 2004;33:137-152.
- Onizuka M, Kunii N, Toyosaki M, et al. Cytochrome P450 genetic polymorphisms influence the serum concentration of calcineurin inhibitors in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant. 2011;46:1113-1117.
- Yaowakulpatana K, Vadcharavivad S, Ingsathit A, et al. Impact of CYP3A5 polymorphism on trough concentrations and outcomes of tacrolimus minimization during the early period after kidney transplantation. Eur J Clin Pharmacol. 2016;72:277-283.
- Nishimoto M, Koh H, Tokuwame A, et al. Drug interactions and safety profiles with concomitant use of caspofungin and calcineurin inhibitors in allogeneic haematopoietic cell transplantation. Br J Clin Pharmacol. 2017;83:2000-2007.
- Groll AH, Townsend R, Desai A, et al. Drug-drug interactions between triazole antifungal agents used to treat invasive aspergillosis and immunosuppressants metabolized by cytochrome P450 3A4. Transpl Infect Dis. 2017;19:e12751. doi.org/10.1111/tid.12751.
- Boswell GW, Bekersky I, Fay J, et al. Tacrolimus pharmacokinetics in BMT patients. Bone Marrow Transplant. 1998;21:23-28.
- Karamperis N, Povlsen JV, Højskov C, et al. Comparison of the pharmacokinetics of tacrolimus and cyclosporine at equivalent molecular doses. Transplant Proc. 2003;35:1314-1318.
- Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295-304.
- Kolb M, Offer K, Jin Z, et al. Risk factors for subtherapeutic tacrolimus levels after conversion from continuous intravenous infusion to oral in children after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:957-961.
- Uberti JP, Cronin S, Ratanatharathorn V. Optimum use of tacrolimus in the prophylaxis of graft versus host disease. BioDrugs. 1999;11:343-358.
- Yule SM, Walker D, Cole M, et al. The effect of fluconazole on cyclophosphamide metabolism in children. Drug Metab Dispos. 1999;27:417-421.
- Chang TK, Weber GF, Crespi CL, Waxman DJ. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res. 1993;53:5629-5637.
- Busse D, Busch FW, Bohnenstengel F, et al. Dose escalation of cyclophosphamide in patients with breast cancer: consequences for pharmacokinetics and metabolism. J Clin Oncol. 1997;15:1885-1896.
- McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043-2048.