Cigarette smoking is the leading cause of preventable death in the United States, accounting for 1 of every 5 deaths annually.1 According to the American Cancer Society, tobacco use is associated with ≥30% of all cancer-related deaths, and 87% and 70% of all lung cancer–related deaths in men and women, respectively.2 In addition, tobacco use is associated with an increased risk for at least 17 types of cancer.3
Tobacco abstinence is imperative in patients with a cancer diagnosis. In addition to the general health benefits observed in all people who quit smoking, tobacco cessation can further benefit patients with cancer by preventing tumor progression, decreasing the risk for a secondary tumor, reducing complications from therapy, and improving morbidity and mortality risks.4
In a review of studies examining tobacco use among patients diagnosed with cancer, the rate of patients who were smokers at the time of their cancer diagnosis ranged from 46% to 75%.4 Among the patients who were smokers at diagnosis, the reviewers estimated that up to 58% of patients continued to smoke after initiating cancer therapy.4 Many factors are involved in a smoker’s ability to quit using tobacco; however, patients with cancer have additional factors that play a role in their decision, motivation, and success in quitting, including time since their cancer diagnosis, stage of disease, and planned cancer treatment.4
Although many studies have examined the effects of behavioral counseling and pharmacologic interventions on tobacco dependence, few prospective studies have focused on the oncology population to date. Four prospective studies involving provider-delivered counseling compared with usual care in hospitalized patients showed that counseling had a positive effect on smoking cessation in patients with cancer.5-8 The major limitations of these studies, however, were that they involved a small sample size, did not use standard-of-care pharmacologic interventions, and had brief follow-up periods.4
The effectiveness of pharmacist-delivered tobacco-cessation programs in achieving abstinence has been demonstrated in multiple studies across various patient populations, but this model is yet to be examined in the oncology population.9 In 2013, the American Society of Clinical Oncology released a statement supporting the increase of federally funded tobacco research to understand how best to implement tobacco-cessation programs,3 illustrating the importance of this topic across the oncology community. In addition, the National Comprehensive Cancer Network published its first guidelines on smoking cessation in 2015.10 This is an important landmark for patients with cancer: the guidelines elucidate the need for smoking cessation, even after a cancer diagnosis.10
Currently at the University of Illinois Hospital in Chicago, healthcare providers may refer their patients to the Tobacco Treatment Center to assist them in quitting tobacco use by placing an order for referral in the electronic ordering system. Patients may also refer themselves to the Tobacco Treatment Center by calling the center directly. Generally, patients are granted an appointment within 1 month of their referral.
The objective of the Tobacco Treatment Center is to increase the likelihood of a patient who uses tobacco to quit by using clinically proven pharmacologic and behavioral techniques. Patients who participate in evidence-based tobacco-dependence therapy have been shown to be twice as likely to quit smoking as patients who try to quit on their own.3
One major barrier that the oncology clinic experiences, however, is a high rate of patients failing to keep their appointments at the Tobacco Treatment Center. By expanding the Tobacco Treatment Center to the oncology clinic at the University of Illinois Hospital, our goal is to eliminate this barrier for patients, by providing these services in conjunction with other oncology clinic appointments.
The primary objective of our study is to analyze the impact of the implementation of a tobacco intervention program in an ambulatory oncology clinic at the University of Illinois Hospital, and to determine the effectiveness of pharmacist-delivered services provided in conjunction with other oncology clinic appointments.
Method
Study design
We conducted a prospective pilot study with historical controls to study the impact of pharmacist-delivered tobacco interventions in an ambulatory oncology clinic. Patients were divided into 2 groups, prospective and retrospective, with 12 patients in each group.
Patients in the prospective group received tobacco interventions from the oncology pharmacy staff in conjunction with their anticancer therapy sessions. Patients in the retrospective group received tobacco interventions at the Tobacco Treatment Center.
Of note, the study design was amended from a prospective, randomized trial to the current study design as a result of poor accrual.
The patients in the prospective group received their tobacco interventions in the oncology clinic. On an average day, the oncology clinic sees 30 patients for infusions and 40 physician visits. The oncology clinic is staffed by 2 full-time clinical pharmacists and 2 to 3 clinic staff pharmacists.
The patients in the retrospective group received their interventions at the Tobacco Treatment Center, which is a pharmacist-run clinic, under the direction of a medical director. The Tobacco Treatment Center is open 2 half-days weekly, and the pharmacist sees an average of 5 patients daily.
Patient population
Patients were eligible for the prospective group if they had a cancer diagnosis, were receiving intravenous anticancer therapy at the time of enrollment, were self-identified as smokers, and expressed interest in quitting smoking. Patients were excluded if they were aged <18 years, pregnant, or prisoners.
Eligible participants were referred for study participation by their primary oncologist or were recruited by study personnel. During the chemotherapy order verification process performed by the clinical pharmacists, electronic health records were reviewed for smoking status to identify potential eligibility.
All patients provided written informed consent. Patients in the retrospective group were eligible if they received tobacco interventions at the Tobacco Treatment Center after January 1, 2000, were aged ≥18 years, and had a documented cancer diagnosis.
The study was approved by the Institutional Review Board and the University of Illinois Cancer Center Protocol Review Committee.
Tobacco intervention program
Patients in the prospective group received the American Cancer Society brochure, “Deciding How to Quit: A Smoker’s Guide,” and met with pharmacists during anticancer therapy sessions or in conjunction with other oncology clinic appointments.
Meetings frequency was based on the patient’s schedule for cancer treatment, but generally occurred every 2 to 4 weeks. The length of a meeting ranged from 15 to 30 minutes. The patient’s carbon monoxide levels were measured using Smokerlyzer breath tests at baseline and at each subsequent pharmacist meeting. A reading of ≤6 parts per million was considered the level for a nonsmoker, as instructed by the Smokerlyzer manufacturer.
At the first visit, patients were asked to complete the Fagerström Test for Nicotine Dependence to assess their level of smoking addiction and readiness to quit. Behavior counseling and/or pharmacologic interventions were tailored to the patient’s specific needs, and aligned with the current standards of practice for tobacco-dependence treatment.
The recommendation to initiate pharmacologic therapy was based on patients’ willingness to attempt this method in their quitting strategy, and was prescribed under the direction of their primary oncologist. The choice of pharmacologic agent was based on patient preference and took into account the patient’s medical history, cancer diagnosis, cancer treatment, and concurrent medications.
At each visit with the pharmacist, adverse effects from pharmacologic therapy were assessed, and therapy was adjusted if intolerable side effects occurred. If a medication was recommended, patients had the option to fill their prescription through the on-site oncology clinic pharmacy.
The patient and pharmacist also worked collaboratively during each session to develop a list of smoking triggers and identify coping mechanisms. The model we used in our study is depicted in the Figure.
The number of visits to the oncology clinic for patients in the prospective arm was not defined; rather, the pharmacists continued to meet with participants based on individual needs. Patients in the prospective arm were asked to complete a survey to assess their satisfaction with the program at their last visit or 3 months after enrollment if they continued to receive treatment. The survey is a 12-item questionnaire adapted from the validated Patient Satisfaction Questionnaire Short-Form.11
Patients in the retrospective group received their nicotine-dependence therapy at the Tobacco Treatment Center. The tobacco intervention model used in the oncology clinic was based on the model developed at the Tobacco Treatment Center. Therefore, patients in the retrospective group received comparable interventions that were tailored to meet the individual patient’s needs.
Statistical analysis
All data collected were analyzed by simple descriptive and comparator statistics.
Results
Patient population
All patients who received anticancer therapy from October 2013 to March 2015 in the oncology clinic were screened by oncology pharmacy staff for participation in the prospective arm of this study. Overall, the recruitment was low, with 12 patients consenting to participate in the prospective arm and 12 patients selected for inclusion in the retrospective arm. Additional patients received tobacco interventions in the oncology clinic, but chose not to participate in the study.
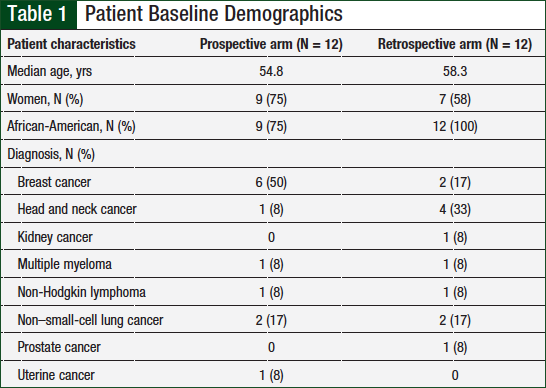
Of the total patient population (N = 24), the majority were women (67%) and African-American (88%). As shown in Table 1, patients with any cancer diagnosis were included, but the most common cancer types included breast cancer (33%), non–small-cell lung cancer (17%), and head and neck cancer (21%).
Tobacco interventions and quit rates
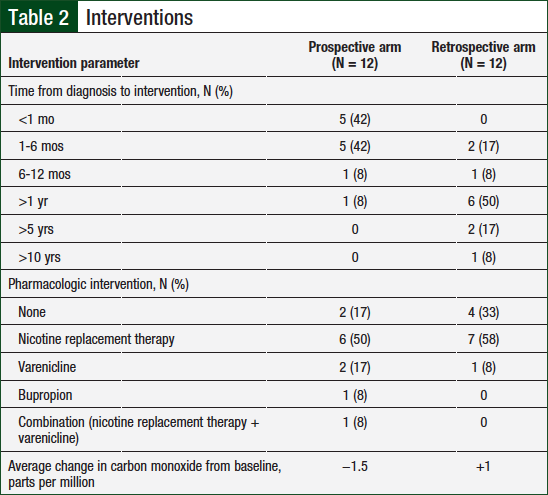
Table 2 lists the type of tobacco interventions used in the study population. The average number of pharmacist visits in the prospective group compared with the historical control group was 3.3 versus 3, respectively.
The majority of interventions in both groups were conducted face-to-face; however, 4 (10%) of the 40 interventions in the prospective group and 5 (13.9%) of the 36 interventions in the retrospective group were conducted via telephone.
The time from cancer diagnosis to first tobacco-dependence intervention was analyzed in both groups, and patients were divided into 6 categories (ie, <1 month, 1-6 months, 6-12 months, >1 year, >5 years, or >10 years). In the prospective arm, 10 patients began receiving tobacco intervention treatment within 6 months (5 patients within the first month) of their diagnosis compared with 2 patients in the retrospective arm. The majority (9) of patients in the retrospective arm began tobacco-dependence treatment at least 1 year after cancer diagnosis.
Carbon monoxide levels were measured at baseline and during each subsequent visit for patients in both groups. Tobacco abstinence was defined as a carbon monoxide level of ≤6 parts per million. Of the patients who participated in carbon monoxide monitoring (1 patient in the prospective arm declined), 4 of 11 (36%) patients in the prospective arm and 3 of 12 (25%) patients in the retrospective arm had carbon monoxide levels consistent with a nonsmoker. The average change in carbon monoxide level from baseline to final assessment in the prospective arm compared with the retrospective arm was −1.5 parts per million versus +1 parts per million, respectively.
Pharmacologic interventions were assessed in both groups (Table 2). In the prospective arm, 10 patients received pharmacologic interventions compared with 8 patients in the retrospective arm. Of the total population, the most common agents used were nicotine-replacement therapies and varenicline.
Patient satisfaction
Of the 12 patients in the prospective arm, only 4 completed the patient satisfaction survey. The other patients declined to complete the questionnaire, never returned the questionnaire, or completed their anticancer therapy before completing the questionnaire.
Of the patients who did complete the survey, the overall results were positive. In regard to general satisfaction, the average score was 4.625 on a 5-point Likert scale.
Discussion
Between October 2013 and March 2015, patients in the oncology clinic were screened for study inclusion by clinical pharmacists. The original study design was to randomize patients to pharmacist-delivered tobacco interventions versus standard of care, which could include physician intervention or referral to the Tobacco Treatment Center. Many patients were identified for inclusion; however, only 1 patient consented for participation.
On multiple occasions patients were interested in quitting smoking, but after being informed of the study design, many potential study participants declined. The major reason for declining was that patients wanted to receive their tobacco-dependence therapy in the oncology clinic during their anticancer infusions rather than potentially being randomized to the standard-of-care group and setting separate appointments in the Tobacco Treatment Center.
In April 2014, we decided to amend the protocol to increase patient enrollment. The updated study design included a prospective group of patients who would receive tobacco-dependence therapy in the oncology clinic and a historical group of patients who received treatment at the Tobacco Treatment Center. Although the original study design would have provided more robust data, the lack of patient enrollment further demonstrated the need for the expansion of these services into the oncology clinic.
One of the main goals of expanding the Tobacco Treatment Center into the oncology clinic was to increase the number of interventions made by pharmacists. The population we selected was comprised of patients who were receiving intravenous anticancer therapy and who, therefore, made frequent visits to the oncology clinic. Our results showed that we were able to make a marginal increase in the number of visits conducted by pharmacists in the oncology clinic compared with the Tobacco Treatment Center. One potential reason for not demonstrating a more significant improvement was that some patients are only seen in the infusion center for a very brief period (eg, <30 minutes), making it difficult for a pharmacist to intervene during each appointment.
Studies have shown that patients who attempt to quit tobacco use by using a tobacco intervention program are significantly more likely to quit smoking than those who try to quit on their own.3 According to the US Department of Health & Human Services, tobacco-quitting rates for patients who use counseling alone, medication alone, or a combination of counseling and pharmacologic interventions are 15%, 22%, and 22% to 28%, respectively.3 The quitting rates demonstrated in our study (36% in the prospective arm vs 25% in the retrospective arm) were comparable to the national average, with a slight improvement in the prospective arm.
In addition, more patients in the prospective group compared with the retrospective group (83% vs 67%, respectively) received a combination of counseling and pharmacologic interventions, which has been shown to be more successful than counseling alone at promoting tobacco abstinence.3
Among the patients who received tobacco interventions at the oncology clinic, the majority (>90%) began the intervention within 6 months of their cancer diagnosis, which is important for several reasons. Continued tobacco use after a cancer diagnosis has been shown to result in increased treatment-related toxicities, increased complication rates from surgery, and reduced treatment effectiveness.3 Thus, even after a cancer diagnosis, tobacco cessation can have a direct association with the ability to treat the cancer, and the treatment tolerability.
In addition, because tobacco use is associated with an increased risk for many cancers, a cancer diagnosis may serve as a “teachable moment” for the patient, and their motivation may be high after a cancer diagnosis. Cox and colleagues demonstrated that patients with cancer who received a tobacco-dependence intervention within 3 months of their diagnosis were more likely to be abstinent at 6 months compared with patients who started receiving tobacco interventions more than 3 months after their diagnosis.12
Our study demonstrates that initiating a tobacco intervention program in our oncology clinic significantly increased the rate of patients who received early intervention (ie, <6 months) after their cancer diagnosis, which could potentially impact the effectiveness and tolerability of their cancer treatment. Patients treated in the oncology clinic, where the pharmacists work in close proximity with the medical oncologists, are often able to initiate tobacco intervention services on the same day as referral.
In addition, expanding the tobacco intervention program to the oncology clinic allowed pharmacists with increased experience in caring for patients with cancer to tailor the program to the needs of the patients. One area in which this was especially important was the selection of pharmacologic agents for the management of tobacco dependence.
In our experience, for patients receiving anticancer agents associated with significant nausea and vomiting, nicotine replacement therapy is preferred to other agents that could increase the risk for gastrointestinal toxicity (ie, varenicline). In addition, combining nicotine replacement therapies (eg, nicotine patch with nicotine gum and lozenges) is an option for patients to reduce cravings.
By contrast, in patients with head and neck cancer who receive concomitant chemoradiation, where mucositis is a significant toxicity, nicotine replacement therapies, such as gum or lozenges, may disrupt the mucosa and lead to more severe toxicities.
These examples demonstrate the ability of oncology pharmacists to use their knowledge of cancer treatment strategies, drug interactions, and associated toxicities when selecting pharmacologic interventions for patients with cancer who wish to quit tobacco use. These interventions could lead to improved outcomes for tobacco abstinence in this unique patient population.
Because of increased screening, detection, and treatment advances, cancer has increasingly become a curable disease or a chronic illness.13 According to the National Cancer Institute and the Centers for Disease Control and Prevention, the number of cancer survivors in the United States increased from approximately 3 million in 1971 to approximately 15.5 million in 2016.13,14 These numbers are anticipated to reach more than 26 million by 2040.14
As a result of striking improvements in cancer care, survivorship has become a major part of cancer centers across the United States.
The role of pharmacists in survivorship and survivorship clinics is still being established; however, tobacco intervention programs are an area of need for survivorship clinics, because of the risk for recurrence and secondary cancers associated with continued tobacco use. Therefore, one way that pharmacists can integrate themselves into survivorship clinics and affect the continued care of cancer survivors is through the delivery of tobacco intervention programs.
Limitations
A major limitation of this study is the small sample size.
In addition, although carbon monoxide monitoring by breath test has its advantages, including being quick, inexpensive, and noninvasive, the results may not be representative of the patient’s smoking status, because the value is greatly affected by the time since the use of the last cigarette. For example, patients might not have smoked in the previous 7 days, but if they smoke immediately before their carbon monoxide test, the value would be significantly higher.
Conclusion
This pilot study demonstrates the feasibility of this type of service in an ambulatory oncology clinic. To strengthen the argument for incorporating a pharmacist-led tobacco intervention in this setting, larger studies should be conducted.
Author Disclosure Statement
Dr Puri is on the Speaker Bureau of Celgene and Novartis Pharmaceuticals, and is on the Advisory Board of the Institute for Cardio Oncology Awareness and Education for Bristol-Myers Squibb. Dr Kimmel, Dr Wilken, and Dr Wirth have no conflicts of interest to report.
References
- Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2015;64:1233-1240.
- American Cancer Society. Cancer facts and figures-2014. www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed June 15, 2016.
- Hanna N, Mulshine J, Wollins DS, et al. Tobacco cessation and control a decade later: American Society of Clinical Oncology policy statement update. J Clin Oncol. 2013;31:3147-3157.
- Cox LS, Africano NL, Tercyak KP, Taylor KL. Nicotine dependence treatment for patients with cancer. Cancer. 2003;98:632-644. Erratum in: Cancer. 2003;98:1104.
- Wewers ME, Bowen JM, Stanislaw AE, Desimone VB. A nurse-delivered smoking cessation intervention among hospitalized postoperative patients—influence of a smoking-related diagnosis: a pilot study. Heart Lung. 1994;23:151-156.
- Stanislaw AE, Wewers ME. A smoking cessation intervention with hospitalized surgical cancer patients: a pilot study. Cancer Nurs. 1994;17:81-86.
- Griebel B, Wewers ME, Baker CA. The effectiveness of a nurse-managed minimal smoking-cessation intervention among hospitalized patients with cancer. Oncol Nurs Forum. 1998;25:897-902.
- Gritz ER, Carr CR, Rapkin D, et al. Predictors of long-term smoking cessation in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 1993;2:261-270.
- Stack NM, Zillich AJ. Implementation of inpatient and outpatient tobacco-cessation programs. Am J Health Sys Pharm. 2007;64:2074-2079.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Smoking Cessation. Version 1.2015. March 10, 2015. https://cancer.osu.edu/~/media/Files/Shared/Press-Releases/Cancer/2015/NCCN-smoking-cessation-guideline.pdf?la=en. Accessed March 23, 2018.
- Thayaparan AJ, Mahdi E. The patient satisfaction questionnaire short form (PSQ-18) as an adaptable, reliable and validated tool for use in various settings. Med Educ Online. 2013;18:21747.
- Cox LS, Patten CA, Ebbert JO, et al. Tobacco use outcomes among lung cancer patients treated for nicotine dependence. J Clin Oncol. 2002;20:3461-3469.
- Centers for Disease Control and Prevention. Cancer survivors—United States, 2007. MMWR Morb Mortal Wkly Rep. 2011;60:269-272.
- Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029-1036.