Angiogenesis, the development of new blood vessels from preexisting vessels, is considered to be one of the central “hallmarks” in most malignancies.1 A tumor must develop an independent blood supply to have the ability to grow beyond 1 to 2 mm in diameter.2 Vascular endothelial growth factor (VEGF) has been identified as the predominant regulator involved in tumor angiogenesis and has become a target of many new antineoplastic drugs, such as bevacizumab.3 Bevacizumab, a recombinant humanized monoclonal antibody, neutralizes VEGF, thereby preventing VEGF from binding to its receptor and subsequently inhibiting the angiogenic process.4
Bevacizumab has been approved for, and has been shown to be effective in, the treatment of advanced or metastatic colorectal cancer (CRC), advanced or metastatic non–small-cell lung cancer (NSCLC), glioblastoma, and renal-cell carcinoma.5-8 Several unique and severe adverse effects are associated with the use of bevacizumab, including, but not limited to, hypertensive crisis, nephrotic syndrome, gastrointestinal tract perforation, wound dehiscence, hemorrhage, arterial thromboembolic events, neutropenia, infection, and congestive heart failure.4,9 Although venous thromboembolism (VTE) has been documented with the use of bevacizumab, it remains controversial whether the drug itself contributes to increased risk for VTE or if specific patient characteristics, such as acquired or congenital risk factors, increase the patients’ risk for venous thrombosis.10,11
VTE is a major cause of morbidity and mortality in patients with cancer. The etiologies of VTE in cancer include the inherent hypercoagulable state, vessel wall trauma, and vessel stasis.1 The frequency of cancer-associated VTE is increased further by the presence of additional risk factors, such as acquired or congenital thrombophilia, prolonged immobilization, surgical procedures, cancer type and disease burden, chemotherapy regimen and duration, comorbid conditions, and concomitant medications. It has been reported that VTE increases the likelihood of death by 2- to 8-fold in patients with cancer.12
The overall incidence of VTE in patients with metastatic or advanced CRC or NSCLC who receive treatment with bevacizumab varies considerably among phase 2 and 3 randomized controlled trials (RCTs; range, 3%-17.6%).13-16 To date, there have been 2 meta-analyses examining the incidence of VTE in patients treated with chemotherapy plus bevacizumab. However, the conclusions of these 2 studies are conflicting. Nalluri and colleagues determined that patients treated with bevacizumab had a significantly increased risk of VTE, relative risk of 1.33 (95% confidence interval [CI], 1.13-1.56;P ‹.001) compared with the risk in the control groups.10 Alternatively, Hurwitz and colleagues concluded that there was no significant increased risk for VTE in patients receiving bevacizumab versus controls (odds ratio, 1.14; 95% CI, 0.96-1.35; P = .13).11
Because of the conflicting results of these meta-analyses, a wide variation in results in previous RCTs, and limited current literature, it is uncertain whether the addition of bevacizumab to chemotherapy increases patients’ risk for VTE. Furthermore, there are no studies directly evaluating the risk for recurrent VTE in patients with a history of VTE who are treated with bevacizumab. In clinical practice, a history of VTE has typically not precluded the use of bevacizumab in patients with cancer.
The purpose of this study is to evaluate the risk for recurrent VTE in patients receiving chemotherapy plus bevacizumab.
Methods
This retrospective cohort chart review included patient data from January 1, 2002, through September 31, 2011. Patients were eligible for inclusion if they were aged ≥18 years, had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, and had a diagnosis of advanced or metastatic CRC or NSCLC and were receiving first-line combination chemotherapy. Patients with previous VEGF inhibitor treatment were excluded.
Patients were identified with the use of International Classification of Diseases, Ninth Revision (ICD-9) codes for NSCLC (162.00) and CRC (153.00). Demographic information collected included age, sex, body mass index (BMI), and baseline ECOG performance status. Risk factors at baseline were also collected, including VTE history and location; baseline medication history, including hormonal therapy; erythropoietin stimulating agents; or megesterol. In addition, medical history of congestive heart failure, myocardial infarction or cerebrovascular accident, platelets ≥350,000, and major or minor surgery within 30 days or while receiving treatment were recorded.
Surgery was defined as major if the surgery lasted at least 1 hour with anesthesia and/or surgery with extensive tissue injury (ie, abdominal, thoracic, or orthopedic surgery, or reconstructive plastic surgery). Minor surgery was defined as a procedure lasting ‹1 hour.17 In addition, baseline use of anticoagulants or antiplatelets, cancer diagnosis, use of a chemotherapy regimen (initiation date and discontinuation date), dose of bevacizumab (if included in a regimen), total number of chemotherapy cycles received, and incidence and location of VTE while receiving treatment and up to 60 days after discontinuation of treatment were collected. The incidence of VTE was further delineated as inpatient or outpatient; inpatient was defined as a patient being admitted to the hospital for >3 days and outpatient for ≤3 days.
Computerized progress notes, pharmacy records, and imaging reports were reviewed. The study was conducted in compliance with the Institutional Review Board and the research and development committee of the Veterans Affairs North Texas Health Care System and Texas Tech University Health Sciences Center.
The primary objective of the study was to evaluate the incidence of recurrent VTE in patients receiving bevacizumab. In addition, the incidence of recurrent VTE in patients receiving chemotherapy without bevacizumab exposure and primary incidence of VTE in patients receiving bevacizumab were evaluated and compared with the primary objective. Group comparison for categorical variables was performed using chi-square or Fisher exact test. Mann-Whitney U test was used to evaluate continuous variables. A multivariate regression model was used to test the relationship between the incidence of VTE and potential confounding factors. All data were analyzed using the Minitab 15 Statistical Software (State College, PA). Statistical significance was defined as P ≤.05.
Results
A total of 1422 patients were initially identified using ICD-9 codes for CRC and NSCLC; of these, 1213 patients were excluded. Of the 209 patients who met the inclusion criteria, 186 patients were treated with chemotherapy plus bevacizumab. Of those 186 patients, 173 had no history of VTE (Group A) and 13 had a history of VTE (Group B). The third group, considered the control group (Group C), included 23 patients with a history of VTE who were treated with chemotherapy, without bevacizumab. Overall, 60.8% of patients had CRC and 39.2% had NSCLC.
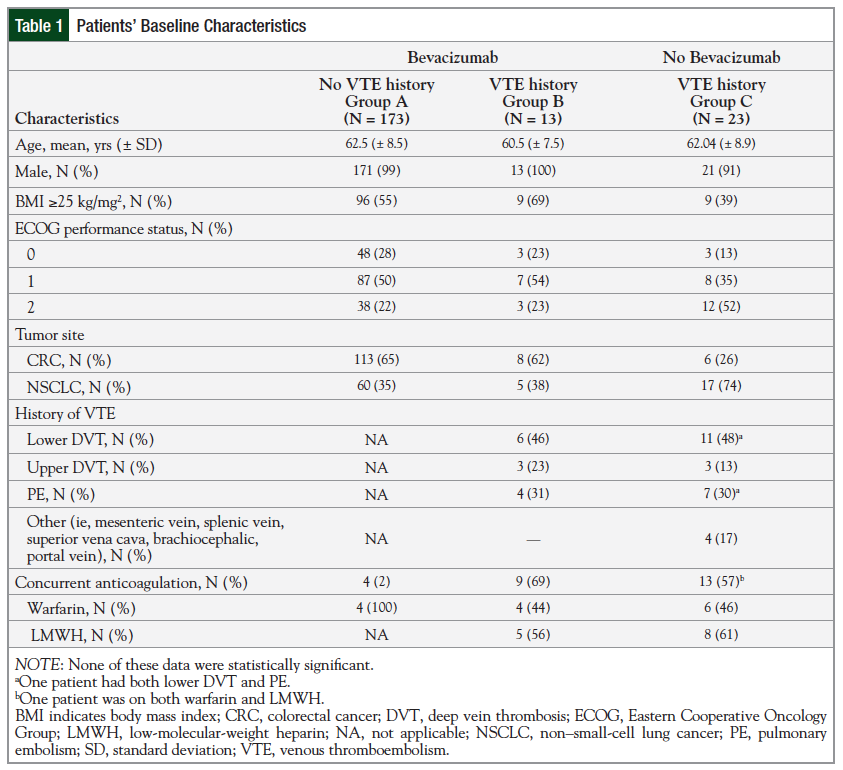
Baseline characteristics are reported in Table 1.
There were no significant differences in baseline characteristics among all 3 groups. The mean age was 62 years, and the majority of the patients were male (98%). The majority of the patients in the bevacizumab-treated groups (Groups A and B) were overweight, with a BMI of ≥25 kg/mg2 compared with those in Group C. The majority of patients in Groups A and B had an ECOG performance status of 1 (50% and 54%, respectively) compared with patients in Group C; 52% of these patients had an ECOG performance status of 2.
In Groups A and B, CRC was more common (65% and 62%, respectively) compared with Group C, in which NSCLC was more common (74%). Considering the 2 groups with a history of VTE (Groups B and C), the most common type of VTE was lower extremity deep vein thrombosis (DVT). These patients were most often treated with a low-molecular-weight heparin (LMWH) and 1 patient in Group C was treated with LMWH and with warfarin at baseline.
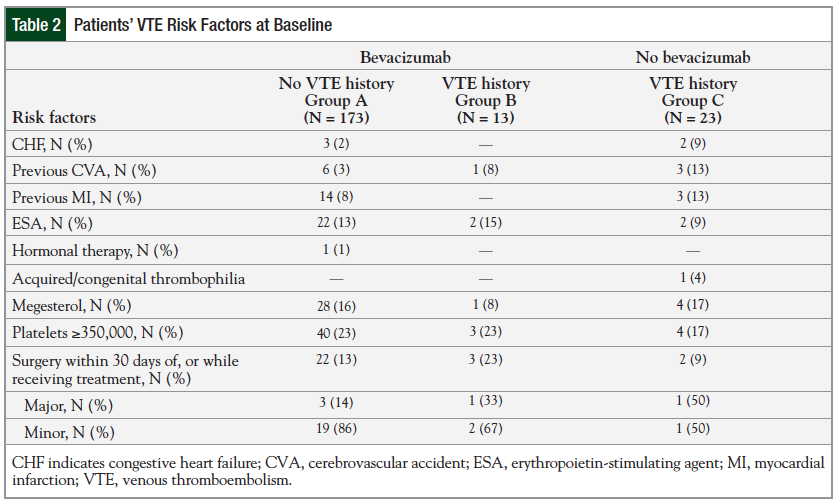
Table 2 reports the VTE risk factors at baseline among the study groups. Very few patients in any of the groups had surgery 30 days before or while receiving treatment.
A total of 22 patients had minor surgeries, including mediport placement (19), cystoscopy (2), and incision and drainage (1); 5 patients had major surgeries, including exploratory laparotomy (2), hemicolectomy (2), and lower abdominal resection (1). Several patients had additional VTE risk factors at baseline; however, a multivariate analysis revealed no significant difference between the bevacizumab-treated and the control group on the effect of these risk factors.
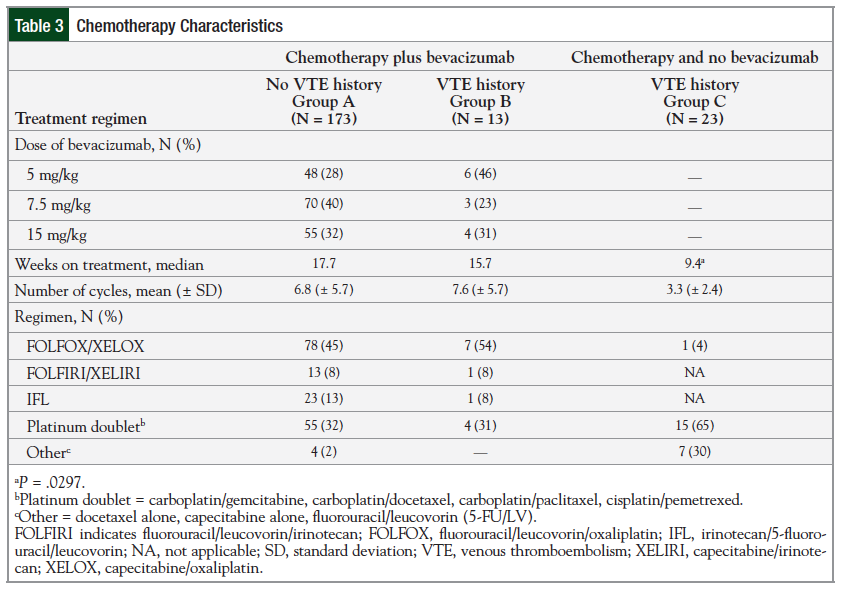
Considering the chemotherapy characteristics, specifically in the bevacizumab-receiving groups, Group A most often (40.5%) received the 7.5-mg/kg dose compared with Group B, in which the most common (46%) dose was the 5-mg/kg dose. Additional chemotherapy characteristics are listed in Table 3.
The patients in Group C received the shortest duration of treatment and subsequently the fewest number of chemotherapy cycles compared with the bevacizumab-treated groups. This difference was significant when comparing the median weeks of treatment between patients in Group B and patients in Group C—15.7 weeks versus 9.4 weeks, respectively (P = .0297).
The most common combination chemotherapy regimen used in all 3 groups was the platinum-based regimens (ie, FOLFOX [fluorouracil, leucovorin, and oxaliplatin], XELOX [capecitabine and oxaliplatin], and platinum doublets).
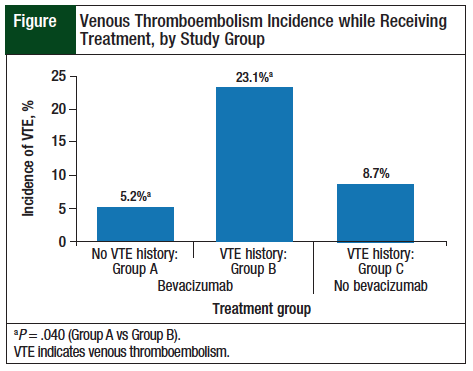
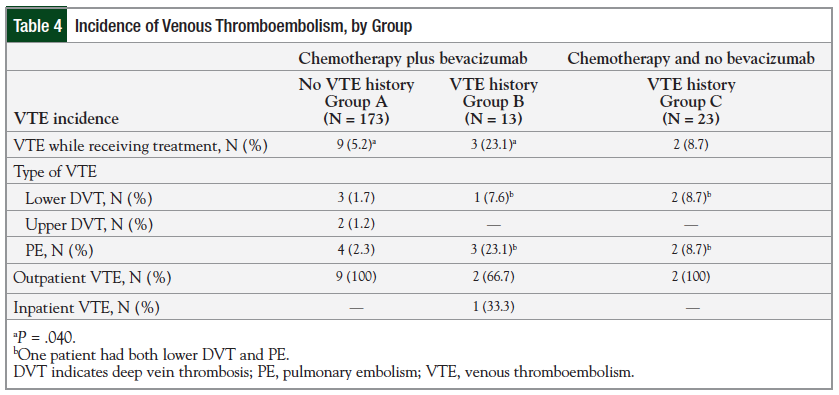
Among the patients treated with bevacizumab, the incidence of VTE was 5.2% (9 of 173 patients) for Group A versus 23.1% (3 of 13) for Group B (P = .040). The incidence was still higher in Group B compared with patients in Group C—23.1% versus 8.7% (2 of 23), respectively; however, this was not significant (P = .328; Figure).
The most common on-treatment VTE manifestation among Groups A and B was pulmonary embolism (PE); in Group C, an equal number of patients had lower DVT and PE. One patient in Group B and 1 in Group C had both a PE and lower DVT. All the on-treatment VTE cases were in the outpatient setting, with the exception of 1 patient in Group B who was classified as inpatient. The incidence of VTE is summarized in Table 4.
Discussion
In patients with cancer, VTE can diminish quality of life and can even result in death. VTE has been reported in patients receiving bevacizumab. We used 2 different comparator groups to better understand the clinical impact of bevacizumab therapy on the incidence of recurrent VTE. The first group (Group A) included patients without a history of VTE who received bevaciz- umab. This group provided a baseline for the incidence of VTE in patients receiving bevacizumab. The comparator group (Group C) was comprised of patients with a history of VTE who received chemotherapy but not bevacizumab. This group provided the baseline risk for recurrent VTE in patients receiving chemotherapy only.
This study used a cutoff period of 60 days to consider at least 3 half-lives of bevacizumab to account for any residual effects of the drug. Our study suggests that the risk for VTE was significantly higher in patients with a history of VTE compared with patients without a history of VTE who were receiving beva-cizumab. Furthermore, the risk for VTE remains high, although not significantly, even when compared with the patients with a history of VTE who were receiving chemotherapy without bevacizumab.
Overall, 69% of patients were receiving active anticoagulation therapy while taking bevacizumab. Our study suggests that there is a consistent increased risk for recurrent VTE in patients receiving bevacizumab. All 3 patients who had recurrent VTE while receiving bevacizumab were also taking therapeutically targeted anticoagulation agents.
We did not detect any significant differences between the group demographics at baseline. However, variations in ECOG performance status and types of cancers were observed between Groups B and C. The majority of the patients in Groups A and B had a performance status ‹1, whereas the majority of patients in Group C had performance status 2. In addition, 62% of the patients in Groups A and B had CRC compared with 26% in Group C. Based on these differences of ECOG performance status and cancer type distributions at baseline between the 2 groups (ie, bevacizumab-treated vs no bevacizu-mab), patients in Group C should have had a higher incidence of VTE. According to the VTE predictive model, patients with lung cancer are at 50% increased risk of having VTE compared with those with CRC.18
No significant difference was noted among the 3 groups regarding the VTE risk factors at baseline (Table 2). There was a higher percentage (23%) of patients with recent surgery in Group B, but the majority of the surgeries were minor. In addition, the surgeries reported occurred within 30 days of chemotherapy or chemotherapy plus bevacizumab administration and did not occur before the incidence of VTE. Patients in Group B had significantly longer duration of cancer treatment (15.7 vs 9.4 weeks; P = .0297) compared with Group C. This difference could be explained by the cancer type distribution and chemotherapy regimen administered in these 2 groups. The majority of patients in Group C had NSCLC and received platinum-based chemotherapy. A typical patient with NSCLC might have received 4 cycles of platinum-based chemotherapy and then might have been followed for observation. By contrast, a typical patient with CRC might have received 5-fluorouracil–based chemotherapy until the progression of cancer.
The increase in incidence of VTE in patients treated with bevacizumab may be attributed to the anti-VEGF mechanism of bevacizumab. Our study revealed a 5.2% incidence of primary VTE in patients with CRC or NSCLC who were treated with bevacizumab (Group A), which is consistent with what is reported in previous studies (incidence range, 3%-17.6%).13-16
The risk for VTE was significantly higher when bevacizumab was administered to patients with a history of VTE (23.1%) in our study compared with those without a history of VTE (5.2%). In addition, the risk for recurrent VTE was higher in patients receiving chemotherapy plus bevacizumab compared with chemotherapy alone (23.1% vs 8.7%; P = .328).
These results are consistent with the results of a 4-arm prospective RCT by Saltz and colleagues that included 1401 patients with metastatic CRC, in which the incidence of primary VTE was 13.5% in the bevacizumab arms.4,5 Among the 116 patients treated with anticoagulation after an initial VTE (73 in the bevacizumab plus chemotherapy arms and 43 in the chemotherapy-alone arms), the bevacizumab arms had higher incidence of recurrent VTE compared with the control arms (31.5% vs 25.6%, respectively).4
Another pivotal first-line trial in patients with metastatic CRC (AVF2107g) evaluated the incidence of recurrent VTE in patients receiving bolus IFL (irinotecan, 5-fluoroucil, and leucovorin) with or without bevacizumab.4,19 In this trial, patients with an incidence of primary VTE received full-dose warfarin therapy. The recurrent VTE occurred in 21% (11 of 53 patients) of patients receiving bolus IFL plus bevacizumab compared with 3% (1 of 30 patients) of patients receiving bolus IFL alone.4,9 The key difference between these 2 trials and this present study is that these 2 trials did not evaluate the incidence of recurrent VTE in patients without previous exposure to bevacizumab.
Limitations
This study has limitations that should be considered when interpreting the results. First, the results of this study are primarily applicable to male patients with cancer, because 98% of the patients were males. Another limitation was the relatively small sample size, which might have contributed to the nonstatistical differences between the groups at baseline, and for the VTE outcomes.
Conclusion
Regardless of the study limitations, this study evaluates an important question, which to our knowledge has not been investigated, that is, the risk of recurrent VTE in patients receiving bevacizumab.
The results of this study suggest that patients with CRC or NSCLC who are receiving bevcizumab are at increased risk for recurrent VTE. This risk may be higher compared with patients receiving chemotherapy without bevacizumab. There are many clinical questions that cannot be answered by the available literature, such as the optimal timing of bevacizumab initiation after a VTE, whether the risk of VTE can be mitigated by appropriate anticoagulation, or if there is a difference in risk of recurrent VTE if patients have a provoked versus an unprovoked VTE. These are questions that still need to be answered to make concrete recommendations in the use of bevacizumab in the setting of a previous VTE. In practice, careful evaluation of risk versus benefit with the use of bevacizumab-based therapy should be considered in the setting of limited literature evaluating the risk of recurrent VTE.
Author Disclosure Statement
Dr Gressett Ussery is an employee of Celgene Corporation; Dr Dowell has received research support from Verastem and MedImmune; Dr Welch, Dr Kelly, and Dr Shah reported no conflicts of interest.
References
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70.
- O’Byrne KJ, Steward WP. Tumour angiogenesis: a novel therapeutic target in patients with malignant disease. Expert Opin Emerg Drugs. 2001;6:155-174.
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027.
- Avastin (bevacizumab) [prescribing information]. South San Francisco, CA: Genentech, Inc; August 2014.
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. Erratum in: J Clin Oncol. 2008;26:3110; and J Clin Oncol. 2009;27:653.
- Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2013;31:3926-3934.
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733-4740.
- Rini BI, Bellmunt J, Clancy J, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J Clin Oncol. 2014;32:752-759.
- Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69(suppl 3):25-33.
- Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277-2285.
- Hurwitz HI, Saltz LB, Van Cutsem E, et al. Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol. 2011;29:1757-1764.
- Heit JA. Cancer and venous thromboembolism: scope of the problem. Cancer Control. 2005;12(suppl 1):5-10.
- Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697-3705.
- Allegra CJ, Yothers G, O’Connell MJ, et al. Initial safety report of NSABP C-08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27:3385-3390.
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer: AVAiL. J Clin Oncol. 2009;27:1227-1234. Erratum in: J Clin Oncol. 2009;27:2415.
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non–small-cell lung cancer. J Clin Oncol. 2004;22:2184-2191.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; for the American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e326S-e350S. Erratum in: Chest. 2012;141:1129.
- Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335-2342.