Pegfilgrastim is a granulocyte colony-stimulating factor (G-CSF) used to promote the growth of white blood cells in patients receiving myelosuppressive chemotherapy. It is approved by the US Food and Drug Administration (FDA) for the prevention of chemotherapy-induced febrile neutropenia. The recommended dose of pegfilgrastim is 6 mg administered subcutaneously at least 24 hours after the completion of chemotherapy.1 Administering pegfilgrastim within 24 hours of chemotherapy is not currently recommended because of the potential for increasing chemotherapy toxicity to myeloid progenitor cells after growth factor stimulation.1,2 Manual injections of pegfilgrastim are given to patients the day after chemotherapy completion, roughly 24 hours after treatment.
An alternative delivery method of pegfilgrastim is necessary, because many factors may contribute to patients being unable to return to the clinic for the manual injection a day later. Patients must make another trip to the clinic to receive their injections at the appropriate time interval. Transportation, as well as physical health, could also be potential barriers to patients receiving their pegfilgrastim. Therefore, another delivery method is needed to ensure patient adherence to protocol. The Onpro device is applied to patients on the same day they receive chemotherapy. The nursing staff will fill the on-body injector with pegfilgrastim using the co-packaged prefilled syringe. The Onpro device is then applied to the skin on the patient’s abdomen or back of the arm. The device administers pegfilgrastim to the patient 27 hours after it is placed, for more than 45 minutes. The McDowell Cancer Institute at Cleveland Clinic Akron General (CCAG) in Ohio began using the Onpro device in April 2015.
The approval of the on-body injector (ie, the Onpro device) was based on a pharmacokinetic clinical trial that compared the maximum concentration (Cmax) and the area under the curve to infinite time (AUC0-inf) of the manual injection versus the Onpro device.3 A total of 262 healthy patients aged 18 to 50 years were randomized into 2 groups. One group received a single dose of subcutaneous pegfilgrastim 6 mg from a prefilled syringe and the other group received the dose from the Onpro device. The study lasted 6 weeks. Pharmacokinetics were measured from participant blood samples, which were collected at baseline, as well as throughout the administration. The primary end points were Cmax and AUC0-inf, and the secondary end points included safety, tolerability, and immunogenicity.3
The mean AUC0-inf was 10,900 ng/mL in the Onpro group and 11,100 ng/mL for the manual injection group, and the mean Cmax was 248 ng/mL and 262 ng/mL, respectively. No deaths occurred during the study. Approximately 85% of patients had ≥1 adverse events, regardless of how they received the pegfilgrastim. Most adverse events were mild to moderate in severity. The adverse events were consistent with pegfilgrastim’s safety profile. Back pain (on-body injector, 49.3% vs manual injection, 50.8%), headache (45.5% vs 43.8%, respectively), arthralgia (11.9% vs 15.6%, respectively), and pain (10.4% vs 12.5%, respectively) were the most frequently reported adverse events. Patients in the Onpro group were more likely to have syringe-related adverse events than those in the manual injection group (13% vs 4%, respectively). Contact dermatitis (3.0% vs 0%, respectively) and injection-site pruritus (0.7% vs 0%, respectively) were also most frequently reported in patients in the Onpro group.3
No pegfilgrastim-neutralizing antibodies were detected in either treatment group. The pharmacokinetics and safety were comparable between the 2 groups. The study concluded that the 2 treatment options were comparable.3
Past clinical trials have evaluated the efficacy of the manual pegfilgrastim injection for primary prevention of chemotherapy-induced febrile neutropenia.4-12 These studies support the use of pegfilgrastim in patients receiving myelosuppressive chemotherapy regimens to prevent febrile neutropenia. However, no studies have been conducted to assess whether the efficacy and adverse events are similar between the Onpro and the manual injection delivery methods.
Theoretically, both delivery methods should result in similar pharmacokinetics, because each method delivers 6 mg of pegfilgrastim via subcutaneous injection. Infusion times differ between the manual injection and the Onpro delivery method. The manual injection is delivered by a subcutaneous push, whereas the Onpro device administers pegfilgrastim subcutaneously over a 45-minute period.13
However, at CCAG, clinicians noticed a trend of lower absolute neutrophil counts (ANCs) in patients who received pegfilgrastim via the Onpro device. In addition, Onpro device failures have occurred, which warrant further investigation of the device.
The Onpro device provides a convenient dosage form of pegfilgrastim that does not require patients to return to the clinic the day after receiving chemotherapy for manual injection. This makes the medication more accessible for patients, especially for those who have difficulty returning to the clinic. Although the Onpro device provides definite advantages for patients, there is the potential for device failure. If this occurs, the patient has to return to the clinic immediately to receive the manual injection. Our study aimed to ensure that patients are receiving the best possible care while allowing pegfilgrastim injection to accommodate their lifestyles.
The primary objective of our study was to compare the incidence of grade 4 neutropenia in patients who received a manual injection of pegfilgrastim versus the use of the Onpro device. The secondary outcomes were to compare the incidence of febrile neutropenia and the frequency of dose reductions and dose delays, define the predictors of Onpro failure, and identify how many patients switched back to the manual injection after using the Onpro device in subsequent cycles of chemotherapy.
Methods
This study was approved by the Institutional Review Board at CCAG, a 511-bed, community teaching hospital in Akron, OH. The McDowell Cancer Institute at CCAG consists of 4 outpatient infusion centers throughout the health system. The infusion centers are serviced by 7 hematology/oncology specialists.
Our study served as a means of quality assurance. Data were obtained through retrospective chart reviews of patients who received pegfilgrastim by means of manual injection or from the Onpro device in addition to their myelosuppressive chemotherapy cycle. A computer-generated list of patients who received pegfilgrastim was used to identify patients for study inclusion. The Onpro group included patients receiving pegfilgrastim via the Onpro device between April 1, 2015, and November 30, 2015.
Patients who received pegfilgrastim via the Onpro device were matched with patients who received the manual injection of pegfilgrastim based on sex, age, cancer type, cancer stage, and chemotherapy regimen. Manual injection records between August 1, 2010, and November 30, 2015, were accessed to optimize patient matching. The time frame was selected based on the implementation of the new electronic medical record (EMR) system. Patients were excluded from the study if they were aged <18 years, were pregnant, or could not be fully matched. Only pegfilgrastim was assessed in our study; patients who received filgrastim or other G-CSF medications were not included.
Data extracted from the EMR included age, sex, cancer type, cancer stage, chemotherapy regimen, use of pegfilgrastim, nadir ANC, dose of chemotherapy, and delay of future chemotherapy cycles. We also documented whether a patient had febrile neutropenia, if the Onpro device failed, or if the patient switched back to the manual injection after receiving pegfilgrastim via the Onpro device. Patients were matched to help account for the variability between cancer types and chemotherapy regimens.
Grade 3 neutropenia was defined as an ANC 500 cells/mm3 to 1000 cells/mm3, and grade 4 neutropenia was defined as an ANC <500 cells/mm3, based on the National Cancer Institute Common Terminology Criteria.14
Statistical AnalysisStatistical analysis was conducted by a statistician using the Statistical Package for the Social Sciences. A Pearson’s chi-square test and a Fisher’s exact test were used to evaluate categorical data, which included the primary and secondary outcomes. Descriptive statistics were used to evaluate the frequency of the Onpro device failure and the percentage of patients who switched back to the manual injection after using the Onpro device. A P value of <.05 was considered significant for all analyses.
Results
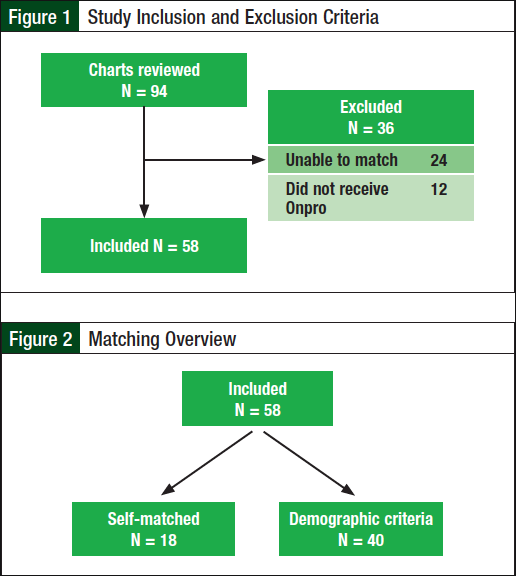
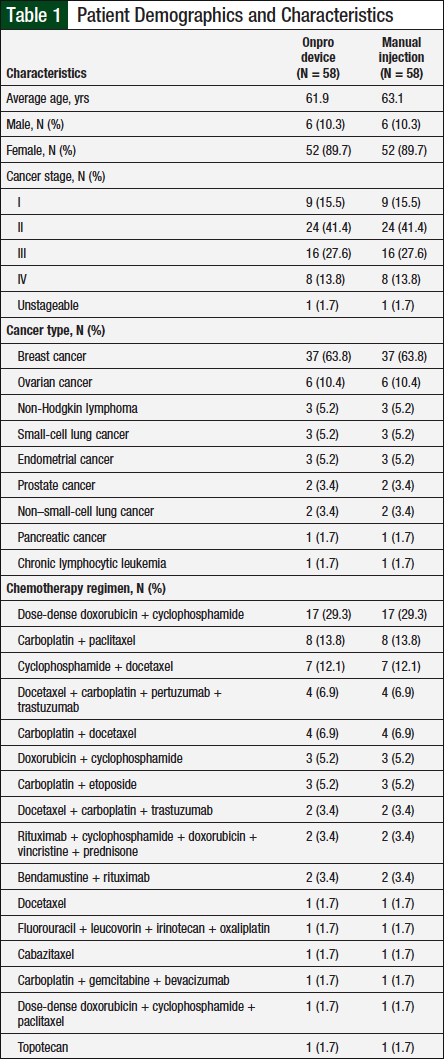
A total of 94 patients were evaluated for eligibility, of whom 58 were included in our study and 36 patients were excluded (Figure 1). The most common reason for exclusion was the inability to match the patient. Patients were excluded if they could not be matched on all 5 criteria—sex, age, cancer type, cancer stage, and chemotherapy regimen, to ensure that there was minimal variability between the 2 treatment groups. A total of 18 patients were self-matched, and the other 40 patients were matched based on demographic criteria (Figure 2). Baseline demographic characteristics between the 2 groups were similar (Table 1).
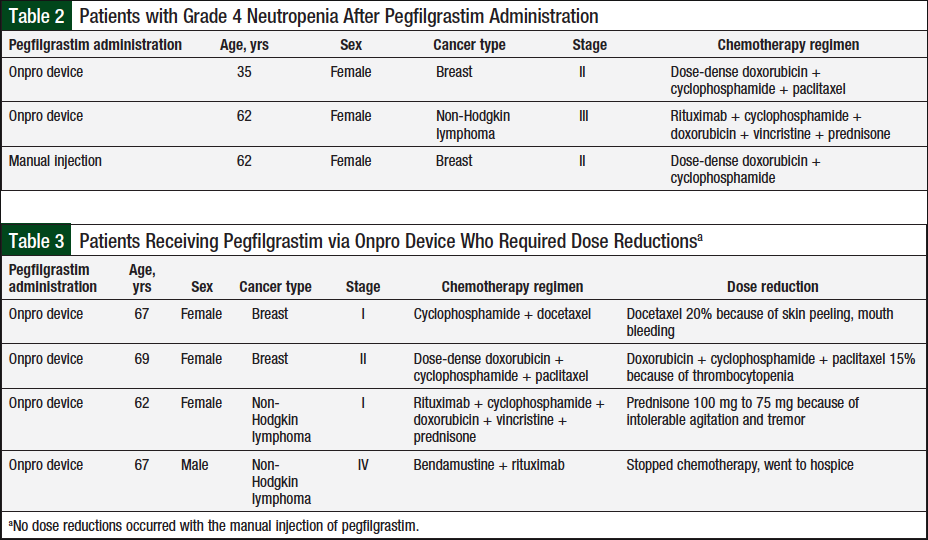
Overall, the rate of grade 3 neutropenia in patients who received the manual pegfilgrastim injection was 1.7% (N = 1) versus 3.4% (N = 2) for the patients who received pegfilgrastim via the Onpro device, and grade 4 neutropenia was 1.7% (N = 1) versus 5.2% (N = 3), respectively (Figure 3). The difference in grade 4 neutropenia between these 2 groups was not significant (P = .618); Table 2 lists the characteristics of patients with grade 4 neutropenia.
With regard to secondary objectives, no febrile neutropenia occurred in patients who received the manual injection and in 1 patient (1.7%) who received the Onpro device (P = 1.00).
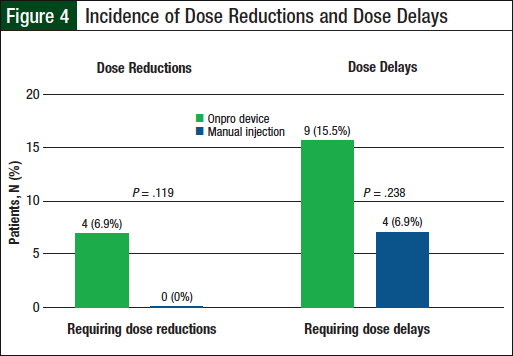
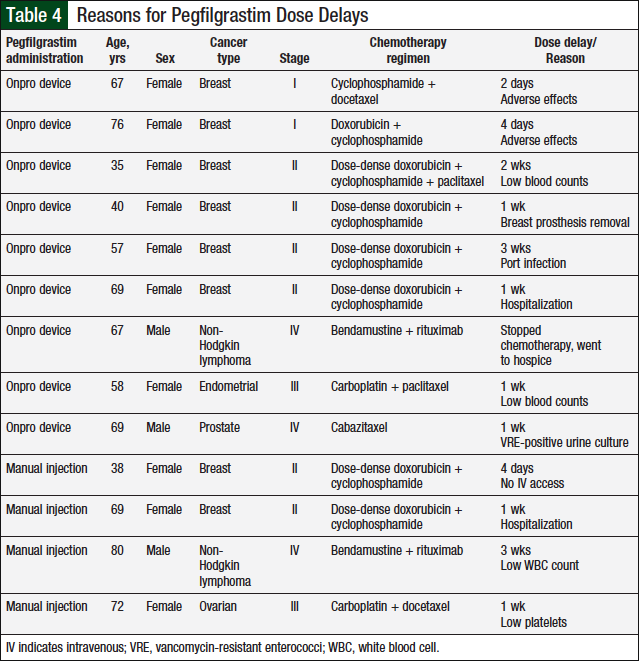
None of the patients in the manual injection group required dose reductions, compared with 4 (6.9%) patients in the Onpro group (P = .119; Table 3 and Figure 4). Dose delays were required for 4 (6.9%) patients in the manual injection group and 9 (15.5%) patients in the Onpro group (P = .238; Table 4 and Figure 4). Patients requiring dose reductions or delays were able to successfully complete their treatment regimens.
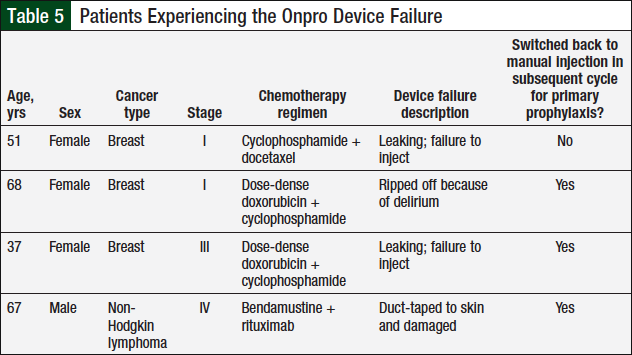
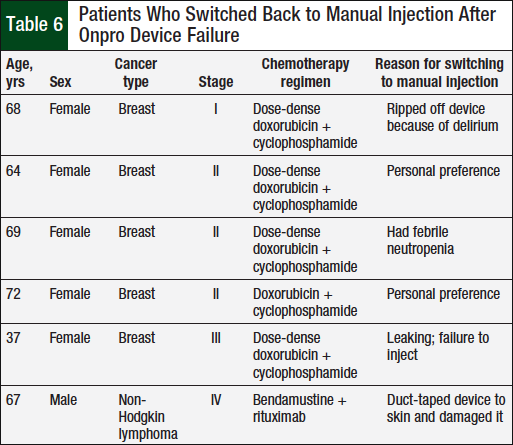
In the Onpro group, 4 (6.9%) patients had a device failure (Table 5), and 6 (10.3%) patients switched back to the manual injection after using the Onpro device (Table 6). Of the 6 patients who switched back to the manual injection, 3 had a device failure; 1 patient who had a device failure continued using the Onpro device without any further issues in subsequent cycles.
Discussion
G-CSF drugs, such as pegfilgrastim, are used to promote the growth of white blood cells in patients receiving myelosuppressive chemotherapy. The use of such medications helps to prevent treatment delays and dose reductions, allowing patients to continue receiving chemotherapy as scheduled. Pegfilgrastim should be administered between 24 hours and 72 hours after the completion of chemotherapy.15
Administering pegfilgrastim within 24 hours of chemotherapy is not currently recommended, because of the potential for increasing chemotherapy toxicity to myeloid progenitor cells after growth factor stimulation.15 This creates a challenge for some patients to return to the clinic a day later. Some patients may have to travel a long distance to receive the manual injection, or may have trouble securing transportation. In addition, they may not feel well enough to keep their appointment. Therefore, another delivery method is needed to ensure patient adherence to treatment.
The results of our study did not show a significant difference in the incidence of grade 4 neutropenia between the 2 pegfilgrastim delivery methods. Furthermore, the results showed no significant difference in secondary outcomes between the 2 delivery methods: the difference in the incidence of febrile neutropenia, frequency of dose reductions, and frequency of dose delays was minimal between the 2 study groups. Based on a previous study of the Onpro device by Yang and colleagues, which showed similar pharmacokinetic and safety data between the 2 delivery methods,3 we expected that the adverse event profile between the devices would be comparable as well.
Overall, only 1 patient in our study had febrile neutropenia among the entire study population. That patient, who used the Onpro device, was a woman aged 69 years who had been diagnosed with stage II breast cancer and received dose-dense doxorubicin/cyclophosphamide. As a result of the febrile neutropenia, the patient’s next cycle of chemotherapy was delayed because of hospitalization. The Onpro device used did not fail, but the patient did switch back to the manual injection for all subsequent chemotherapy cycles. The patient had no other episodes of febrile neutropenia throughout the course of chemotherapy.
Another goal of the study was to establish predictors of the Onpro device failure. Only 4 device failures occurred in the study, which makes it difficult to establish trends, especially because only 2 of the failures were truly attributed to the Onpro device (Table 5). The only commonality between the 2 Onpro device failures was that the patients were women who had been diagnosed with breast cancer. However, women with breast cancer comprised 64% of the study population. By determining predictors of the Onpro device failure, we anticipated that specific criteria for use could be developed. However, as a result of the limited device failures, we were unable to develop specific criteria for use. These data may be revisited in the future if additional device failures are observed.
The final objective of our study was to evaluate the percentage of patients who switched back to the manual injection after using the Onpro device (Table 6). Of the 6 patients who switched back to the manual injection, 2 did so because of an issue with the Onpro device. One patient had a device failure, and although the other patient’s device did not fail, that patient had febrile neutropenia after using the Onpro device. Two patients switched back to the manual injection because they were not appropriate candidates for the device. One patient had dementia and pulled the device off because she did not remember the purpose of the Onpro device, and another patient was afraid that the device would fall off and duct-taped it to his skin, which damaged the Onpro device. The other 2 patients switched back to manual injection based on personal preference. The only similarity between these 6 patients was that 5 of them were women with a diagnosis of breast cancer.
This study primarily serves as a means of quality assurance. Based on the results, providers can consider working with their patients to select the method that best fits the patients’ lifestyles, because both methods provide patients with an efficacious treatment option with no difference in risk for grade 3 or grade 4 neutropenia.
Limitations
There are several limitations to this study. The amount of data collected was limited by the recent (ie, March 2015) approval of the Onpro device. CCAG began using the device in April 2015, 1 month after its approval by the FDA. Patients were collected over an 8-month period before analysis of the results began.
Furthermore, for patients to be included in the study, they had to be matched on all 5 demographic criteria, which excluded 36 potential patients from the study. Having more patients could have contributed additional depth to the study results. Patients included in the study were manually matched; human error should be taken into consideration.
When the results were calculated with only the self-matched patients (N = 18), the results were similar compared with the entire population (N = 58); P values were .494 and .618, respectively. This helps to illustrate that matching bias might not have been a factor in this study.
Moreover, the study was only conducted at a single center; therefore, the results may not correlate with the general population. The accuracy of the study was reliant on past documentation in the EMR notes. The installation of the new EMR system in 2010 limited the pool of patients who were available to match the patients in the Onpro group.
Conclusion
The Onpro device removes potential barriers for patients who receive pegfilgrastim, and it also benefits the infusion clinic administering the medication. When the Onpro device is used, patients will not have to occupy a clinic appointment the day after chemotherapy. Therefore, the use of the Onpro device will help with the scheduling availability for other patients.
The Onpro device is a safe alternative to the manual injection and provides a more convenient delivery method for patients to receive their dose of pegfilgrastim after chemotherapy. The results of this study are based on a single institution and warrant further research to determine the incidence of grade 4 neutropenia within a larger patient population.
We found no significant difference between the 2 delivery methods with regard to grade 4 neutropenia, because the rates of grade 4 neutropenia were similar between patients who used the Onpro device and those who received manual injection of pegfilgrastim. As a result, patients can select the delivery method that best fits with their lifestyle, and providers can be assured that each delivery method will provide patients with an equally effective treatment option.
Author Disclosure StatementDr Townley, Dr Porter, and Dr McMullen have no conflicts of interest to report.
References
- National Comprehensive Cancer Nerwork. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Myeloid Growth Factors. Version 1.2017. April 28, 2017. www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf. Accessed June 1, 2017.
- Weycker D, Li X, Tzivelekis S, et al. Burden of chemotherapy-induced febrile neutropenia hospitalizations in US clinical practice, by use and patterns of prophylaxis with colony-stimulating factor. Support Care Cancer. 2017;25:439-447.
- Yang BB, Morrow PK, Wu X, et al. Comparison of pharmacokinetics and safety of pegfilgrastim administered by two delivery methods: on-body injector and manual injection with a prefilled syringe. Cancer Chemother Pharmacol. 2015;75:1199-1206.
- Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23:1178-1184.
- Aarts MJ, Peters FP, Mandigers CM, et al. Primary granulocyte colony-stimulating factor prophylaxis during the first two cycles only or throughout all chemotherapy cycles in patients with breast cancer at risk for febrile neutropenia. J Clin Oncol. 2013;31:4290-4296.
- Balducci L, Al-Halawani H, Charu V, et al. Elderly cancer patients receiving chemotherapy benefit from first-cycle pegfilgrastim. Oncologist. 2007;12:1416-1424.
- Green MD, Koelbl H, Baselga J, et al; for the International Pegfilgrastim 749 Study Group. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14:29-35.
- Yang BB, Kido A, Salfi M, et al. Pharmacokinetics and pharmacodynamics of pegfilgrastim in subjects with various degrees of renal function. J Clin Pharmacol. 2008;48:1025-1031.
- Yang BB, Kido A. Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet. 2011;50:295-306.
- Lyman GH, Dale DC, Culakova E, et al. The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2013;24:2475-2484.
- Burris HA, Belani CP, Kaufman PA, et al. Pegfilgrastim on the same day versus next day of chemotherapy in patients with breast cancer, non-small-cell lung cancer, ovarian cancer, and non-Hodgkin’s lymphoma: results of four multicenter, double-blind, randomized phase II studies. J Oncol Pract. 2010;6:133-140.
- Cheng C, Gallagher EM, Yeh JY, Earl MA. Rates of febrile neutropenia with pegfilgrastim on same day versus next day of CHOP with or without rituximab. Anticancer Drugs. 2014;25:964-969.
- Neulasta (pegfilgrastim) injection [prescribing information]. Thousand Oaks, CA: Amgen; December 2016.
- Kluetz PG, Chingos DT, Basch EM, Mitchell SA. Patient-reported outcomes in cancer clinical trials: measuring symptomatic adverse events with the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ Book. 2016;35:67-73.
- Diri R, McBride A, Lee C, et al. Efficacy of same-day vs. next-day pegfilgrastim for the prevention of chemotherapy-induced (febrile) neutropenia (CIN/FN): a meta-analysis. Blood. 2015;126:4764.