Ovarian cancer has an annual incidence of 11.4 per 100,000 women in the United States, with a 5-year overall survival of 47.6% for all stages.1 In patients with fallopian tube or primary peritoneal ovarian cancer, a platinum-based (ie, carboplatin, cisplatin) regimen is the chemotherapy of choice in the metastatic or the adjuvant setting.2 Ovarian cancer is highly sensitive to platinum-based therapy, with a 5-year survival rate of 90% for stage I disease.3
Ovarian cancer with a disease-free period of ≥6 months between the last platinum-based chemotherapy and disease recurrence is considered to be platinum-sensitive.4-6 Ovarian cancer that recurs within 6 months is considered platinum-resistant; recurrence between 6 and 12 months is considered intermediately resistant.4,5,7
Patients in the platinum-sensitive or intermediately resistant ovarian cancer groups typically receive treatment with a platinum-based combination in the second-line setting.4,5 The use of a platinum-based combination in the second-line setting has shown response rates of more than 30%, making it the preferred first-line treatment option.2,5,6
In the setting of chemotherapy-naïve recurrent endometrial cancer, platinum agents, anthracyclines, and taxanes are preferred because of their activity, with reported response rates of 20%.8 Nagao and colleagues aimed to apply the concept of platinum sensitivity to recurrent endometrial cancer, and concluded that a platinum-free interval was a predictor of response to second-line platinum-based chemotherapy, as well as a predictor of survival.9 More recently, Pignata and colleagues presented prospective data showing superior outcomes in patients with platinum-sensitive ovarian carcinoma that was managed with a platinum-based regimen in the second-line setting (MITO8 trial)7; therefore, this remains the preferred second-line treatment when feasible.
With increased exposure to platinum-based chemotherapy, the risk for hypersensitivity reactions (HSRs) also increases.10 Carboplatin is often the preferred platinum over cisplatin, because of its relative lack of nephrotoxicity, neurotoxicity, and emetogenicity.11 The incidence of carboplatin HSR is greater with prolonged exposure (>6 cycles), or with the administration of carboplatin as a component of initial chemotherapy and in the second-line setting.11 Hypersensitivity to carboplatin is rarely seen in the first-line setting, and peaks in the third-line setting. HSRs to carboplatin are typically immunoglobulin E–mediated allergic reactions, with prominent flushing, urticaria, bronchospasm, pruritus, and hypotension or hypertension. Cisplatin has similar incidences and characteristics of hypersensitivity to carboplatin.11
The incidence of carboplatin hypersensitivity ranges from 1% to 44% in patients with ovarian cancer, but it generally varies depending on the treatment setting, with third-line retreatment showing the highest incidence (44%).11 In addition, more than 50% of HSRs to carboplatin are classified as severe.12 This has previously limited the treatment options in patients with HSRs to non–platinum-based therapy.
The National Comprehensive Cancer Network (NCCN) guidelines recommend that desensitization can be considered in the setting of mild to life-threatening platinum drug reactions.2 However, drug desensitization is a procedure associated with some risk, requiring an individually designed protocol and close monitoring for each patient.2,12-14
Patients who may be candidates for desensitization should preferably be evaluated in an academic setting with expertise in the area.2,13 Increasingly, protocols for safe desensitization are being used, allowing this subset of patients to receive further carboplatin dosing but no single standard desensitization protocol has been recommended.15
Regardless of which carboplatin desensitization protocol is being used, survival data are lacking in patients undergoing desensitization. Choi and colleagues discussed the potential efficacy of their desensitization protocol based on the pharmacokinetics of carboplatin, which preserved efficacy based on tumor marker response.16
Our study compared the overall survival and time to progression (TTP) among patients who underwent carboplatin desensitization after HSR, those who received a non–platinum-containing regimen after HSR, and patients without HSR who received carboplatin-based therapy. We hypothesized that the ability to administer further carboplatin cycles in platinum-sensitive patients with the use of safe desensitization protocols would improve overall survival, by increasing exposure to platinum-based therapy, and that the TTP would be longer.
Methods
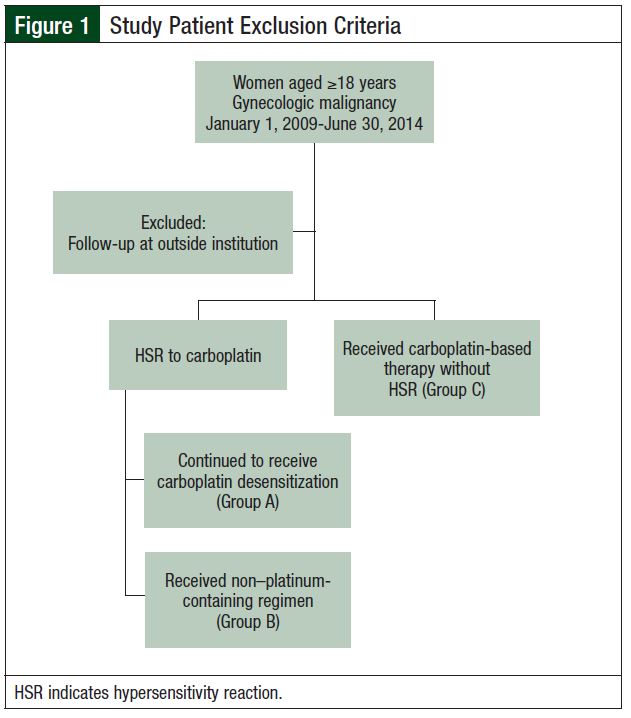
Women aged 18 years or older who had a confirmed diagnosis of an advanced-stage (defined as the International Federation of Gynecology and Obstetrics [FIGO] stage III/IV disease) or recurrent platinum-sensitive gynecologic oncology malignancy (ovarian, fallopian tube, primary peritoneal, or endometrial cancer) between January 1, 2009, and June 30, 2014, were eligible for study inclusion. Patients were excluded if they did not meet the inclusion criteria or if they continued follow-up at a medical institution other than Rush University Medical Center.
This single-center, retrospective, case control study was conducted at a tertiary medical center and was approved by the hospital’s Institutional Review Board in January 2015. Patients meeting inclusion criteria were selected through the hospital’s electronic database. Selected patients were categorized into 3 groups. Group A included patients who had an HSR to carboplatin and underwent carboplatin desensitization. Group B included patients who had an HSR to carboplatin and received a non–platinum-containing regimen. Group C included patients with no documented HSRs who received additional treatments or additional lines of carboplatin-based chemotherapy (Figure 1).
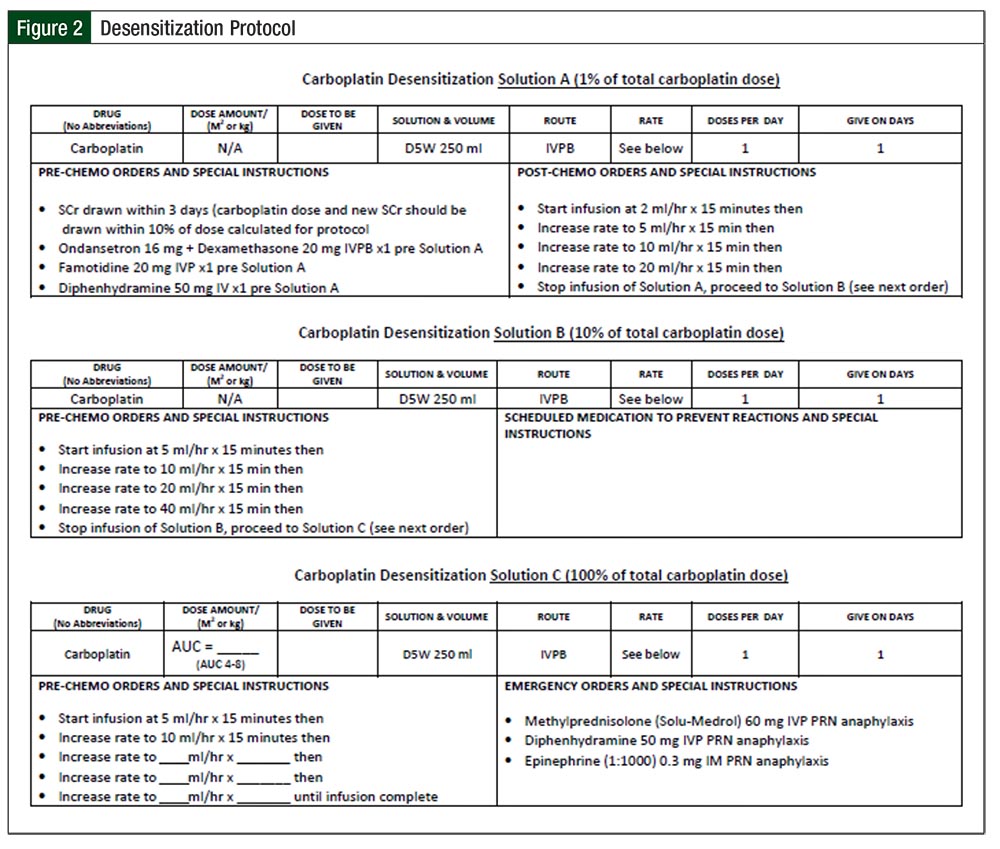
Our institution, Rush University Medical Center, follows a 6-hour, 12-step desensitization protocol.14 Allergy and immunology service is consulted and involved in the process for each desensitization cycle. The initial desensitization is performed in the medical intensive care unit (ICU), and subsequent desensitizations are performed on an outpatient basis, based on patient tolerance. Three infusion solutions (A, B, and C) containing X/100 mg, X/10 mg, and X mg of carboplatin are infused, with the rate of infusion adjusted every 15 minutes with each step until the final step, which is maintained at a predetermined constant rate of infusion for the remainder of Solution C. Solution A is used for steps 1 to 4, Solution B for steps 5 to 8, and Solution C for steps 9 to 12. Rescue medications include methylprednisolone, diphenhydramine, and epinephrine. Figure 2 provides the protocol and rescue medication details.
Patient demographics, treatment, HSRs, and desensitization data were collected from the electronic and paper medical records. Data collected included age, oncologic diagnosis, performance status, histology, desensitization and its tolerability, BRCA mutation status, HSRs, and survival outcomes. Overall survival was measured as the time from date of diagnosis to death or last follow-up. The TTP was determined by the individual physician’s assessment in chart review, combined with time to next treatment or death from any cause.
Study End Points
The primary end points were to determine overall survival and TTP in patients who underwent carboplatin desensitization for HSRs (Group A) compared with those receiving non–platinum-containing regimens for HSRs (Group B) and with those receiving standard infusions of carboplatin-based therapy without HSRs (Group C). The secondary end point was to assess the frequency of carboplatin HSRs in patients undergoing desensitization, as well as their severity, graded according to the Common Terminology Criteria for Adverse Events Version 4.0, which was the published version at the time this study was completed.17
We estimated overall survival and TTP curves using the Kaplan-Meier method and compared them across study groups by the log-rank test. To assess the association of covariates and predictors on overall survival and TTP, we used the Cox proportional hazards regression model. The difference in response rates across the study groups was assessed by exact chi-square tests and the association of covariates with response rate was assessed using logistic regression.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics, Version 19.0 (IBM; Armonk, NY) and the R statistical software (the R Foundation for Statistical Computing; Vienna, Austria). All reported P values are 2-sided, and P values <.05 were considered statistically significant.
Results
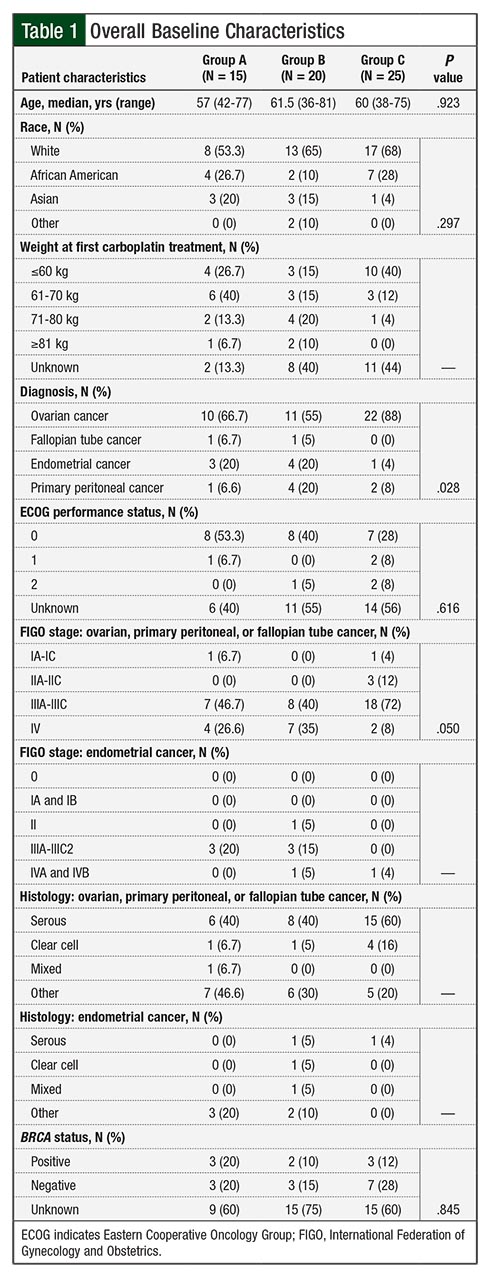
A total of 60 patients met the study inclusion criteria between January 1, 2009, and June 30, 2014. Patients in Group A (N = 15) had a documented HSR and had undergone carboplatin desensitization(s). Patients in Group B (N = 20) had a documented HSR and had received further treatment with non–platinum-based therapy (standard of care was followed; the regimen and/or agent were selected based on physician preference). Patients in Group C (N = 25) had no documented HSR and received additional treatments or additional lines of carboplatin-based chemotherapy.
The median number of carboplatin cycles before an HSR event was 8 for patients in Group A and 9 for those in Group B. The mean age among all 3 groups was approximately 59 years. Overall, 53% of patients in Group A had an Eastern Cooperative Oncology Group performance score of 0 versus 40% in Group B and 28% in Group C (Table 1).
The majority of patients had a primary diagnosis of ovarian cancer (67% in Group A, 55% in Group B, and 88% in Group C). Among patients in Group A with a diagnosis of ovarian, primary peritoneal, or fallopian tube cancer, 47% had FIGO stage IIIA-IIIC cancer, and 40% of those had a serous histology. In Group B, 40% of the patients with those diagnoses had FIGO stage IIIA-IIIC disease, and 40% of them had a serous histology. In Group C, 72% of the patients with the same diagnoses had FIGO stage IIIA-IIIC disease and 60% had a serous histology (Table 1).
The majority of the patients with a diagnosis of endometrial cancer in Group A and Group B had FIGO stage IIIA-IIIC2 disease (20% in Group A, 15% in Group B), with a distribution of serous, clear cell, mixed, and other histology, as seen in Table 1. One patient with an endometrial cancer diagnosis in Group C had FIGO stage IVB disease, with serous histology (Table 1).
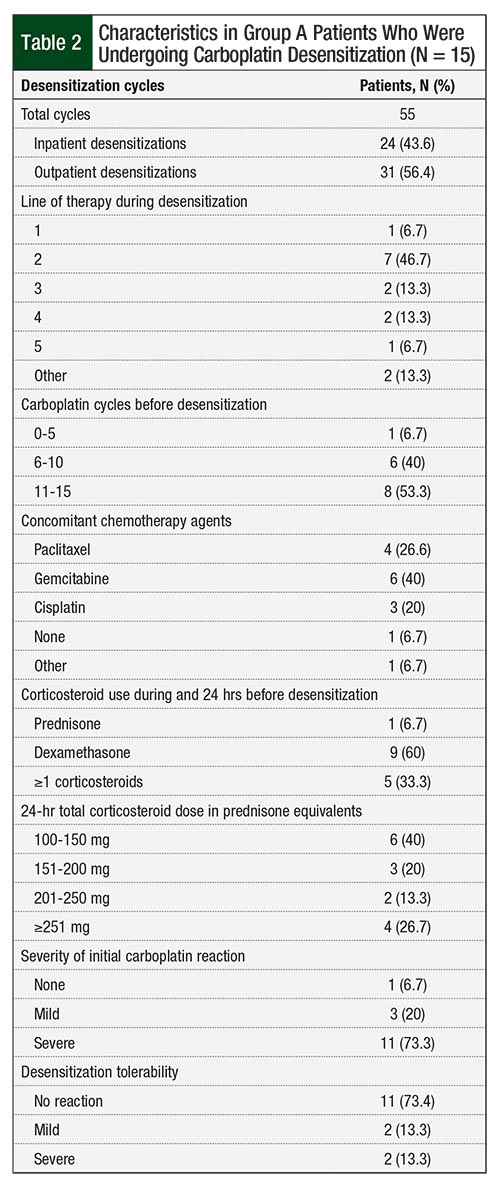
Table 2 provides information on patient characteristics in Group A, who underwent carboplatin desensitization. In terms of HSR severity in Group A, 11 (73.3%) patients had an initial severe HSR, 3 (20%) patients had mild HSRs, and 1 (6.7%) patient underwent desensitization based on previously documented allergy. Among patients undergoing a desensitization, 7 of the 15 (46.7%) were receiving carboplatin as second-line therapy. All 15 eligible patients in Group A successfully completed 55 carboplatin desensitizations; 24 (43.6%) of those were performed in the inpatient setting and 31 (56.4%) were completed in the outpatient setting. Overall, 7 (47%) patients underwent initial desensitization during their second line of therapy (Table 2).
The majority (53.3%) of patients in Group A received 11 to 15 carboplatin cycles before the initial desensitization; 6 (40%) received concomitant gemcitabine during the desensitization protocol and 4 (27%) received paclitaxel. Carboplatin desensitization was tolerated, with 11 (73%) patients having no reaction. A mild or severe reaction consisting of rash, pruritus, and tongue/throat swelling occurred in 4 (26.6%) patients; 2 of the 4 patients who had an HSR during the desensitization received the subsequent desensitization cycle as inpatients rather than as outpatients (Table 2).
During the study period, 19 (31.7%) patients in all 3 groups died of the primary gynecologic oncologic disease and 57 (95%) had disease recurrence. In all, 40% (6 of 15) of deaths occurred in Group A, 45% (9 of 20) in Group B, and 16% (4 of 25) in Group C.
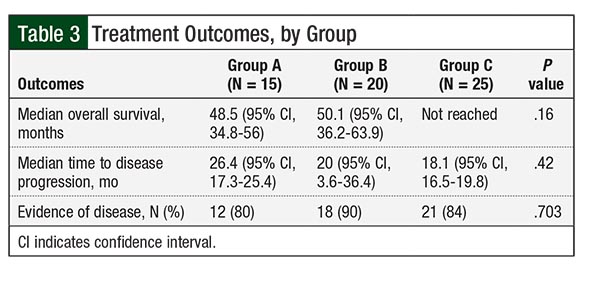
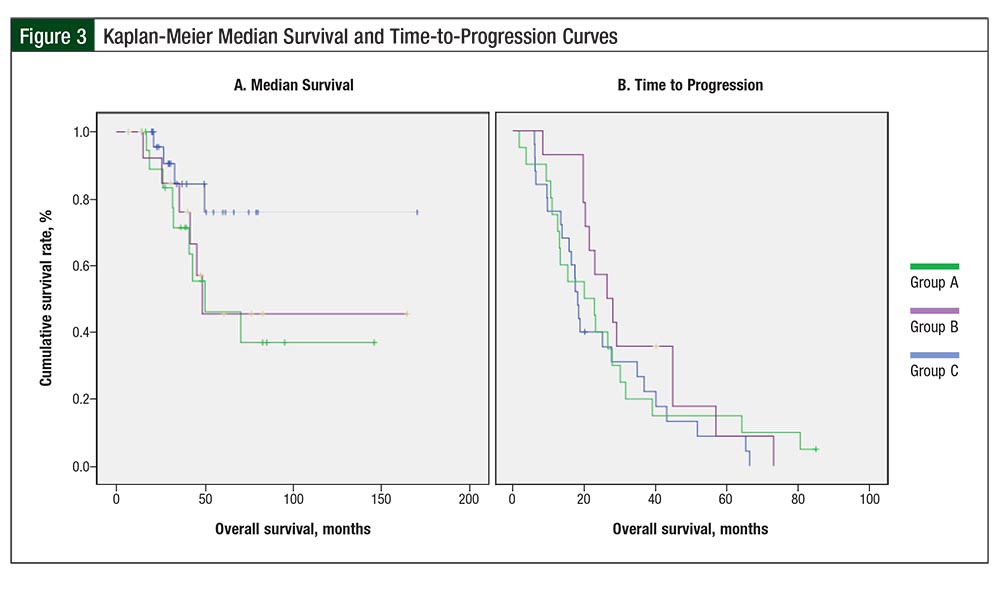
Treatment outcomes by group are listed in Table 3. The differences in overall survival between Group A (median overall survival, 48.5 months) and Group C (median overall survival, not reached) and between Group A and Group B (median overall survival, 50.1 months) were not significant (Table 3; hazard ratio [HR], 0.62; P = .13; HR, 1.16; P = .78, respectively).
The difference in overall survival between Group C (median overall survival, not reached) and Group B (median overall survival, 50.1 months) suggested a trend but was not significant (HR, 0.35; P = .07; Figure 3). When groups A and B were combined, the difference in overall survival between Group C and groups A and B combined suggested a trend but ultimately was not significant (HR, 0.36; P = .06). The difference in overall survival among the groups remained nonsignificant in multivariate analysis after adjustment for age.
Disease progression during follow-up occurred in 93% of the patients in Group A, in 95% of patients in Group B, and in 96% of patients in Group C. The differences in TTP among patients in Group A (median TTP, 26.4 months), Group B (median TTP, 20 months), and Group C (median TTP, 18.1 months) were not significant. In addition, 12 (80%) patients in Group A, 18 (90%) in Group B, and 21 (84%) in Group C had evidence of disease at the end of follow-up (P = .703; Table 3).
Discussion
Platinum-based regimens are highly effective in gynecologic malignances and are used as first-line treatment options in recurrent or advanced ovarian or endometrial cancer.2 The downside of platinum-based treatment is that approximately 16.8% of patients who receive carboplatin for gynecologic malignancies will have an HSR.7 The risk for HSR in patients who receive more than 7 cycles is as high as 25% and approaches 50% by the third-line retreatment.7,18 When this occurs, patients face the option of switching to a non–platinum-based chemotherapy regimen or undergoing a carboplatin desensitization procedure.
The procedure often entails a referral to a tertiary care center and admission to an ICU for close monitoring, steroid administration, and a prolonged infusion of carboplatin.2,15,16 According to our institution’s steroid administration guidelines and doses shown in Figure 2, if the initial inpatient desensitization is successful, patients will undergo a 6-hour outpatient carboplatin desensitization, with subsequent chemotherapy cycles. Inpatient desensitization admissions are more expensive and inconvenient than outpatient administration of alternative chemotherapy agents.
Although initially cumbersome, desensitization procedures have been streamlined, with increasing use of outpatient protocols.11,12,15 However, despite the use of platinum desensitization protocols,14-16 no evidence shows that this approach prolongs survival.14 To our knowledge, our study is the first in attempting to determine if undergoing desensitization in this patient population will improve overall survival compared with receiving a non–platinum-based treatment. Our retrospective analysis showed no difference in overall survival between patients who underwent desensitization and were able to receive additional carboplatin and patients who were switched to an alternative regimen.
Efforts have been made to predict which patients are at risk for HSR before treatment. Several studies have demonstrated that the strongest risk factors for a reaction are cumulative carboplatin dose and/or the number of cycles received.19-21 Schwartz and colleagues stated that longer duration since the last treatment with platinum therapy increases the risk for a reaction.12 Skin testing has been proposed as a potential predictor of future hypersensitivity, but no standard approach is yet available.22
Most important, a skin test can be negative immediately before an HSR, thereby limiting its clinical utility. More recent retrospective data show that repeated skin testing may eliminate false-negatives, but these data need further validation.23 Other known risk factors for HSR include a history of allergic disorders and BRCA-positive mutation status.12,21,24 We collected BRCA status, but it was unknown in ≥60% of patients in all 3 groups (Table 1). This was expected, based on the retrospective time period of the data evaluated.
Future studies that evaluate the effect of carboplatin desensitization on cancer survival should be controlled for BRCA status, because BRCA status is a risk factor for HSR, as well as increased sensitivity of ovarian cancer to platinum therapy.24
Our study presents an original design and has the advantage of including all patients with the listed gynecologic cancers who had received carboplatin at our institution (ie, patients in real-life setting), as opposed to the highly selected patient populations typically enrolled in clinical trials. In addition, patients who had multiple lines of platinum-based therapy were also included, in contrast to the previously published studies, which focused only on patients undergoing platinum retreatment in the second line.20
Future prospective studies with a larger number of patients may provide valuable information about the utility of platinum or carboplatin desensitization, given the complexity of these procedures.
Limitations
Several limitations of our study could have influenced the oncologic outcomes of the 3 study groups. Endometrial cancer diagnosis was included in the study analysis despite its different pathology, because of its platinum sensitivity.
Because of the timing of this study, the majority of the patients in all 3 groups did not have BRCA testing. Nevertheless, it is unlikely that BRCA status would differ between the 3 study groups and cause significant imbalances in the baseline patient characteristics among the 3 study groups.
In addition, our study had a relatively short overall follow-up and a small sample size, and it was retrospective in nature. Because combination platinum-based chemotherapy is the NCCN preferred treatment in the setting of first HSR recurrence,2 it was not possible to conduct a randomized controlled trial to evaluate the overall survival difference in patients who had developed platinum hypersensitivity and underwent or did not undergo desensitization.
Conclusion
Platinum-based chemotherapy remains the standard of care for the first-line treatment of women diagnosed with ovarian, fallopian tube, or primary peritoneal cancers, as well as those with endometrial cancer. It also remains the standard of care for second-line therapy in women with platinum-sensitive ovarian, fallopian tube, or primary peritoneal cancers. As a result of the additional cycles of carboplatin, many patients have an HSR and require a desensitization procedure to tolerate subsequent treatment with carboplatin. Despite the small size of our study, our findings show comparable oncologic outcomes in patients undergoing carboplatin desensitization who were switched to an alternative regimen or continued with a platinum-based regimen without an HSR. Our findings do not show superiority of carboplatin desensitization over treatment with other chemotherapy agents approved for these cancers. Therefore, these results may help patients and their physicians make decisions about chemotherapy options based on risk (a small risk for HSR during carboplatin desensitization) and convenience (inpatient carboplatin desensitization versus outpatient administration of alternative agents). Larger prospective studies with longer follow-up are needed to confirm these findings.
Acknowledgments
We would like to acknowledge the generous contributions of Mr and Mrs Eugene and Shirley Deutsch, Mr and Mrs Mark and Maha Halabi Ditsch, Mr George Ruwe, Mr and Mrs Douglas and Sarah Criner, and the Clow Foundation.
Author Disclosure Statement
Dr Tollkuci, Dr Chalmers, Dr Schultz, Dr Basu, Dr Dewdney, and Dr Usha have no conflicts of interest to report.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. Bethesda, MD. April 2019. https://seer.cancer.gov/csr/1975_2016/. Accessed January 29, 2020.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 3.2019. November 26, 2019. www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed August 29, 2014.
- Chuang LT, Temin S, Berek JS. Management and care of women with invasive cervical cancer: American Society of Clinical Oncology Resource-Stratified Clinical Practice Guideline summary. J Oncol Pract. 2016;12:693-696.
- Fung-Kee-Fung M, Oliver T, Elit L, et al. Optimal chemotherapy treatment for women with recurrent ovarian cancer. Curr Oncol. 2007;14:195-208.
- Parmar MK, Ledermann JA, Colombo N, et al; ICON and AGO Collaborators. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699-4707.
- Pignata S, Scambia G, Raspagliesi F, et al. The MITO8 phase III international multicenter randomized study testing the effect on survival of prolonging platinum-free interval (PFI) in patients with ovarian cancer (OC) recurring between 6 and 12 months after previous platinum-based chemotherapy: a collaboration of MITO, MANGO, AGO, BGOG, ENGOT, and GCIG. J Clin Oncol. 2016;34(15 suppl):5505.
- Thigpen JT, Blessing JA, Homesley H, et al. Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 1989;33:68-70.
- Nagao S, Nishio S, Michimae H, et al. Applicability of the concept of “platinum sensitivity” to recurrent endometrial cancer: the SGSG-012/GOTIC-004/Intergroup study. Gynecol Oncol. 2013;131:567-573.
- Markman M, Kennedy A, Webster K, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141.
- Makrilia N, Syrigou E, Kaklamanos I, et al. Hypersensitivity reactions associated with platinum antineoplastic agents: a systematic review. Met Based Drugs. 2010. pii: 207084.
- Schwartz JR, Bandera C, Bradley A, et al. Does the platinum-free interval predict the incidence or severity of hypersensitivity reactions to carboplatin? The experience from Women and Infants’ Hospital. Gynecol Oncol. 2007;105:81-83.
- Picard M, Matulonis UA, Castells M. Chemotherapy hypersensitivity reactions in ovarian cancer. J Natl Compr Canc Netw. 2014;12:389-402.
- Lee CW, Matulonis UA, Castells MC. Carboplatin hypersensitivity: a 6-h 12-step protocol effective in 35 desensitizations in patients with gynecological malignancies and mast cell/IgE-mediated reactions. Gynecol Oncol. 2004;95:370-376.
- Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122:574-580.
- Choi J, Harnett P, Fulcher DA. Carboplatin desensitization. Ann Allergy Asthma Immunol. 2004;93:137-141.
- US Department of Health & Human Services, National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. May 28, 2009. www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed April 30, 2015.
- National Comprehensive Cancer Network. NCCN Clinical Guidelines in Oncology (NCCN Guidelines) Uterine Neoplasms. Version 5.2019. December 23, 2019. www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed August 29, 2014.
- Sugimoto H, Iwamoto T, Murashima Y, et al. Risk factors contributing to the development of carboplatin-related delayed hypersensitivity reactions in Japanese patients with gynecologic cancers. Cancer Chemother Pharmacol. 2011;67:415-419.
- Gadducci A, Tana R, Teti G, et al. Analysis of the pattern of hypersensitivity reactions in patients receiving carboplatin retreatment for recurrent ovarian cancer. Int J Gynecol Cancer. 2008;18:615-620.
- Navo M, Kunthur A, Badell ML, et al. Evaluation of the incidence of carboplatin hypersensitivity reactions in cancer patients. Gynecol Oncol. 2006;103:608-613.
- Lax T, Long A, Banerji A. Skin testing in the evaluation and management of carboplatin-related hypersensitivity reactions. J Allergy Clin Immunol Pract. 2015;3:856-862.
- Wang AL, Patil SU, Long AA, Banerji A. Risk-stratification protocol for carboplatin and oxaliplatin hypersensitivity: repeat skin testing to identify drug allergy. Ann Allergy Asthma Immunol. 2015;115:422-428.
- Moon DH, Lee JM, Noonan AM, et al. Deleterious BRCA1/2 mutation is an independent risk factor for carboplatin hypersensitivity reactions. Br J Cancer. 2013;109:1072-1078.