Autologous stem-cell transplantation (ASCT) extends overall survival for patients with multiple myeloma1 and is a potentially curative treatment option for patients with non-Hodgkin lymphoma (NHL).2 CD34+ is expressed on peripheral blood stem cells. This marker can be used to measure the amount of stem cells that are available for collection. Target levels of CD34+ in the peripheral blood must be met to proceed with stem-cell collection. Patients who do not reach target CD34+ levels, or have enough stem cells for ASCT, require additional mobilization attempts, blood monitoring, and treatment; this lengthens the stem-cell collection process, and increases resource expenditure.2
Plerixafor (Mozobil), an inhibitor of CXC chemokine receptor type 4, is a hematopoietic agent that mobilizes stem cells into the peripheral blood.3 The drug is approved for patients with NHL or multiple myeloma who undergo ASCT.3,4 In combination with filgrastim (Neupogen), plerixafor produces a predictable peak of CD34+ cells in the peripheral blood, approximately 10 hours after administration.3
In a clinical study of plerixafor plus granulocyte colony-stimulating factor (G-CSF) versus G-CSF plus placebo, the median time to reach the collection goal (≥6 × 106 CD34+ cells/kg) in patients with multiple myeloma was 1 day for the plerixafor plus G-CSF group, and 4 days for the placebo plus G-CSF group.5 In another clinical study, the median time to reach the collection goal (≥5 × 106 CD34+ cells/kg) in patients with NHL was 3 days for the plerixafor group; it could not be evaluated in the placebo group.6
Despite the predictable response—resulting in target levels of CD34+ cells in less time—the cost of plerixafor has restricted its use.2 The average wholesale package price for one 1.2-mL vial of plerixafor is $8652.68.7 Costa and colleagues found that using a risk-adaptive protocol for poor mobilizers is more cost-effective, and projected a savings of $2589 per patient.8 Vishnu and colleagues concluded that using a risk-adaptive strategy for plerixafor significantly reduced the frequency of mobilization failures, and estimated a cost-savings of $19,300 per patient, owing to fewer collection days and more patients being able to undergo transplantation.9
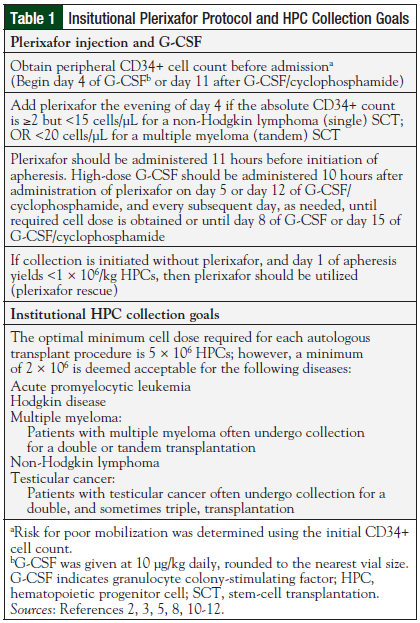
Given these results, a protocol was initiated at University Health Shreveport, LA, to limit the use of plerixafor to patients who are at risk for poor mobilization, including patients with prolonged exposure to lenalidomide (Table 1).2,3,5,8,10-12
Prior to protocol implementation, all eligible patients received plerixafor as part of their stem-cell mobilization regimen. Although many institutions have developed algorithms and protocols to improve stem-cell mobilization efficacy and cost-effectiveness,9,11,13 to our knowledge, there are no known comparison studies of mobilization costs before and after implementation of the protocol.
The primary objective was to determine the median number of collection days before and after initiating the risk-based plerixafor protocol. Secondary objectives were (1) to evaluate mobilization response (ie, the number of cells collected and the number of collection days), and (2) to analyze the cost-effectiveness of implementing the protocol.
Methods
This study was a single-center, retrospective chart review conducted at a tertiary-care medical center, and approved by the Institutional Review Board before initiation. Medical record numbers of eligible patients were obtained from stem-cell transplantation records—which were used to maintain accreditation—and database registries. Adult patients diagnosed with multiple myeloma or any type of NHL who underwent stem-cell collection for ASCT between July 1, 2009, and January 24, 2011 (before protocol implementation), or between January 25, 2011, and July 31, 2014 (after protocol implementation), were included. Patients aged ≤18 years and patients who were incarcerated at time of stem-cell collection were excluded from the study.
Per the protocol, patient risk was categorized using the initial CD34+ cell count on day 4 of G-CSF, or on day 11 of G-CSF plus cyclophosphamide. Patients were deemed to be at poor risk for mobilization if their absolute CD34+ count prior to collection was ≥2 but <15 cells/µL for a single stem-cell transplant or <20 cells/µL for a tandem stem-cell transplant. All patients who were given plerixafor, both pre- and postprotocol implementation, received 24 mg (to use the entire dose in the vial and minimize medication waste) regardless of weight, unless they had renal impairment that required dose adjustment. G-CSF was dosed at 10 μg/kg per dose, and rounded to the nearest vial size in all patients regardless of when the stem cells were collected (ie, before or after plerixafor protocol implementation). The institution’s optimal collection goal was 5 × 106 CD34+ cells per ASCT procedure.
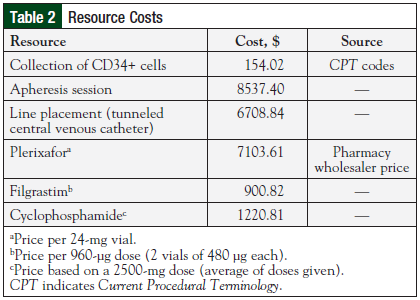
Patient data, which were collected using electronic health records, included age, weight, medical record number, diagnosis, mobilization regimen and duration, and CD34+ levels before collection, as well as the number of collection and clinic/hospital days, apheresis sessions, and transplantation procedures. Information on previous therapies that may have affected mobilization response, including chemotherapy and radiation, were also collected. Billing codes were used to collect cost data pertaining to drawing the CD34+ cells, line placement, apheresis sessions, and hospital/clinic days (Table 2). These costs were assumed to remain constant over time. Medication costs were based on pharmacy wholesaler cost information.
The median number of collection days before and after protocol implementation were compared using the independent-samples Mann-Whitney U test. We determined that our sample size of 101 patients had 80% power to detect a difference of 0.57 collection days. P values <.05 were considered significant. Secondary end points were exploratory, and, thus, were not analyzed by descriptive statistics. Statistical analyses were performed using SPSS software (version 22; IBM, Armonk, NY).
Results
During the study period, 152 patients underwent stem-cell collection for ASCT; of these patients, 101 met the inclusion criteria. The primary reasons for exclusion were diagnoses other than NHL or multiple myeloma, and stem-cell collection outside the study period. Included patients were divided into 2 groups: preprotocol (n = 39) and postprotocol (n = 62). Baseline characteristics are listed in Table 3.
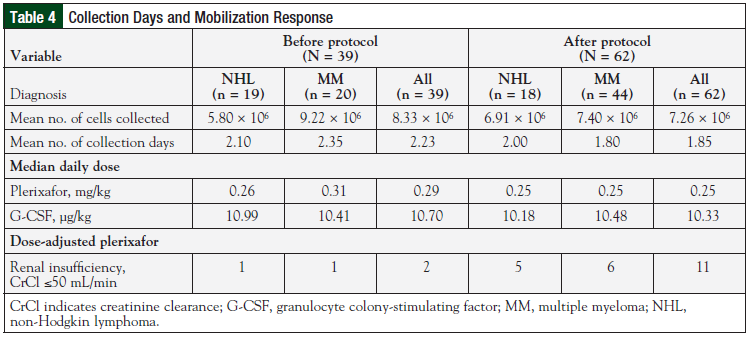
The median number of collection days was 2.00 for the preprotocol group and postprotocol group, respectively (P = .192). The mean number of collection days were also compared for these groups (Table 4). Before protocol implementation, the mean number of collection days was 2.23; the average was 2.10 days among patients with NHL, and 2.35 days among those with multiple myeloma. After protocol implementation, the mean number of collection days was 1.85; the average was 2.00 days for patients with NHL, and 1.80 days for patients with multiple myeloma.
Mobilization responses were evaluated for the pre- and postprotocol groups, and for the subsets of patients with multiple myeloma or NHL in each group (Table 4). Before protocol implementation, the mean number of cells collected was 8.33 × 106; the average was 5.80 × 106 cells among patients with NHL, and 9.22 × 106 cells among those with multiple myeloma. After protocol implementation, the mean number of cells collected was 7.26 × 106, with an average of 6.91 × 106 cells for patients with NHL, and 7.40 × 106 cells for patients with multiple myeloma.
Because the median number of collection days did not differ, it was assumed that nonmedication costs related to the mobilization process (eg, line placement, apheresis sessions) would approximately be the same for the pre- and postprotocol groups. Therefore, the focus of the cost analysis became medication expenses before and after initiation of the plerixafor protocol. In the preprotocol group, $532,000 was spent on plerixafor.
During the 1.5 years that plerixafor was used at our institution before implementation of the protocol, 76 doses were administered. In the postprotocol group, $364,000 was spent on plerixafor during the 3.5-year postprotocol period. During the preprotocol period, 55 doses of plerixafor were administered in 1 year.
During the postprotocol period, only 52 doses of plerixafor were administered during the first 3.5 years of protocol use. Using the risk-based protocol reduced the overall plerixafor cost by 32% (pre- vs postprotocol), and decreased plerixafor use by 77% from 2010 to 2011 (ie, the years immediately before and after protocol initiation).
Discussion
Risk-based use of plerixafor did not lengthen the duration of stem-cell collection. Although the use of the protocol had no significant effect on the median number of collection days, it did reduce the administration and cost of plerixafor.
Mobilization response, defined by the number of cells collected and the number of collection days, did not differ substantially between the study groups. This result was unexpected because all patients in the preprotocol group received plerixafor as part of their mobilization regimen. It was predicted that the preprotocol group would have more cells collected, and, therefore, have better mobilization responses. Risk factors for poor mobilization differed between pre- and postprotocol patients with multiple myeloma. More postprotocol patients with multiple myeloma had been treated previously with radiation, or had histories of lenalidomide (Revlimid) and radiation therapy.
The number of patients who underwent chemomobilization with cyclophosphamide, G-CSF, and plerixafor was higher in both subgroups (NHL and multiple myeloma) of preprotocol patients than among postprotocol patients. These differences in baseline characteristics, coupled with the patient-specific nature of stem-cell mobilization—including greater difficulty of mobilization with NHL—may have affected mobilization response.
Weight-based dosing (0.24 mg/kg) is used for plerixafor, and the maximum dose is 40 mg daily.3 In the present study, 20.7% of patients weighed >100 kg; however, they received only 24 mg daily to utilize the total dose in the plerixafor vial and eliminate medication waste. Technically, these patients could have received higher doses of plerixafor, potentially changing their mobilization response.
The number of renally adjusted plerixafor doses differed between the study periods. Before protocol implementation, only 2 patients received renally adjusted plerixafor doses; after protocol implementation, 11 patients received renally adjusted doses. This difference may be attributable to the difficulty in obtaining information from medical records before 2012.
Cost differences between the preprotocol and postprotocol groups were plerixafor-dependent. Use of the risk-based protocol reduced plerixafor usage, and, therefore, total mobilization costs.
Limitations
There are several limitations to this study. After data collection began, the first purchase of plerixafor was determined to be in July 2009. Therefore, we excluded all patients who underwent stem-cell collection before July 2009, because they did not receive plerixafor and their inclusion could have altered the preprotocol mobilization results.
In 2012, there was an institutional change to a new electronic health record system. Data collection for patients who underwent stem-cell collection prior to 2012 consisted primarily of notes and assumptions, as there was no reliable record of medication administration.
Adherence to G-CSF and plerixafor administration in these patients, was determined primarily by using notes from outpatient visits for injection. After the institutional change to the new electronic health record system, medication administration data were utilized to determine adherence to the mobilization regimen. If there were no visits or notes for the patients collected prior to 2012, it was assumed that the medications were administered, which may have affected the results and comparisons.
In addition, the CD34+ levels for these patients were reported as absolute values, whereas the new electronic health record system implemented in 2012 reported the CD34+ levels as percentages. Calculations were made to convert absolute values to percentages, which increased the risk for errors in actual precollection CD34+ levels.
Mobilization costs were assumed to be constant over time, and do not reflect possible price changes that may have occurred during the study period.
Conclusion
The use of a risk-based plerixafor protocol produced no significant difference in the number of collection days; however, the protocol greatly reduced medication costs without prolonging the stem-cell collection process, or affecting the mobilization response.
Author Disclousure Statement
The authors have no conflicts of interest to report.
References
1. Dispenzieri A, Lacy MQ, Kumar S. Multiple myeloma. In: Greer JP, Arber DA, Glader B, et al, eds. Wintrobe’s Clinical Hematology. 13th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014:2046-2097.
2. Sinha S, Gastineau D, Micallef I, et al. Predicting PBSC harvest failure using circulating CD34 levels: developing target-based cutoff points for early intervention. Bone Marrow Transplant. 2011;46:943-949.
3. Mozobil [package insert]. Cambridge, MA: Genzyme Corporation; 2014.
4. Lexi-Comp, Inc. Plerixafor. July 23, 2014. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1312540. Accessed May 15, 2015.
5. DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113: 5720-5726.
6. DiPersio JF, Micallef IN, Stiff PJ, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4767-4773.
7. Red Book Online [database online]. Greenwood Village, CO: Truven Health Analytics, Inc. Updated periodically. Accessed May 15, 2015.
8. Costa LJ, Alexander ET, Hogan KR, et al. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transplant. 2011;46:64-69.
9. Vishnu P, Roy V, Paulsen A, Zubair AC. Efficacy and cost-benefit analysis of risk-adaptive use of plerixafor for autologous hematopoietic progenitor cell mobilization. Transfusion. 2012;52:55-62.
10. Micallef INM, Ho AD, Klein LM, et al. Plerixafor (Mozobil) for stem cell mobilization in patients with multiple myeloma previously treated with lenalidomide. Bone Marrow Transplant. 2011;46:350-355.
11. Duong HK, Savani BN, Copelan E, et al. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014;20:1262-1273.
12. Majhail NS, Farnia SH, Carpenter PA, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21:1863-1869.
13. Abhyankar S, DeJarnette S, Aljitawi O, et al. A risk-based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transplant. 2012;47:483-487.