Tumor lysis syndrome (TLS) is a life-threatening complication of hematologic malignancies and some solid tumors. This syndrome occurs after tumor cells break down spontaneously or after exposure to radiation or chemotherapy.1 Lysis of tumor cells will release intracellular contents into the bloodstream, leading to hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. If not treated immediately, these electrolyte changes may lead to acute renal failure, cardiac arrhythmias, seizures, and death. The clinical significance of TLS occurs 12 to 72 hours, and sometimes up to 168 hours, after starting treatment with chemotherapy.1-3
Rasburicase is a recombinant urate-oxidase enzyme, which converts uric acid to allantoin (an inactive and soluble metabolite of uric acid); it does not inhibit the formation of uric acid. Rasburicase is recommended for the prevention or management of hyperuricemia associated with TLS in patients who are at high risk.4,5
The dose of rasburicase currently approved by the US Food and Drug Administration (FDA) for the prevention or treatment of hyperuricemia associated with malignancy is 0.2 mg/kg, administered intravenously once daily, for up to 5 days.5 However, many studies have reported efficacy and cost-savings using single dose rasburicase.6-9
Reeves and Bestul conducted a prospective open-label study to compare the efficacy of a single 7.5-mg dose of intravenous rasburicase versus an intravenous dose of 0.15 mg/kg for the prevention or treatment of hyperuricemia associated with TLS.7 The investigators concluded that a single 7.5-mg dose of intravenous rasburicase had effects similar to those of a single 0.15-mg/kg intravenous dose, and that the low 7.5-mg fixed dose also has the potential to reduce costs.7
Trifilio and colleagues conducted the largest retrospective single-center study of 247 adults, with 224 receiving a single 3-mg fixed dose of rasburicase to treat 287 episodes (51 episodes required >1 dose).9 The single dose was effective in normalizing uric acid levels (to ≤7 mg/dL) 24 hours after rasburicase administration in 72% of the patients, and substantially reducing levels (to ≤5 mg/dL) in 48% of patients. At these doses, the institutional total cost was less than $400,000, which is significantly lower than the estimated total cost of $7 million to $9 million for the FDA-approved dosing of rasburicase, based on 2009 prices. The renal function remained stable: no correlation was found between uric acid level at 24 hours and the percent change in serum creatinine levels from baseline to 24 hours.9
Giraldez and Puto conducted a retrospective study of a single 6-mg fixed dose of rasburicase in 15 adults, 2 of whom had a second 6-mg dose, because the uric acid levels remained >8 mg/mL more than 48 hours after the first dose.10 The mean uric acid levels were 10.76 mg/dL before rasburicase administration and 3.2 mg/dL after. The cost-savings of a single dose was >$18,500 per patient.10
The Princess Noorah Oncology Center in Jeddah, Saudi Arabia, is one of the largest tertiary care referral oncology centers in the western region of Saudi Arabia, where different types of cancer, including hematologic malignancies, such as acute leukemia, lymphomas, and multiple myeloma, are being managed. These hematologic malignancies are often associated with TLS.
In 2007 and 2008, rasburicase was not yet approved by the FDA or by the Saudi Arabia Institutional Pharmacy and Therapeutic Committee for the prevention or treatment of hyperuricemia associated with malignancy in adults. However, published data supported its off-label use in adults with cancer.7,11 Although the optimal rasburicase dose for adults had not been determined at that point, a review of earlier studies revealed that a single fixed dose of 3 mg to 7.5 mg of rasburicase had comparable efficacy to the 0.2-mg/kg multiple-day dosing7,9,10 that was approved by the FDA in 2009.12
Therefore, based on the study by Giraldez and Puto, which concluded that rasburicase 6-mg fixed dose is effective in reducing uric acid to <4 mg/dL by day 3,10 and another study reporting similar efficacy with rasburicase 3-mg fixed-dose strategy,9 our Princess Noorah Oncology Center started using a single 6-mg fixed dose of rasburicase for the prevention and management of hyperuricemia associated with TLS in patients with high-risk hematologic malignancies.
In 2013, we planned this retrospective study to assess the effectiveness of a single 6-mg fixed dose of rasburicase on reducing serum uric acid and serum creatinine levels when used for the prevention or management of hyperuricemia associated with TLS in adults with cancer. Our primary objective was to determine the effectiveness of a single 6-mg fixed dose of rasburicase to lower uric acid levels to <7 mg/dL from baseline to 24 and 48 hours after rasburicase administration.
Secondary objectives were to determine the ability of a single 6-mg fixed dose of rasburicase to lower uric acid levels to <7 mg/dL at 24 and 48 hours after rasburicase administration, and the serum creatinine level from baseline to <1.5 mg/dL at 96 hours after rasburicase administration; to determine the number of rasburicase doses that are required for the prevention or management of hyperuricemia associated with TLS; and to discuss the cost-savings with a single fixed dose of rasburicase.
Methods
This study was a retrospective single-center chart review that was approved by the Institutional Review Board of our institution. We examined the electronic medical records (EMR) system for eligible patients who received rasburicase therapy between January 2008 and December 2012 at our oncology center. Inclusion criteria required that patients be aged 14 years or older (the Saudi healthcare system defines adults as aged more than 14 years), have received a single 6-mg fixed dose of rasburicase, and have serum uric acid and serum creatinine levels measured before and after rasburicase administration. Patients who received a weight-based dose of rasburicase, as recommended by the FDA, were excluded from the study.
Demographic information, concurrent medications use, and laboratory parameters were collected for each patient. In addition, we collected uric acid levels after rasburicase administration with repeated serum uric acid and serum creatinine levels recorded for up to 96 hours after rasburicase administration. We have been using specific handling procedures for the measurement of serum uric acid after the administration of rasburicase as per our center’s existing TLS guidelines, because rasburicase causes enzymatic degradation of the uric acid in blood samples if left at room temperature, resulting in spuriously low uric acid levels. Our TLS guidelines recommend accurate measurements of uric acid by collecting blood into prechilled tubes containing heparin anticoagulant, and then immediately immersing and maintaining in an ice-water bath; plasma samples are assayed within 4 hours of sample collection, as per our TLS guidelines and hence serum uric acid data collected retrospectively after rasburicase administration were reliable.
Hyperuricemia was defined as a uric acid level of >7 mg/dL. Acute renal dysfunction was defined as serum creatinine of >1.5 mg/dL. Because we had been using an off-label single 6-mg fixed dose of rasburicase in our institution, we also compared the cost of a single dose with the cost of the FDA-approved dose of intravenous rasburicase of 0.2 mg/kg daily for 5 days for the same indication.5
Data Collection
The study method included many steps. We used a data collection sheet to record the following variables and laboratory values for each patient, including patient demographics (ie, age, sex, weight, height); type of cancer; medication (ie, rasburicase, allopurinol, sodium bicarbonate hydration); serum uric acid levels; serum creatinine levels; serum lactate dehydrogenase levels; serum potassium levels; serum phosphorus; and serum calcium levels—all at baseline, prerasburicase, and postrasburicase at 24 hours, 48 hours, 72 hours, and 96 hours.
The patient charts in the EMR system were accessed to obtain patient demographics, by using QuadraMed
(our hospital information system) to obtain the data regarding rasburicase and allopurinol administration. Laboratory data, such as serum uric acid levels, serum creatinine, and electrolytes, were also accessed from the EMR using the QuadraMed system.
All data were then analyzed. Serum uric acid levels were assessed at baseline as prerasburicase administration, and at 24 to 96 hours postrasburicase doses. Serum creatinine, serum potassium, serum phosphorus, corrected serum calcium, and serum lactate dehydrogenase were collected retrospectively, and the cost impact was calculated.
The mean and standard deviations were reported for continuous variables; numbers and percentages were reported for nominal or categorical data.
Results
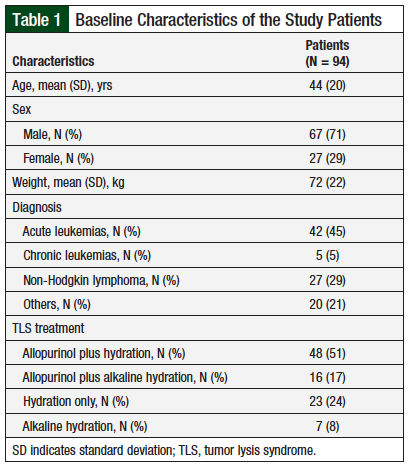
A total of 94 adults were included in the study during the 5-year period. The study population is described in Table 1. The patients’ mean age was 44 years, with 71% (N = 67) men, and 29% (N = 27) women. The patients’ mean weight was 72 kg.
In all, 42 (45%) patients had a diagnosis of acute leukemia; 27 (29%) had non-Hodgkin lymphoma; 5 (5%) had chronic leukemia; and 20 (21%) had a solid tumor diagnosis. Overall, 48 (51%) patients received allopurinol plus hydration; 23 (24%) patients received hydration alone; 16 (17%) patients received allopurinol plus alkaline hydration; and 7 (8%) patients received alkaline hydration alone (Table 1).
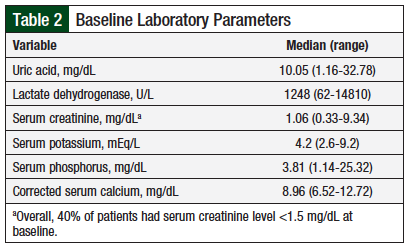
The baseline laboratory parameters are described in Table 2. We documented the 6 laboratory parameters that are frequently affected by TLS, including serum uric acid, serum creatinine, serum potassium, serum lactate dehydrogenase, serum phosphorus, and corrected serum calcium. Our findings showed that 2 of the 6 mean parameters were abnormally elevated, possibly indicating that the patients had TLS, or they were at high risk for TLS. Overall, 38 (40%) patients had elevated (>1.5 mg/dL) serum creatinine.
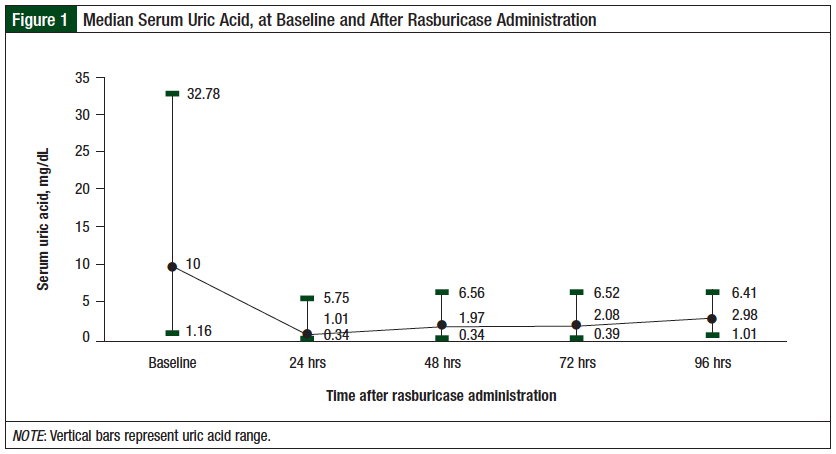
All patients had received a single 6-mg fixed dose of rasburicase. We recorded all serum uric acid levels that were obtained before and after the first dose of rasburicase, for up to 96 hours. At 24 hours, 79% (75) of the patients had normalization of serum uric acid to <7 mg/dL, with a median of 1.00 mg/dL (range, 0.33-5.74 mg/dL). Approximately 81% (77) of the patients had normalization of serum uric acid to <7 mg/dL at 48 hours, with a median of 1.96 mg/dL (range, 0.33-6.55 mg/dL). The results of the study end points are presented in Figure 1.
Uric acid reduction was maintained for 96 hours, and 87% of the patients had normalization of serum uric acid, with a median of 2.97 mg/dL (range, 1-6.40 mg/dL). Normalization of serum creatinine below 1.5 mg/dL was achieved in 70% of patients at 96 hours, with a median of 0.62 mg/dL (range, 0.31-1.28 mg/dL).
Four patients died by 96 hours after treatment, and 6 patients did not have serum creatinine measured at 96 hours. These patients were nevertheless included in the final analysis (Figure 2). The results demonstrated that 78% (73) of the patients required a single 6-mg fixed dose of rasburicase, and 22% (21) required more than 1 dose.
Subgroup Analysis
Acute renal dysfunction was defined as serum creatinine higher than 1.5 mg/dL. We found that 11 of 21 (52%) patients who required more than 1 single 6-mg fixed dose of rasburicase had acute renal dysfunction at presentation, suggesting a potential correlation between acute renal dysfunction and a requirement for more than 1 rasburicase dose.
Patients with serum uric acid higher than 7 mg/dL during the 96-hour period after receiving the first dose of rasburicase were considered appropriate candidates for a repeated dose of rasburicase. However, we found that a repeated dose of rasburicase was appropriate in only 7 of 21 (33%) patients, and approximately 67% (14) of the patients received a repeated dose of rasburicase unnecessarily, because their serum uric acid was lower than the normal limit of the institution.
Cost-Savings Benefit
Based on our analysis, the Ministry of National Guard-Health Affairs’ budget reflected a significant cost-savings of $320,000 in 94 patients using the single 6-mg fixed dose of rasburicase compared with the multiple-day dosing recommended by the FDA, suggesting that this strategy is cost-effective in addition to being clinically beneficial.
Discussion
TLS is a lethal complication of hematologic malignancies and some solid tumors. It occurs after tumor cells break down spontaneously or after exposure to radiation or chemotherapy.1 Rasburicase significantly decreases uric acid levels in patients with hyperuricemia related to TLS and is recommended for the management or prevention of hyperuricemia in patients at high risk for TLS.3,4
Another treatment option for the management or prevention of hyperuricemia associated with TLS is allopurinol, a xanthine oxidase inhibitor; however, this agent is only used for patients at low- or intermediate-risk for TLS.11 Furthermore, alkaline intravenous fluids are not recommended for the prevention or management of any level of TLS risk; rather, nonalkaline intravenous fluids must be administered.3
Screening patients before rasburicase administration for glucose-6-phosphate dehydrogenase deficiency (which would preclude rasburicase use) is crucial, because patients may have serious adverse events, such as severe hemolytic reactions, within 2 to 4 days after rasburicase initiation.13
Rasburicase was initially approved by the FDA in 2002 to reduce uric acid levels in children receiving chemotherapy who are at high risk for TLS.2,5 In 2009, rasburicase was granted FDA approval for weight-based dosing in the initial management of TLS in adults,5 based on a randomized phase 3 study of patients with hematologic malignancies at risk for hyperuricemia and TLS.12 The current FDA-approved dose of rasburicase for prevention or management of hyperuricemia associated with TLS is 0.2 mg/kg administered intravenously once daily for ≤5 days.5
Published guidelines suggest the use of 0.2 mg/kg for 1 to 7 days,4,6,11 but many studies have reported efficacy and cost-savings with the use of rasburicase at lower doses, or for shorter durations.6-10
Our finding showed that the median daily serum uric acid and serum creatinine levels remained stable at low levels during the 24- to 96-hour period (Figure 1 and Figure 2). Approximately 80% of patients had normalization of uric acid at 24 hours and 48 hours after rasburicase administration, and the uric acid reduction was maintained until 96 hours, with 87% maintaining normal levels of uric acid (Figure 1 and Figure 2).
Our findings showed a positive correlation between a repeated dose of rasburicase and acute renal dysfunction (ie, >1.5 mg/dL) at initial presentation. Patients who receive a single 6-mg fixed dose of rasburicase should continue to be monitored for TLS and have their uric acid checked daily, for up to 96 hours, to assess the need for a repeated dose of rasburicase. A repeated dose should be used if the serum uric acid level is above 7 mg/dL during the 96 hours after receiving the first dose of rasburicase.
Overall, approximately 22% (21) of the patients received a repeated 6-mg dose of rasburicase, but based on our findings, this was appropriate in only 33% (7) of the patients, meaning that it was unnecessary in approximately 67% (14) of these patients.
Furthermore, our analysis found a significant cost-savings with the use of a single 6-mg fixed dose of rasburicase compared with the multiple-day dosing recommended by the FDA.
Limitations
This study had some limitations. The small sample size is a potential limitation, as is the use of data from a single center. Future prospective studies are needed that include a large sample size to compare the efficacy of rasburicase 6-mg single fixed dose versus multiple days of rasburicase dosing.
Conclusion
Our findings demonstrated that a single 6-mg fixed dose of rasburicase is effective in preventing or managing hyperuricemia in patients at high risk for TLS and maintaining uric acid reduction for 96 hours; furthermore, it is as effective as using weight-based doses for multiple days. This is similar to the findings in previously published studies of a single fixed dose of rasburicase. The use of a single 6-mg fixed dose of rasburicase is also cost-effective. Based on these data, we recommend using the single 6-mg fixed-dose strategy for the prevention or management of hyperuricemia associated with TLS, in our institution as well as in other institutions.
Patients who receive a single 6-mg fixed dose of rasburicase should have continued TLS monitoring and have their uric acid checked on a daily basis for up to 96 hours to assess the potential need for repeated doses of rasburicase. Larger, randomized, prospective studies are needed to compare the use of a single 3-mg fixed dose of rasburicase versus a single 6-mg fixed dose of rasburicase and compare them to the FDA-approved dose of rasburicase to determine the optimal dosing schedule.
Author Disclosure Statement
Mr Khan, Dr Alshamrani, Dr Aseeri, Dr Al Saeed, Mr Alhamdan, and Dr Masari have no conflicts of interest to report.
References
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844-1854. Erratum in: N Engl J Med. 2018;379:1094.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11.
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55(5 Suppl 3):S1-S13; quiz S14-S19.
- Cairo MS, Coiffier B, Reiter A, Younes A; for the TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
- Elitek (rasburicase) for injection for intravenous use [prescribing information]. Bridgewater, NJ: Sanofi-Aventis; September 2017.
- Coiffier B, Altman A, Pui CH, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26:2767-2778. Erratum in: J Clin Oncol. 2010;28:708.
- Reeves DJ, Bestul DJ. Evaluation of a single fixed dose of rasburicase 7.5 mg for the treatment of hyperuricemia in adults with cancer. Pharmacotherapy. 2008;28:685-690.
- Campara M, Shord SS, Haaf CM. Single-dose rasburicase for tumour lysis syndrome in adults: weight-based approach. J Clin Pharm Ther. 2009;34:207-213.
- Trifilio SM, Pi J, Zook J, et al. Effectiveness of a single 3-mg rasburicase dose for the management of hyperuricemia in patients with hematological malignancies. Bone Marrow Transplant. 2011;46:800-805.
- Giraldez M, Puto K. A single, fixed dose of rasburicase (6 mg maximum) for treatment of tumor lysis syndrome in adults. Eur J Haematol. 2010;85:177-179.
- Cortes J, Seiter K, Maziarz RT, et al. Superiority of rasburicase versus allopurinol on serum uric acid control in adult patients with hematological malignancies at risk of developing tumor lysis syndrome: results of a randomized comparative phase III study. Blood (ASH Annual Meeting Abstracts). 2008;112. Abstract 919.
- PRNewswire. FDA approved Elitek (rasburicase) for management of plasma uric acid levels in adults with leukemia, lymphoma, solid tumors receiving anti-cancer therapy. October 16, 2009. www.news.sanofi.us/press-releases?item=118501. Accessed November 29, 2018.
- Sonbol MB, Yadav H, Vaidya R, et al. Methemoglobinemia and hemolysis in a patient with G6PD deficiency treated with rasburicase. Am J Hematol. 2013;88:152-154.