Although more than 36% of Americans are obese, minimal guidance exists for weight-based drug dosing in obese patients1Further controversy exists in the setting of highly toxic medications, such as cytotoxic chemotherapy. There may be an increased risk for severe chemotherapy toxicity when administering larger doses of chemotherapy as calculated by actual body weight in obese patients.2 Because of this perceived risk, some institutions choose to calculate the doses of chemotherapy using adjusted body weight instead of actual body weight.
Although the American Society of Clinical Oncology released recommendations to dose chemotherapy for solid malignancies based on actual body weight in the obese population in 2012,3 optimal chemotherapy dosing strategies for obese patients with hematologic cancers who have had a transplant remain unclear. Excessive chemotherapy dosing may contribute to transplant-related mortality, whereas underdosing may lead to suboptimal transplant outcomes. The American Society of Bone Marrow Transplant (ASBMT) released chemotherapy dosing guidelines in 2014 that acknowledged the lack of evidence to make strong dosing recommendations, but it did recommend the use of actual body weight in calculating melphalan doses for transplant conditioning.4
In the autologous hematopoietic stem-cell transplantation (HSCT) setting, however, some studies have suggested that elevated body mass index (BMI) does not have a negative effect on progression-free survival (PFS), overall survival, or non–relapse-related mortality.5,6 In fact, obese and severely obese patients who received melphalan and total body irradiation conditioning have had superior PFS and overall survival compared with normal-weight or overweight patients.5,6 Limited studies have also evaluated the impact of melphalan dosing adjustments in obese patients.6-8
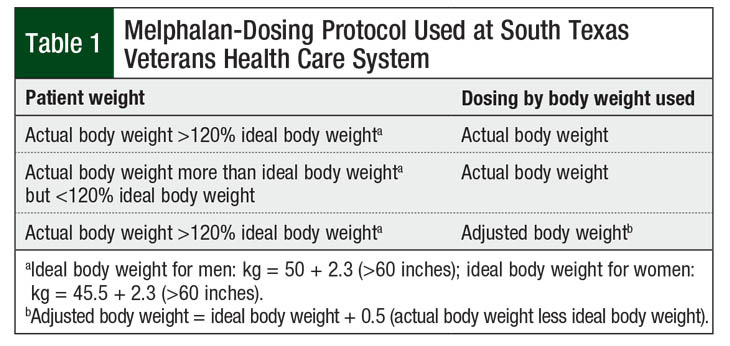
At the South Texas Veterans Health Care System (STVHCS), all patients with multiple myeloma who undergo HSCT are hospitalized during the duration of the transplant. Patients who weigh >20% of their ideal body weight receive melphalan doses based on adjusted body weight. Per STVHCS protocol, the adjusted body weight is calculated using a 50% correction (Table 1). All other patients receive melphalan doses based on their actual body weight.
The goal of this current study was to compare the outcomes between the use of actual and adjusted body weight in patients with multiple myeloma who receive a melphalan conditioning regimen during autologous HSCT.
Methods
This retrospective, single-center study is based on STVHCS’s electronic medical record and hematopoietic transplant database. Historical chart reviews were performed to identify patients with multiple myeloma who underwent autologous HSCT between January 1, 2010, and January 1, 2015. Patients received melphalan conditioning dosing based on actual body weight or based on adjusted body weight. Despite only a moderate emetic risk associated with melphalan, STVHCS’s providers were given the option, per the institution’s order sets, to use a high-emetic-risk prophylactic regimen based on the patient history and risk factors.9
Autologous HSCT recipients aged ≥18 years with multiple myeloma who received melphalan-based conditioning regimens before transplant during the prespecified time period were included in this study. The key exclusion criteria were a history of previous autologous HSCT and having a planned tandem autologous HSCT.
The patients were divided into 2 groups based on the melphalan dosing used according to the patient’s weight (actual body weight or adjusted body weight). The primary outcome was the 3-year PFS. The secondary end points were 3-year overall survival, time to disease progression, time to death, non–relapse-related mortality, response at transplantation day 100, time to neutrophil engraftment, hospitalization length of stay, melphalan dose reductions of >5% compared with dosing based on actual body weight, and the rates of adverse events.
The categorical data were compared using Fisher’s exact test or chi-square test, as appropriate. The continuous data were compared using a student t-test or Wilcoxon rank sum test, as appropriate. The Kaplan-Meier method was used to estimate the distributions of PFS and overall survival rates. The 3-year PFS and overall survival rates were also compared between the 2 groups using the log-rank test. Statistical significance was defined as P <.05, with Bonferroni correction used for multiple comparisons.
This study was deemed by the local Institutional Review Board to be exempt from review before the initiation of data collection.
Results
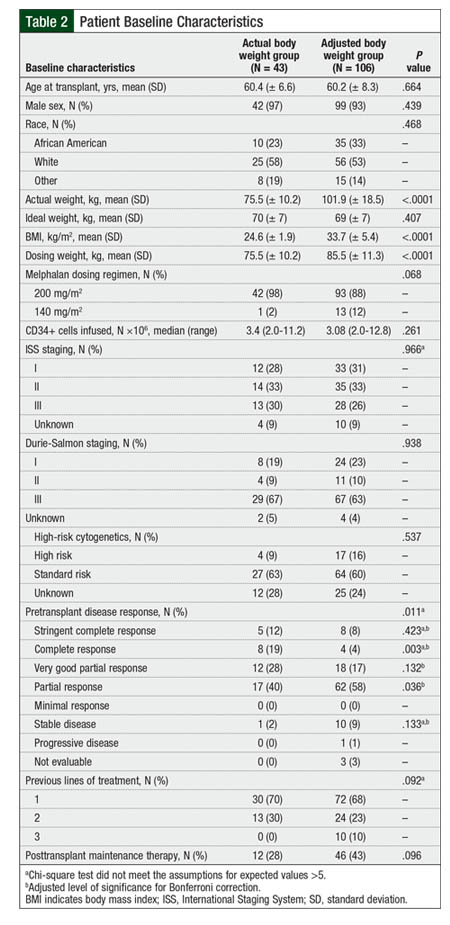
Between January 1, 2010, and January 1, 2015, a total of 174 patients received a melphalan-based conditioning regimen and underwent autologous HSCT for the treatment of multiple myeloma. Of these patients, 25 had received previous autologous HSCT and were excluded from the study, resulting in a total of 149 patients who were eligible for the study. Of those 149 patients, 42 patients received melphalan doses based on their actual body weight, and 106 patients received melphalan doses based on their adjusted body weight. The baseline characteristics between the groups were relatively well-balanced (Table 2).
At baseline, significantly more patients in the actual body weight group had achieved a complete response before transplant compared with patients in the adjusted body weight group. As expected, the dosing weights in the adjusted body weight group were significantly greater than those in the actual body weight group.
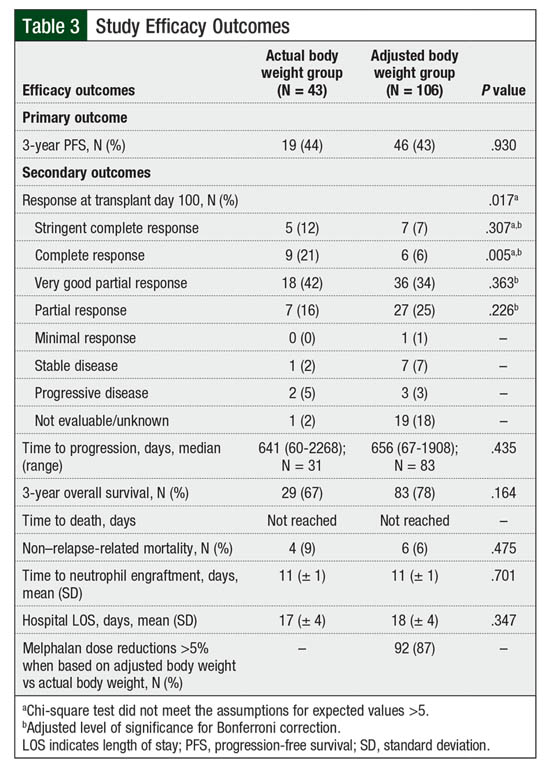
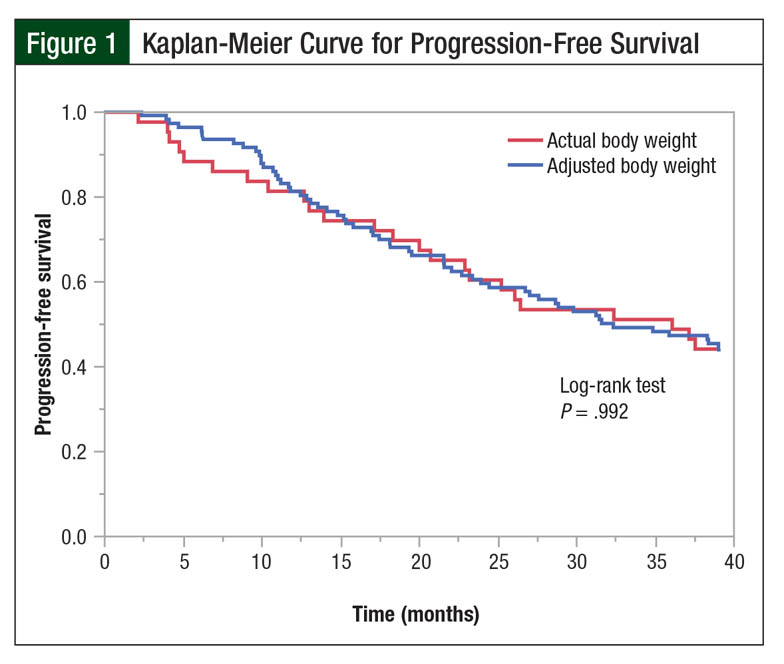
A total of 19 patients in the actual body weight group and 46 patients in the adjusted body weight group had a 3-year PFS (44% vs 43%, respectively; P = .93; Table 3). There were no differences in the Kaplan-Meier PFS curves (log-rank test, P = .992; Figure 1).
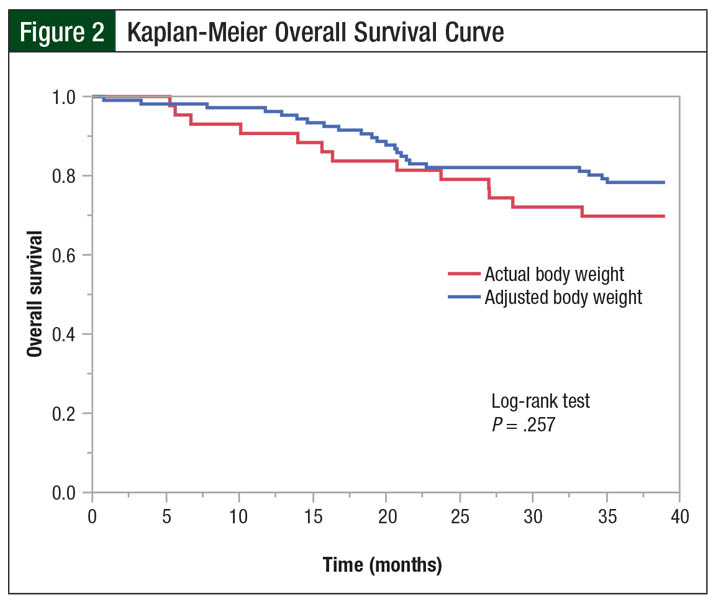
The median time to disease progression was 641 days in the actual body weight group compared with 656 days in the adjusted body weight group (P = .435). Although no difference was found in the 3-year overall survival rates (67% vs 78%, respectively; P = .164) and Kaplan-Meier overall survival curves (log-rank test, P = .257; Figure 2) between the actual and adjusted body weight groups, there was a trend toward greater overall survival in the adjusted body weight group.
The median time to death was not reached in either group, and no difference was found in the rates of non–relapse-related mortality between the actual body weight and adjusted body weight groups (9% vs 6%, respectively; P = .475). Consistent with their baseline characteristics, patients were more likely to achieve a complete response at day 100 after transplant in the actual body weight group than in the adjusted body weight group (21% vs 6%, respectively; P = .005). During the transplant hospitalization, no differences were identified between the groups in time to neutrophil engraftment or in the length of hospitalization.
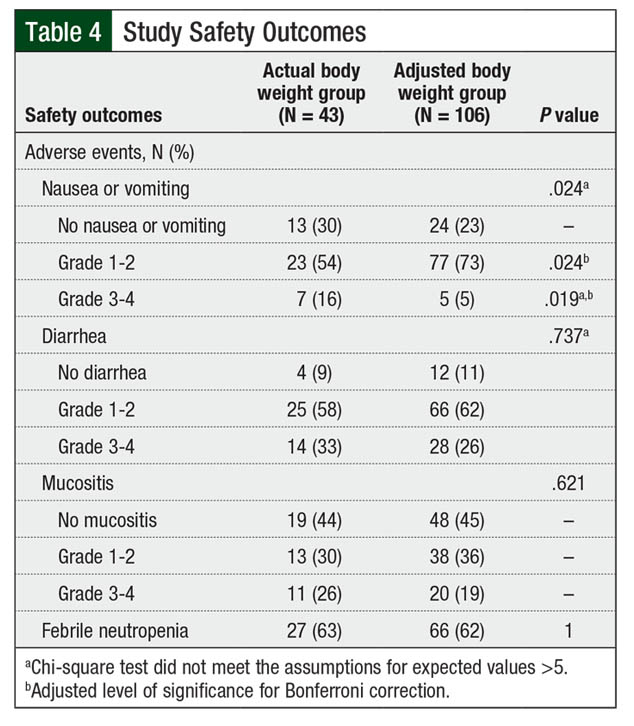
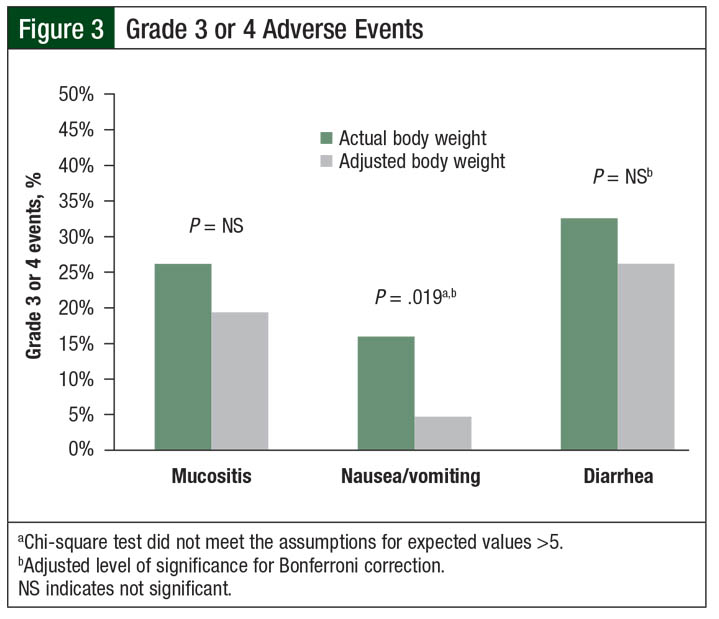
During the transplant-related hospital stay, no differences were found between the groups in the rates of diarrhea, mucositis, or febrile neutropenia (Table 4). However, the rates of grade 3 or 4 nausea or vomiting were higher in the actual body weight group than in the adjusted body weight group (16% vs 5%, respectively; P = .019; Figure 3). The clinical significance of this difference in nausea or vomiting is difficult to interpret, given the inconsistent use of moderate- or high-risk antiemetic regimens among the patients.
Discussion
Based on the results of this retrospective, single-center study, the use of adjusted body weight for different melphalan dosing strategies in obese patients with multiple myeloma who are undergoing autologous HSCT results in similar outcomes compared with the use of actual body weight dosing strategies in nonobese patients. Although theoretically, the consequences of underdosing obese patients using adjusted body weight would result in inferior survival and transplant outcomes, in our study, the group of obese patients who received melphalan dosing based on adjusted body weight dosing showed a trend toward better overall survival than the nonobese patients who received melphalan dosed by actual body weight.
This is consistent with a previous study that has demonstrated superior PFS and overall survival in obese and severely obese patients.5 Furthermore, the nonobese patients who were dosed based on actual body weight had more toxicity than the adjusted body weight group, in terms of significantly higher rates of grade 3 or 4 nausea or vomiting.
Although the validity of improved PFS and overall survival outcomes in obese patients is unknown, an evidence-based optimal melphalan dosing by weight is unclear. In a retrospective study by Frimpong and colleagues, dosing melphalan based on actual body weight was associated with a decreased risk for non–relapse-related mortality (hazard ratio, 0.38; 95% confidence interval, 0.19-0.72; P = .003).6 By contrast, other studies have not identified any differences in outcomes from dose reductions in obese patients.7,8,10
In patients with a BMI of ≥30 kg/m2 who received a ≥20% dose reduction relative to melphalan dosing based on actual body weight, Brunstein and colleagues found no differences in survival, PFS, relapse, or treatment-related mortality.7 Ellent and colleagues also did not identify any differences in transplant outcomes—such as days to engraftment, adverse effects, or time to relapse—in overweight or obese patients who received dosing based on ≥95% of their actual body weight.8
In a study by Shultes and colleagues, the 3-year event-free survival rate in obese patients who received melphalan dosed by adjusted body weight was noninferior to that of nonobese patients who were primarily dosed by ideal body weight per their institution protocol (51% vs 40%, respectively; P = .0025 for noninferiority).10 That study also showed no difference in the 3-year overall survival rate (59.1% vs 63.6%, respectively; P = .52).10
Although the available studies are conflicting and heterogeneous in their definitions of obesity in relation to melphalan, the drug’s dose reduction thresholds, and comparators, the overall data and results from our analysis suggest that melphalan dosing based on adjusted body weight in patients with multiple myeloma does not lead to inferior outcomes.7,8,10
One interesting result of our study was the significantly increased rate of complete response in the actual body weight group at day 100 after transplant versus the adjusted body weight group (21% vs 6%, respectively; P = .005), despite the lack of differences in 3-year PFS and overall survival. This increased response in the actual body weight group is likely a result of the imbalance of complete response rates at baseline in our study. At baseline, 19% of the patients in the actual body weight group achieved a complete response before transplant compared with only 4% (P = .003) in the adjusted body weight group; this difference remained consistent at day 100 after transplant (21% vs 6%, respectively; P = .005).
Therefore, this significant difference in complete response rate at baseline does not suggest any benefit from the use of actual body weight for melphalan dosing in terms of disease response rates. In addition, 13 patients in the adjusted body weight group received a reduced dose of 140 mg/m2 of melphalan compared with 1 patient in the actual body weight group (12% vs 2%, respectively), which may also contribute to the increased rate of complete responses in the actual body weight group at day 100 after transplant, although there was no significant difference in dosing found between the groups (Table 2).
Limitations
This study has several limitations that affected the applicability and interpretation of the results. The study included a relatively small sample size, used a retrospective design, and had a lack of statistical power.
Per STVHCS protocol, a correction factor of 50% was used to calculate the adjusted body weight, as has been used in the literature for other conditioning agents, such as busulfan, carmustine, cyclophosphamide, and etoposide.4 Although correction factors of 25% or 40% have been frequently used for dosing in the literature,4 the extrapolation of a 50% correction factor for melphalan dosing in our study demonstrates that despite lower dosing weights, similar outcomes are achieved in obese and nonobese patients.
It is prudent to note that a difference of more than 5% existed between the calculated doses based on actual and adjusted body weight for 92 (87%) patients in the adjusted body weight group. After considering chemotherapy rounding, 91 (86%) patients in the adjusted body weight group received melphalan doses beyond the 5% dosing variation relative to the calculated dose based on actual body weight. Chemotherapy doses are often rounded for cost-optimization purposes, and per STVHCS dose-rounding guidance, doses of melphalan were typically rounded to the nearest vial size (10 mL) as long as the doses were within a 5% dosing variation. A 5% dosing variation was selected based on local protocol and cytotoxic chemotherapy-rounding guidance in the curative setting.11 Therefore, 86% of patients in this study received a clinically significant dose reduction based on adjusted body weight; the remaining 13% of patients received an acceptable dose based on chemotherapy-rounding principles, which limited the interpretation of the results.
Furthermore, a total of 69 (65%) patients in the adjusted body weight group received melphalan doses within a 10% dosing variation allowance, which is suggested by a dose-rounding position statement released by the Hematology/Oncology Pharmacy Association.11 This introduces the idea that a larger variation of 10% may be acceptable when dose rounding melphalan, which can lead to cost minimization, without compromising patients’ outcomes.
Finally, this study did not address the comparison of using actual and adjusted body weight dosing exclusively in an obese population, because patients weighing within 120% of their ideal body weight were never dosed by adjusted body weight. When defining obesity based on a BMI of ≥30 kg/m2, no patients in the actual body weight group and 70 (66%) patients in the adjusted body weight group were categorized as obese.12
Therefore, the possibility of excess toxicity when using actual body weight versus adjusted body weight for melphalan dosing in obese patients cannot be excluded. If greater toxicity were to result from actual body weight–based dosing without any survival advantage, adjusted body weight–based dosing would be the optimal dosing strategy in obese patients based on safety and efficacy, contrary to the current ASBMT recommendations.
Conclusion
In this study, melphalan dosing based on adjusted body weight compared with actual body weight for patients with multiple myeloma who have had autologous HSCT did not affect the efficacy and did not worsen the 3-year PFS or overall survival, or increase the time to disease progression. Dosing based on adjusted body weight, however, did improve safety: obese patients who were dosed based on adjusted body weight had significantly fewer grade 3 or 4 nausea or vomiting events. These results provide evidence for the safety and efficacy of using adjusted body weight dosing contrary to the current melphalan dosing recommendations of ASBMT for obese patients.
Therefore, dosing melphalan based on adjusted body weight for autologous HSCT conditioning regimens in obese patients may be the same as actual body weight–based dosing strategies, and with less severe toxicity. Although this study supports the use of adjusted body weight dosing, these results should be confirmed in a larger randomized, controlled study that directly compares dosing based on actual body weight versus adjusted body weight in an exclusively obese population.
Acknowledgments
The authors thank Christopher Frei, PharmD, MSc, for his assistance with statistical analyses, and Juan Toro, MD, for his assistance with patient recruitment via database access.
Author Disclosure Statement
Dr Yeung and Dr Malamakal have no conflicts of interest to report.
References
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. National Center for Health Statistics Data Brief No. 219. Hyattsville, MD: National Center for Health Statistics. 2015.
- Lyman GH. Commentary: chemotherapy dosing in obese patients with cancer-the need for evidence-based clinical practice guidelines. J Oncol Pract. 2011;7:17-18.
- Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553-1561.
- Bubalo J, Carpenter PA, Majhail N, et al. Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation practice guideline committee. Biol Blood Marrow Transplant. 2014;20:600-616.
- Vogl DT, Wang T, Pérez WS, et al. Effect of obesity on outcomes after autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2011;17:1765-1774.
- Frimpong JO, Tombleson R, Alsina M, et al. Impact of obesity in patients with multiple myeloma receiving high-dose melphalan followed by autologous hematopoietic cell transplantation [abstract]. Blood. 2016;128:4636.
- Brunstein CG, Pasquini MC, Kim S, et al. The effect of conditioning regimen dose reduction in obese patients undergoing autologous transplantation [abstract]. Blood. 2017;130 suppl 1:S3292.
- Ellent E, Pai SG, Chasick A, et al. Effect of dose on melphalan used in conditioning regimen on outcomes of autologous stem cell transplantation in overweight/obese patients with multiple myeloma. J Clin Oncol. 2015;33:e18014.
- National Comprehensive Cancer Network. NCCN Guidelines for Supportive Care (NCCN Guidelines): Antiemesis. Version 2.2020. www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed April 20, 2020.
- Shultes KC, Arp C, Stockerl-Goldstein K, et al. Impact of dose-adjusted melphalan in obese patients undergoing autologous stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:687-693.
- Fahrenbruch R, Kintzel P, Bott AM, et al. Dose rounding of biologic and cytotoxic anticancer agents: a position statement of the hematology/oncology pharmacy association. J Oncol Pract. 2018;4:e130-e136.
- Centers for Disease Control and Prevention. Defining adult overweight & obesity. Overweight & obesity. Updated June 16, 2016. www.cdc.gov/obesity/adult/defining.html. Accessed January 30, 2019.