Hypercalcemia occurs in 20% to 30% of patients with active malignancy.1,2 Hypercalcemia sequelae encompass neurologic, renal, and cardiovascular manifestations.1,2 If an ionized serum calcium level is not available, a corrected calcium level must be calculated in patients with hypoalbuminemia or hyperalbuminemia to estimate the true physiologic free calcium concentration, because serum calcium is primarily bound to albumin.2,3 Most laboratories in the United States suggest that a normal physiologic serum calcium level is between 8.5 mg/dL and 10.2 mg/dL.3 A serum calcium level >14 mg/dL is considered to be severe hypercalcemia, in which case injectable calcitonin is often recommended.3,4 The degree of hypercalcemia is classified by the total serum calcium level, as follows: mild, 10.5 mg/dL to 11.9 mg/dL; moderate, 12 mg/dL to 13.9 mg/dL; and severe, >14 mg/dL.1,3
The optimal management strategy for hypercalcemia of malignancy is prompt treatment of the underlying malignancy and discontinuation of any contributing agents, such as calcium, vitamin D, thiazide diuretics, and lithium.1-3,5 However, chemotherapy initiation may not always be immediately feasible.5 Rapid-acting anti-hypercalcemia therapies are often required as a temporary measure to control moderate-to-severe hypercalcemia and its associated symptoms.2,5
The degree of hypercalcemia and presence or absence of symptoms must be considered when evaluating treatment options for hypercalcemia related to a malignancy.1,2 Mild hypercalcemia can often be managed by promoting renal calcium excretion via intravenous (IV) fluids, namely, normal saline. Fluid replacement should be considered a first-line therapy for most patients with hypercalcemia, regardless of the degree of the hypercalcemia.1,2
For patients with mild hypercalcemia refractory to volume expansion or moderate, severe, and/or symptomatic hypercalcemia, therapy should be aimed at reducing bone resorption.1,3,5 Administration of an IV bisphosphonate is considered a first-line therapy and should be done within 48 hours of diagnosis.1,2 The onset of effect is approximately 48 hours to 96 hours, and the effects persist for 1 to 3 weeks.1
Zoledronic acid and pamidronate are the 2 agents approved by the US Food and Drug Administration for mild-to-severe hypercalcemia related to a malignancy.1,2 Although zoledronic acid was found to be superior to pamidronate, either option is acceptable.1,2,6 Denosumab is a very costly agent that should be reserved for patients with hypercalcemia refractory to bisphosphonates.3,7,8 Dialysis or continuous renal replacement therapy can be considered in patients unresponsive to all other therapy options.2
Injectable calcitonin is indicated for early adjunctive treatment of hypercalcemic emergencies.9 The onset of effect is within 1 to 4 hours after administration and the peak effect occurs within 24 to 48 hours.3,9 Injectable calcitonin is generally used in combination with IV hydration and an IV bisphosphonate for emergent treatment of severe and/or symptomatic hypercalcemia, because combination therapy can decrease calcium levels more quickly than either agent alone.2,10 We deemed any symptomatic hypercalcemia to be an indication for injectable calcitonin, to facilitate rapid normalization of calcium levels.
Injectable calcitonin rapidly reduces serum calcium levels, whereas bisphosphonates have a longer time to onset but provide a prolonged duration of action. Injectable calcitonin may hasten reduction in serum calcium levels compared with other agents; however, its effects are limited and short-lived. It may reduce serum calcium levels by an average of 2 mg/dL to 3 mg/dL.5,11
Prolonged use of injectable calcitonin is limited by tachyphylaxis, because tolerance to the calcium-lowering effect generally occurs after 48 to 72 hours of calcitonin administration, resulting from calcitonin receptor downregulation in osteoclasts.5,11-14 Aside from its limited effectiveness, injectable calcitonin is also a costly agent.15 The sudden price increase in 2015 prompted our institution to create and implement formulary restriction criteria to ensure optimal and cost-effective use.15 The objective of this study was to assess injectable calcitonin utilization reduction after the implementation of the formulary restrictions at West Virginia University Hospital.
Methods
On September 27, 2016, West Virginia University Hospital in Morgantown, West Virginia, implemented formulary restrictions to ensure judicious use of injectable calcitonin as a result of its price increase in 2015.15 Injectable calcitonin was restricted at our institution for use only in patients with a corrected calcium >14 mg/dL regardless of symptoms or any corrected calcium >10.2 mg/dL with symptoms (ie, neurologic, renal, and/or cardiac abnormalities).
We made additional changes to the injectable calcitonin order in our institution’s electronic medical record (EMR), including defaulting to a stop date of 48 hours from first dose of injectable calcitonin and incorporating automatic dose rounding to the nearest 50 units. Additional recommendations included capping the dosing weight at 100 kg, but this change was not made a default, because of system limitations within the EMR.
The Institutional Review Board at West Virginia University approved this study. The primary end point was to assess injectable calcitonin utilization before (ie, pre–formulary restriction group) and after (ie, post–formulary restriction group) implementation of the formulary restrictions. Secondary end points were the change in expenditures associated with the intervention, compliance with formulary restrictions, and the number of instances injectable calcitonin was reordered, or its duration of use was extended beyond 48 hours.
We standardized several end points, including number of patients who received injectable calcitonin, doses administered, units administered, and vials used by 1000 patient-days to account for the different number of patients in the pre–formulary restriction and post–formulary restriction groups, resulting from hospital expansion. This standardization allowed for a more accurate interpretation of the results.
We then conducted a retrospective chart review of adult patients who received injectable calcitonin between August 2015 and June 2016 (ie, pre–formulary restriction period) and between October 2016 and July 2017 (ie, post–formulary restriction period).
We estimated the incidence rates of patients administered injectable calcitonin per 1000 patient-days based on the number of new patients who received injectable calcitonin, divided by total number of person-days at risk, which is the actual time at risk of all patients in the study. We used a chi-square test to assess the association between 2 categorical variables. The Wilcoxon rank sum test was used to assess differences of continuous variables between the 2 groups.
Results
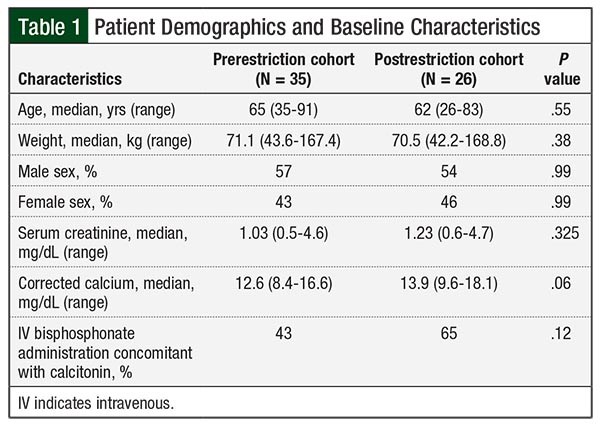
As a result of our hospital expansion, the hospital population was 128,281 adult patient-days in the pre–formulary restriction arm and 150,026 in the post–formulary restriction arm. A total of 35 patients received injectable calcitonin in the prerestriction time period (ie, the prerestriction cohort) and 26 patients received it in the postrestriction time period (ie, the postrestriction cohort). The number of patients administered injectable calcitonin was 0.273 and 0.173 per 1000 patient-days before and after implementation (ie, 35 patients vs 26 patients, respectively; P = .10). The baseline characteristics were similar between the 2 groups (Table 1).
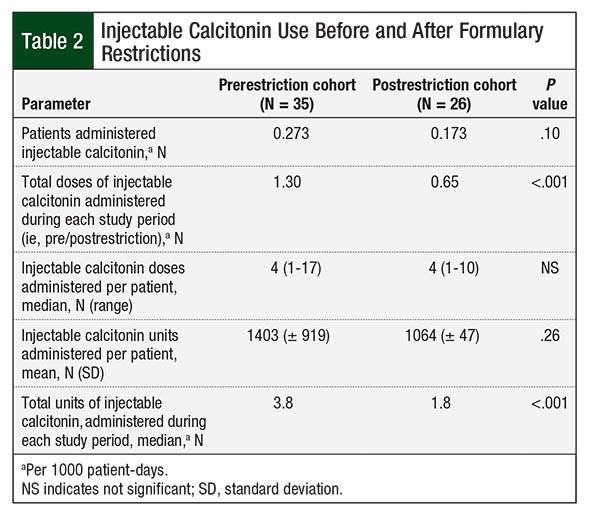
The majority of the patients in the study—83% before and 89% after the formulary restriction implementation—received injectable calcitonin at a dose of 4 units/kg every 12 hours. In the postrestriction group, 32 (84.2%) orders met the restriction criteria for use, with only 5 (13.2%) orders continuing beyond 48 hours. In all, 3 patients reached the capped dosing weight of 100 kg, and 2 (66.7%) of these patients had their doses capped at the 400-unit vial size. A net total of 96 units were saved from the automatic dose rounding and recommended dose capping. Significantly fewer doses and units of injectable calcitonin were administered in the post–formulary restriction group. Table 2 compares the utilization of injectable calcitonin in the 2 groups.
The median corrected calcium levels at baseline were 12.6 mg/dL (range, 8.4-16.6 mg/dL) and 13.9 mg/dL (range, 9.6-18.1 mg/dL) before and after implementation, respectively (P = .06). The median corrected calcium levels at 72 hours were 11.0 mg/dL (range, 8.3-13.1 mg/dL) and 10.5 mg/dL (range, 8.5-13 mg/dL) before and after implementation, respectively (P = .63).
Depending on waste practices with each dose prepared (ie, how many units are discarded with each vial), the formulary intervention resulted in a savings of $241,600 to $427,700 over the study period based on an average wholesale price of $3244 per 400-unit vial of injectable calcitonin. When extrapolated to 12 months, the intervention resulted in an estimated annual savings between $290,000 and $513,000.
Discussion
The implementation of formulary restrictions and a defaulted 48-hour stop date for injectable calcitonin resulted in a significant reduction in the number of doses administered per 1000 patient-days and total units administered, yielding an estimated cost-savings of up to $513,000 annually. The intervention did not significantly reduce the number of patients who were administered injectable calcitonin per 1000 patient-days, number of doses administered per patient, or units administered per patient, but a trend toward reduction in each of these parameters contributed to an overall utilization reduction when the number of patients was standardized. The range of the number of doses administered per patient was smaller after the intervention, which likely was reduced by the automatic stop date.
After the intervention, the majority of injectable calcitonin orders met the criteria for use, few orders were extended beyond 48 hours, and corrected calcium levels at 72 hours were similar between the 2 arms. To decrease the number of orders incongruent with the formulary restrictions, we used education that was included within the injectable calcitonin order in the EMR to alert prescribers and pharmacists.
Our results suggest that the restricted duration was adequate for the treatment of hypercalcemia in conjunction with IV fluids and IV bisphosphonate therapy. In addition, these results highlight the effectiveness of a defaulted stop date in the order entry system. Defaulting to a 48-hour stop date also minimizes waste during order discontinuation.
Two of the formulary restriction strategies implemented did not yield much benefit. Despite having a historically obese patient population, very few patients reached the recommended dose-capping weight of 100 kg. As a result, limiting the dose to 1 vial (400 units) did not prevent a significant number of vials from being used. Furthermore, rounding doses to the nearest 50 units resulted in a nominal number of net vials saved. The degree to which doses were rounded up or down essentially balanced out, which nullified the benefit of the strategy. In contrast to the other formulary restriction strategies implemented, these specific initiatives did not have a significant impact on decreasing our injectable calcitonin expenditures.
Limitations
The limitations to the study include the retrospective nature of the study and the single-center experience.
In addition, it was difficult to measure accurately the cost-savings attributed to the waste of prepared injectable calcitonin doses that were discarded on order discontinuation before injectable calcitonin administration.
The estimated cost-savings were based on the average wholesale price, which differs from the actual price paid by the institution based on individual contracts.
Conclusion
Injectable calcitonin utilization and expenditures can be significantly decreased by the implementation of formulary restrictions, without affecting hypercalcemia outcomes. Listing specific criteria for use and defaulting to an automatic 48-hour stop date likely had the greatest impact on curbing use at our institution. In addition, education should be provided to the sterile compounding team, addressing the high cost of injectable calcitonin and the importance of reusing the multidose vials for up to 28 days when possible. Altogether, incorporating measures to restrict the use of injectable calcitonin is safe and feasible, leading to significant cost-savings and more judicious use of an increasingly expensive medication.
Acknowledgment
This study was supported, in part, by ACS 16-143-07-IGR grant from the American Cancer Society.
Author Disclosure Statement
Dr Walchack, Dr Hill, Dr Wen, and Dr Cumpston have no conflicts of interest to report.
References
- Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373-379.
- Goldner W. Cancer-related hypercalcemia. J Oncol Pract. 2016;12:426-432.
- Mirrakhimov AE. Hypercalcemia of malignancy: an update on pathogenesis and management. N Am J Med Sci. 2015;7:483-493.
- Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959-1966.
- Davidson TG. Conventional treatment of hypercalcemia of malignancy. Am J Health Syst Pharm. 2001;58(suppl 3):S8-S15.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558-567.
- Salahudeen AA, Gupta A, Jones JC, et al. PTHrP-induced refractory malignant hypercalcemia in a patient with chronic lymphocytic leukemia responding to denosumab. Clin Lymphoma Myeloma Leuk. 2015;15:e137-e140.
- CADTH. Cost comparison table for denosumab for the treatment of bone metastases in patients with other solid tumours. In: Common Drug Review. Table 1: Denosumab (Xgeva). Ottowa, Ontario: Canadian Agency for Drugs and Technologies in Health; November 2016. www.ncbi.nlm.nih.gov/books/NBK409942/table/T34/. Accessed February 27, 2020.
- Miacalcin (calcitonin salmon) injection [prescribing information]. Rockford, IL: Mylan; July 2018.
- Minisola S, Pepe J, Piemonte S, Cipriani C. The diagnosis and management of hypercalcaemia. BMJ. 2015;350:h2723. doi.org/10.1136/bmj.h2723. Accessed June 24, 2018.
- Nussbaum SR. Pathophysiology and management of severe hypercalcemia. Endocrinol Metab Clin North Am. 1993;22:343-362.
- Purdue BW, Tilakaratne N, Sexton PM. Molecular pharmacology of the calcitonin receptor. Receptors Channels. 2002;8:243-255.
- Seccareccia D. Cancer-related hypercalcemia. Can Fam Physician. 2010;56:244-246, e90-e92.
- Wada S, Yasuda S. Appropriate clinical usage of calcitonin escape phenomenon and intermittent v.s. daily administration of calcitonin. Clin Calcium. 2001;11:1169-1175.
- Elvidge S. AHA: Inpatient Drug Costs Skyrocketing. Biopharma Dive; October 12, 2016. www.biopharmadive.com/news/aha-inpatient-drug-costs-skyrocketing/428105/. Accessed February 28, 2020.