Oral chemotherapy utilization and availability have increased in the past several years. Of the new oncologic agents in development, 30% to 35% are oral preparations.1 With the increasing development of new oral anticancer therapies, there have also been growing concerns regarding adherence, toxicity, and cost. The World Health Organization defines adherence as “the extent to which a person’s behavior—taking medication, following a diet, and/or executing lifestyle changes—corresponds with agreed recommendations from a healthcare provider.”2 Few studies have addressed adherence rates in patients receiving oral chemotherapy. For example, in clinical trials of oral therapies (eg, tamoxifen, a hormonal agent used to treat breast cancer), adherence has ranged from 72% to 96%. However, patients participating in clinical trials are usually highly motivated and closely monitored.3 Overall, adherence rates in patients with cancer remain variable from 20% to 100%, depending on patient demographics, chemotherapy regimens, and definitions of adherence.4
Adherence is a critical factor when treatment options include oral chemotherapy. Nonadherence can lead to disease resistance and disease progression. For example, in a study examining patients receiving adjuvant cyclophosphamide, methotrexate, and fluorouracil, a combined oral and intravenous regimen, patients receiving >85% of the optimal dose had improved overall and progression-free survival compared with patients receiving <85% of the optimal dose.5 Moreover, inappropriate or overuse of oral chemotherapy may lead to more toxicities. Although patients often prefer oral to intravenous chemotherapy because of decreased medical visits and perceived lack of toxicities, the burden of treatment administration is the responsibility of the patient.6 In general, when treatment is self-administered in the patient’s home, it is more difficult to assess adherence and monitor toxicities.
Studies have evaluated adherence rates in patients receiving oral chemotherapy, and the healthcare professional’s role in improving patient compliance. Unlike intravenous chemotherapy, oral chemotherapy is self-administered by the patient at home and without direct medical supervision. Therefore, adherence to therapy is often assessed through patient self-reporting, prescription refill records, and pill counts. Tsang and colleagues reviewed patient pharmacy claim records for patients prescribed imatinib, and found that although overall compliance was 75%, only 50% of patients were 100% compliant.7 Spoelstra and colleagues conducted a study evaluating the effect of an automatic voice recording system on adherence.8 They found that 42% of patients were nonadherent to oral chemotherapy, and that missed doses increased with complexity of the treatment regimen. Factors affecting adherence include the patient’s knowledge and understanding of his or her disease, the quality of the patient’s interaction with their healthcare provider, the patient’s financial and social resources, and their treatment regimen’s complexity.9 Healthcare providers need to support patients with education, and ensure that patients understand their treatment and any toxicities associated with the therapy.
To address concerns associated with adherence, toxicity, and cost related to oral chemotherapy, several studies have evaluated interventions to improve adherence. Mathes and colleagues conducted a systematic review of 7 clinical trials evaluating the effectiveness of an intervention program addressing adherence in patients receiving oral chemotherapy. Interventions included educational programs managed by nurses or pharmacists, and prefilled pillboxes for each treatment cycle. Three studies showed favor toward intervention programs, and one study showed identical adherence rates in controlled and intervention groups.4 However, because of the heterogeneity of the studies included in the review, the authors concluded that adherence-enhancing programs could be promising, but that high-quality studies are still needed.
The cost of oral chemotherapy is another concern with increased utilization of oral anticancer agents. In general, oral chemotherapy has the potential to be cost-effective compared with intravenous chemotherapy (eg, dispensing oral therapy can alleviate staffing requirements traditionally needed for intravenous administration). However, increased educational requirements needed for patients receiving oral chemotherapy requires that additional phone calls and follow-ups be made by healthcare providers.5
In response to concerns about adherence, toxicity, and increasing costs associated with oral chemotherapy, the Veterans Health Administration released guidelines for a 14-day dispensing protocol.10 According to the guidelines, patients starting new regimens receive a 14- day supply of oral chemotherapy until adherence and tolerability are established. The goals of the guidelines were to reduce unnecessary costs and toxicities, and to improve patient education and adherence to the regimen. In order to ascertain the impact of the protocol on patient outcomes, it is necessary to evaluate adherence, toxicity, and costs after institution of the guidelines.
Methods
Audie L. Murphy Veterans Health Care Center adopted an oral chemotherapy protocol in January 2013. The protocol allows for a 14-day supply of oral chemotherapy for 2 fills until tolerability and adherence are established.10 All patients initiating oral chemotherapy are required to pick up their medication in person so that they receive counseling. Patients are assessed every 2 weeks before the next fill is released. Exceptions to the protocol are made on an individual basis depending on patient travel and inventory issues. Furthermore, therapy should not exceed a 30-day supply, nor should it be refillable.
The aim of this study was to evaluate the effectiveness of a 14-day versus 30-day dispensing protocol on adherence, toxicity, and cost. The primary objective of the study was to compare adherence rates in a 14-day versus 30-day dispensing protocol. Secondary objectives included evaluating the percentage of patients who experienced hematologic and nonhematologic toxicities on the 2 dispensing protocols, and the cost-savings of a 14- day dispensing protocol.
The project was designed as a retrospective chart review, and received approval from the Institutional Review Board at The University of Texas Health Science Center at San Antonio, and the Research and Development Committee at the South Texas Veterans Health Care Center (STVHCS). Patients receiving oral chemotherapy according to the oral chemotherapy policy at the Audie L. Murphy Veterans Health Care Center from June 1, 2012, to June 1, 2013, were identified using the STVHCS database, which maintains patient health information, and by collecting documented information from oncology/hematology outpatient clinic notes. Oral chemotherapy agents included in the program are listed in Table 1. A set of predefined variables were collected from the STVHCS database, including patient demographics, diagnosis, oral chemotherapy drug name, line of therapy, pharmacy refill records, laboratory values, and nonhematologic toxicities. Nonhematologic toxicities and laboratory values were collected from oncology/ hematology clinic notes and patient medical records after the first and final prescription fills were recorded. The percentage of patients with nonhematologic and hematologic toxicities was reported for each protocol. Hematologic toxicity was graded using the Common Terminology Criteria for Adverse Events version 4.0. The total drug cost was calculated by adding the cost per fill for each patient on the 14-day versus 30-day dispensing protocol.
Medication Possession Ratios
Adherence was measured using medication possession ratios (MPRs), which were defined as the sum of the days supply divided by the number of days between the first fill and last fill dates during the 12-month study period, with the addition of the days supply for the last prescription fill. MPR = Total days supply dispensed/[(Last fill date – first fill date) + last prescription day supply].
The patients’ length of therapy during the study period was used as the denominator. For example, patient X received imatinib from August 1, 2012, to November 29, 2012. Each refill was for a 30-day supply.
Patient X’s MPR = (30 days supply × 4 fills)/[November 29, 2012 – August 1, 2012 + 30 days)] = 120/150 = 0.80.
Patient X’s MPR is 80%, and he or she is therefore considered to be adherent to imatinib. If a patient’s length of therapy extended beyond the study’s time frame, the last refill date within the study period was used. For oral chemotherapy given in cycles, treatment breaks were added to the overall days supply.
Study Population
All outpatient veterans with a diagnosis of solid tumor or hematologic malignancy receiving care from the Audie L. Murphy Division oncology service, and receiving oral chemotherapy as part of the oral chemotherapy policy from June 1, 2012, through June 1, 2013, were included. Any patients receiving oral chemotherapy prescribed outside the oncology service, including hormonal agents for breast and prostate cancers, oral chemotherapy for nonmalignant causes, oral experimental agents, patients receiving concurrent parenteral chemotherapy, and patients receiving only 1 fill of medication, were excluded from the study.
Study Analyses
From June 1, 2012, to June 1, 2013, there were 190 patients receiving oral chemotherapy agents listed in the Audie L. Murphy Veterans Health Care Center oral chemotherapy protocol. With 80% power and a 5% margin of error, the number of patients needed to detect a significant change in adherence rates for a 14-day versus 30-day dispensing protocol was 89. Descriptive statistics were used to illustrate the baseline characteristics of the study population. Medians with interquartile ranges were used to express MPRs, and the Wilcoxon rank sum test was used to evaluate differences between the 2 protocol groups for the primary objective. Secondary objectives were analyzed using at test.
Results
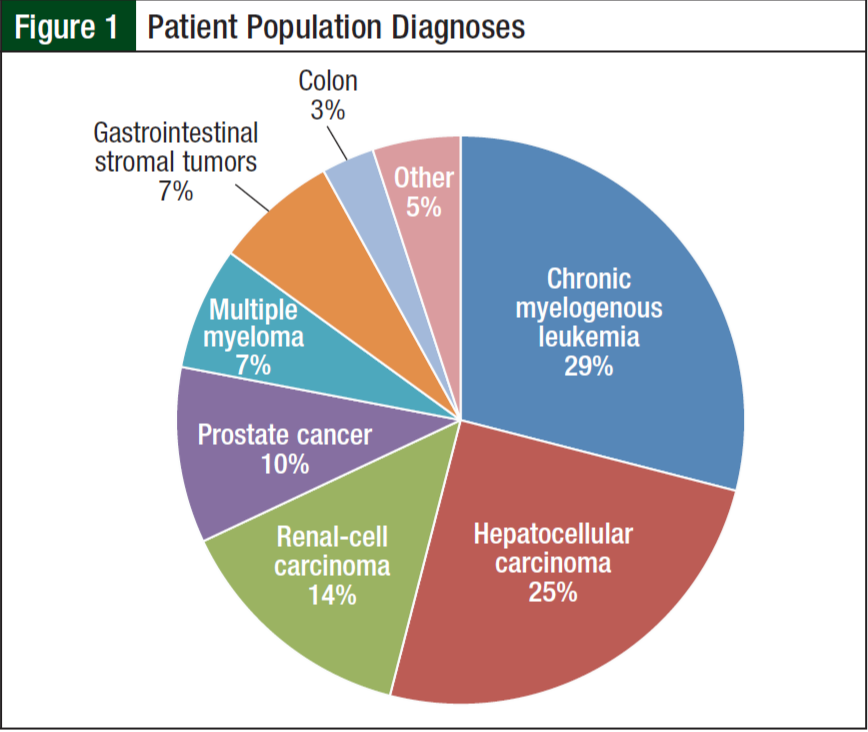
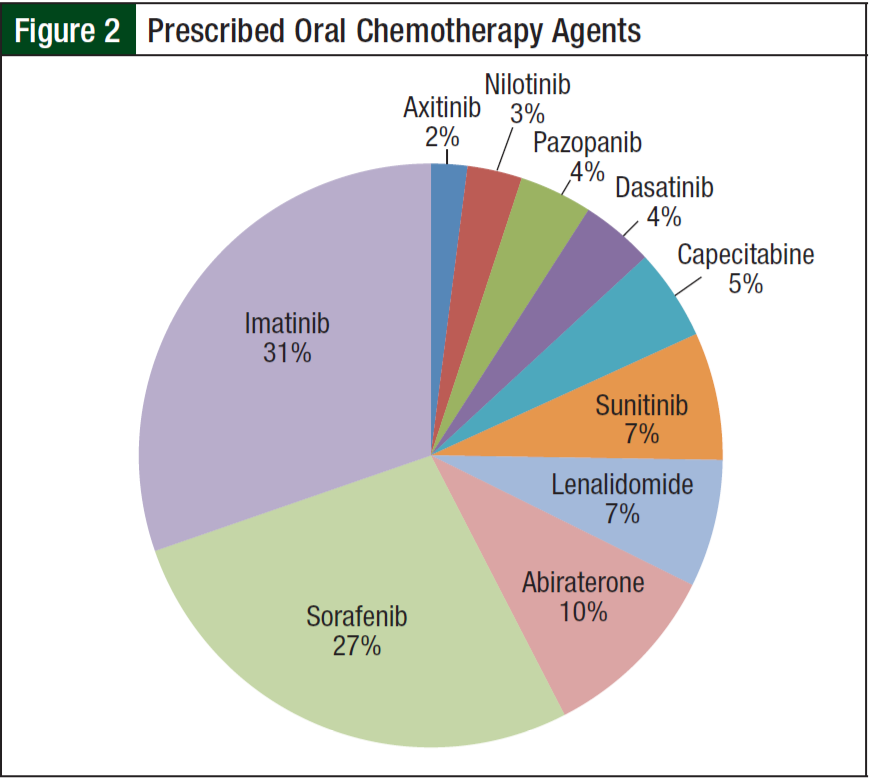
From June 1, 2012, to June 1, 2013, 84 of the 190 patients receiving oral chemotherapy met inclusion criteria. Five patients receiving lenalidomide were excluded because they received a 21-day supply according to the myeloma treatment regimen. The majority of patients (99%) were men, and more than half (68%) were white. The average age was 65 years. The 3 most common diagnoses included chronic myelogenous leukemia (29%), hepatocellular carcinoma (25%), and renal- cell carcinoma (14%) (Figure 1). Imatinib (31%) and sorafenib (27%) were the most commonly prescribed oral chemotherapy agents (Figure 2). More than half (69%) of the oral chemotherapy agents were prescribed in the first line of therapy.
Patients on the 14-day dispensing protocol had an MPR of 95% ± 9% versus 87% ± 14% on the 30-day protocol during the 12-month study period (P = .02). Eighty-five percent of patients on the 14-day supply protocol had an MPR >90% versus 47% of patients on the 30-day protocol (P <.001). The MPR for patients receiving tyrosine kinase inhibitors was 93% ± 9% versus other oral chemotherapy at 90% ± 13% (P = .18). No difference was found in MPR between solid tumor and hematologic malignancy, and both cancers had an MPR of 90% ± 13%. Finally, there was no difference in MPR based on the zip code for the region in which patients lived at the time of the study. Patients living >40 miles from the Veterans Health Administration had an MPR of 93% ± 11% versus 89% ± 13% (P = .06).
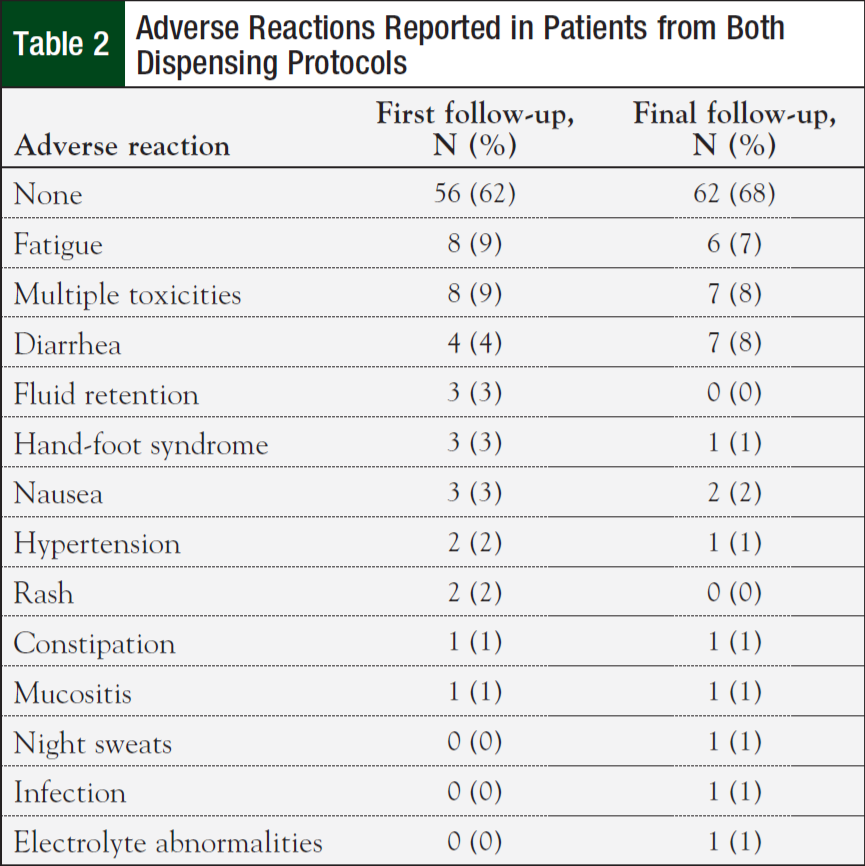
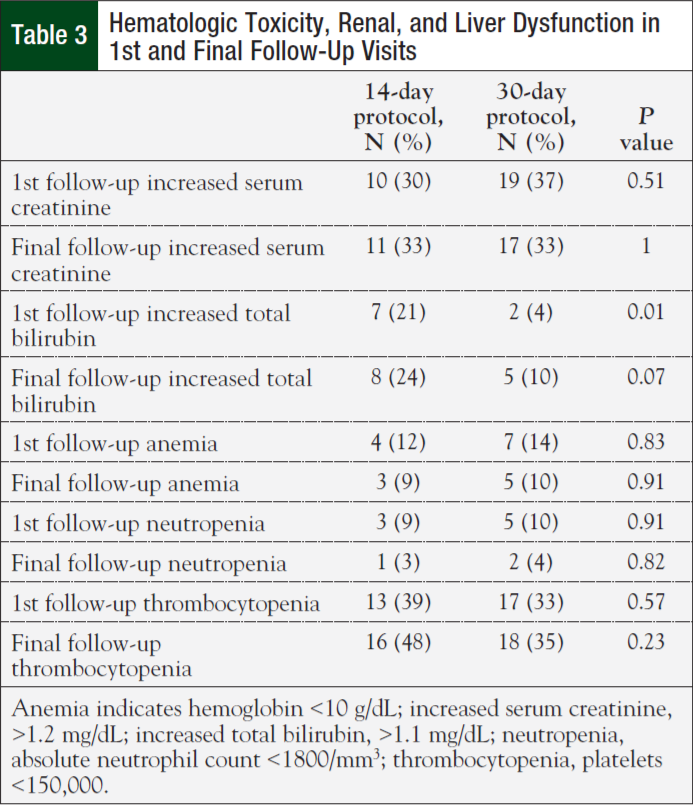
Initial nonhematologic toxicities reported at the first clinic follow-up were lower in patients on the 14-day (42%) versus 30-day protocol (75%; P = .003). However, most patients (62%) reported having no toxicities at their initial follow-up visit. Fatigue was the most commonly reported adverse reaction (Table 2). There were no significant differences in nonhematologic toxicities reported at the final clinic visit—61% for the 14-day protocol versus 75% for the 30-day protocol (P = .17). There was no difference in anemia, neutropenia, thrombocytopenia, renal, and liver dysfunction between the 2 groups (Table 3). Finally, the median cost for treatment under the 14-day protocol was $6479.25 ($4034, $13,718) versus $22,106.43 ($10,390, $29,791) for the 30-day protocol (P <.001).
Discussion
The high adherence rates among patients receiving oral chemotherapy in this study are similar to other studies. Santoleri and colleagues conducted a retrospective study to evaluate adherence of patients treated with imatinib, nilotinib, or dasatinib, and found that patient adherence ranged from 80% to 90%.11 In our study, adherence rates were increased by providing a 2-week supply of medication, and requiring that patients return to the clinic for follow-up to receive more medication. However, no difference in adherence was noted for different oral chemotherapeutic agents, cancer being treated, or for patients living >40 miles from the Veterans Affairs hospital. This study suggests that closer monitoring of patients can improve adherence rates. At the Audie L. Murphy Veterans Health Care Center, patients not only follow up in the clinic 2 weeks after initiating treatment, but are also followed up on a regular basis via telephone by a nurse case manager. These strategies have prove to be effective in increasing adherence and lowering costs.
Patient education is also a key component of the 14- day dispensing protocol. Patients meet with a pharmacist before the medication is dispensed. Administration, drug and food interactions, storage, missed/extra doses, and adverse reactions are discussed extensively with the patient. Patients also receive written education materials on the medication. Patients follow up with the pharmacist or physician in 2 weeks to discuss experienced toxicities and assess adherence. Factors associated with adherence include patients’ understanding of the treatment plan, perceived benefit of treatment, and overall health literacy.6 Education has shown to promote a better understanding of the treatment regimen, and it has established a collaborative relationship between the healthcare provider and patient.
The National Comprehensive Cancer Network (NCCN) also identified several misconceptions surrounding oral chemotherapy and the need for patient education.12 The perception that oral chemotherapy is less toxic than conventional intravenous chemotherapy is a common misconception. It is often up to the patient to be aware of toxicities and to know when to seek attention. The NCCN recommends that oral chemotherapy should only be given to well-motivated and health-literate patients who are able to understand complex regimens. In addition, oral chemotherapy requires significant patient education when starting therapy, and extensive telephone calls thereafter. This requires additional staff to manage toxicities, monitor drug interactions, and assess adherence.12
There were less nonhematologic toxicities reported at the initial follow-up visit by patients with the 14-day versus 30-day protocol. The majority of patients did not report adverse reactions during the initial follow-up on both protocols. This may be because some adverse reactions have a cumulative effect (eg, hand-foot syndrome), which is seen with oral multiple kinase inhibitors.13 Onset of hand-foot syndrome ranges from 24 hours to 10 months after initiating the causative medication. In addition, patients may not have wanted to report adverse reactions because of the perceived benefit from therapy.
Nonadherence can lead not only to toxicity and disease progression, but also to waste of healthcare resources. 14 Patients who are nonadherent to therapy may have disease progression that can prompt more laboratory testing, imaging, and healthcare utilization. This may cause a change in therapy if disease progression is assumed to be from lack of treatment efficacy instead of nonadherence. The 14-day protocol was shown to decrease costs by allowing only a limited supply of medication until tolerability and adherence were established. This method allows the provider to ensure compliance before more medication is prescribed to the patient. The significant difference in cost can be contributed to patients’ treatment being discontinued sooner because of toxicity, adherence issues, and worsening disease states.
The results of this study show that a 14-day oral chemotherapy dispensing protocol is effective in improving adherence and decreasing costs. The key aspect of this protocol is patient education and communication, which are interventions known to improve adherence. According to McCue and colleagues, comprehensive oral chemotherapy education should consist of drug and indication, dose, dosing schedule, administration, missed doses, food and drug interactions, adverse reactions and management, safe handling of medications, and clinic contact information.14 Verbal and written communication is essential for patient education.
There are several limitations to this study. External validity is limited because the study was conducted at a single site. Adherence rates were measured using pharmacy refill records, which do not necessarily correlate with actual consumption. MPRs do not take into account treatment breaks from hospitalization, infection, or patient preference. In addition, although MPRs indicate that patients are filling their medications on time, it cannot measure whether patients are actually taking them. Despite reporting increased adherence rates with the 14-day dispensing protocol, the study was unable to assess whether patients were taking the medication appropriately. Only oral chemotherapy agents included in the Audie L. Murphy Veterans Health Care Center policy were included in the study, which is another limitation. Baseline comorbidities, medication burden, and performance status were not obtained, which may affect adherence rates. The study was only conducted over 1 year, and studies have shown that adherence rates may decrease with chronic therapy.6 The total costs only included drug costs, and do not account for additional staffing and clinic follow-up required for the 14-day dispensing protocol.
Conclusion
Adherence is a significant concern with increased utilization of oral chemotherapy. With rates being highly variable in the literature, and measured using different techniques, it is difficult to establish true adherence to oral chemotherapy. Several methods have been studied to help improve patient compliance, including reminder tools, simplifying medication regimens, patient education, closer follow-up, and managing toxicity.14 The findings of our study confirm that a 14-day oral chemotherapy dispensing protocol improves adherence and decreases costs compared with a 30-day dispensing protocol. Important components of this protocol include patient education through verbal and written communication, and frequent follow-ups. By identifying the effectiveness of a 14-day protocol, it has become another tool, along with patient education, that can be instituted in clinical practices to help prevent nonadherence and decrease costs.
Dr Young is a Clinical Pharmacy Specialist, Oncology, at VA Texas Valley Coastal Bend Health Care System, Harlingen, TX. Dr Zigmond is a Clinical Pharmacy Specialist, Oncology/ Hematology, and Ms Lee is a Statistician at South Texas Veterans Health Care System, San Antonio, TX.
Author Disclosure Statement
The authors reported no conflicts of interest.
Acknowledgments
Dr Koselke, Dr Walker, Ms Allore, and Dr Mackler performed this work during affiliation with the University of Michigan Comprehensive Cancer Center.
References
- Weingart SN, Brown E, Bach PB, et al. NCCN task force report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(suppl 3):S1-S14.
- Halfdanarson TR, Jatoi A. Oral cancer chemotherapy: the critical interplay between patient education and patient safety. Curr Oncol Rep. 2010;12:247-252.
- Neuss MN, Polovich M, McNiff K, et al. 2013 updated American Society of Clinical Oncology/Oncology Nursing Society Chemotherapy Administration Safety Standards including standards for the safe administration and management of oral chemotherapy. J Oncol Pract. 2013;9(suppl 2):5s-13s.
- Chan A, Tan SH, Wong CM, et al. Clinically significant drug-drug interactions between oral anticancer agents and nonanticancer agents: a Delphi survey of oncology pharmacists. Clin Ther. 2009;31(pt 2):2379-2386.
- Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401-5411.
- Tschida SJ, Aslam S, Lal LS, et al. Outcomes of a specialty pharmacy program for oral oncology medications. Am J Pharm Benefits. 2012;4:165-174.
- Red Book Online. Greenwood Village, CO: Truven Health Analytics. www.micro medexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidenc expert/CS/9228A9/ND_AppProduct/evidencexpert/DUPLICATIONSHIELD SYNC/149B89/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/ PFActionId/redbook.FindRedBook?navitem=topRedBook&isToolPage=true. Accessed June 18, 2015.
- Neuss M, Gilmore TR, Kadlubek P. Tools for measuring and improving the quality of oncology care: the Quality Oncology Practice Initiative (QOPI) and the QOPI certification program. Oncology (Williston Park). 2011;25:880, 883, 886-887.
- Weingart SN, Flug J, Brouillard D, et al. Oral chemotherapy safety practices at US cancer centres: questionnaire survey. BMJ. 2007;334(7590):407.
- MacLeod A, Branch A, Cassidy J, et al. A nurse-/pharmacy-led capecitabine clinic for colorectal cancer: results of a prospective audit and retrospective survey of patient experiences. Eur J Oncol Nurs. 2007;11:247-254.
- Wong SF, Bounthavong M, Nguyen C, et al. Implementation and preliminary outcomes of a comprehensive oral chemotherapy management clinic. Am J Health Syst Pharm. 2014;71:960-965.
- Hlubocky JM, Stuckey LJ, Schuman AD, et al. Evaluation of a transplantation specialty pharmacy program. Am J Health Syst Pharm. 2012;69:340-347.
- 13. Flanagan P, Kainth S, Nissen L. Satisfaction survey for a medication management program: satisfaction guaranteed? Can J Hosp Pharm. 2013;66:355-360.
- Moczygemba LR, Barner JC, Brown CM, et al. Patient satisfaction with a pharmacist- provided telephone medication therapy management program. Res Social Adm Pharm. 2010;6:143-154.