Neuroendocrine tumors (NETs) represent a spectrum of rare neoplasms derived from cells of the nervous and endocrine systems. Although these malignancies may arise from nearly any anatomic site, they most commonly are diagnosed in organs of the gastrointestinal tract and can be subdivided broadly according to their ability to produce and secrete hormonal products at supraphysiologic levels.1 The status of a NET as functional or nonfunctional is solely dependent on clinical manifestations resulting from excessive concentrations of peptide hormones, neuropeptides, and neurotransmitters leading to the characteristic carcinoid syndrome and is not determined by pathologic profiling.2 Carcinoid syndrome—defined as flushing, diarrhea, and abdominal pain—can be disabling in some patients and commonly occurs in approximately half of all diagnosed patients, especially those with advanced disease that includes liver metastases.3-5 The specific patterns of hormone production, degree of aggressiveness, and response to therapy result in a heterogeneous class of rare tumors that make clinical trial design and standardization of therapy difficult.
In 2000, the World Health Organization published guidelines to clarify the histopathologic categorization of NETs. This system divides these malignancies into three classifications: well-differentiated NETs, well-differentiated carcinomas, and poorly differentiated carcinomas.6 Well-differentiated NETs, including carcinoid tumors, are relatively indolent and may be benign, whereas welldifferentiated carcinomas imply malignant behavior with the possibility of metastasis. Poorly differentiated or high-grade carcinomas, including small-cell carcinomas, may be aggressive in pattern of growth and typically confer a poor prognosis.6-8
According to an analysis of the Surveillance, Epidemiology, and End Results database, NETs have an age-adjusted incidence of 5.25 cases per 100,000 people per year, representing approximately 1% to 2% of all malignant tumors.9 Although the rate of diagnosis of NETs has appeared to increase over the past 30 to 40 years (2.5% of all gastric neoplasms in the 1970s; 6% in 1999), improvements in the clinical management of these patients has lagged.10
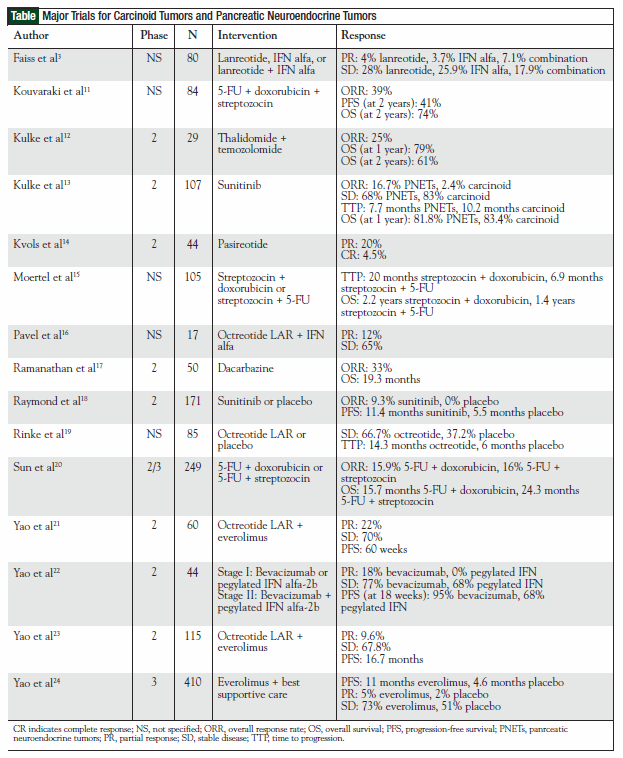
This review summarizes clinical trials (Table) that have established the contemporary management of patients with carcinoid tumors and pancreatic NETs (PNETs) highlighting promising treatments that likely will affect patient care in the foreseeable future.
Surgery
Surgery remains the preferred treatment modality and only curative option for patients with localized NETs. For patients with metastatic disease, consideration should be given to removal of the primary tumor, regional lymph nodes, and any isolated liver lesions.25 Debulking has been described to result in increased overall survival in a small subset of patients.26 Surgical management for patients with localized and metastatic carcinoid tumors and PNETs has been described extensively in the literature and, although beyond the scope of this review, should be the first consideration for any patient with a new diagnosis of NET.25,27-29
Somatostatin Analogs
Somatostatin is an endogenous peptide secreted by neuroendocrine cells found throughout the central and peripheral nervous systems. It produces negative regulatory effects on digestive secretions and the release of hormones including serotonin, somatostatin, and vasoactive intestinal peptide, among others throughout the gastrointestinal system via exocrine, endocrine, paracrine, and autocrine activity. This results in symptomatic control of the hormonally mediated syndromes frequently observed in patients with metastatic carcinoid tumors and PNETs.30-32 Therapeutic interest in somatostatin lies in the knowledge that, to varying degrees, all NETs express the five somatostatin receptor subtypes (SSTR1 to SSTR5), each capable of regulating various biological effects via signal transduction pathways.33 Unfortunately, the therapeutic use of native human somatostatin is limited severely by a half-life of less than 3 minutes.34 For this reason, somatostatin analogs, such as octreotide and lanreotide, were first developed in the early 1980s as short-acting formulations, requiring multiple daily administrations. More recently, sustained-release formulations have been introduced extending the dosing interval to every 1 month or 3 months. These somatostatin analogs largely are believed to exert their biological activity via binding to SSTR2, with lower affinity to SSTR3 and SSTR5.33 More specifically, apoptotic effects of somatostatin analogs are mediated via SSTR2 and antiangiogenic effects via SSTR2 and SSTR3.35
Although somatostatin analogs have long been used in the management of carcinoid syndrome, as well as other hormonally mediated syndromes observed in patients with metastatic gastroenteropancreatic (GEP) NETs, until recently these compounds rarely had been documented to stabilize the growth of NETs or to result in the regression of these tumors because of their slowgrowing nature.
The publication of the Placebo Controlled, Double- Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine MiDgut Tumors (PROMID) represents a new era for the use of somatostatin analogs in the management of patients with carcinoid tumors.19 This trial conducted in Germany enrolled 85 treatment-naïve patients with a locally inoperable or metastatic NET of midgut origin who were assigned to treatment with octreotide LAR 30 mg intramuscularly (IM) monthly or to placebo. Patients were evaluated by computed tomography/magnetic resonance imaging until disease progression was documented. This study demonstrated a 67% reduction in risk of disease progression favoring octreotide LAR (26 progressions in the octreotide LAR group; 41 in the placebo group; P = .000015). In addition, median time to progression was superior for octreotide LAR compared with placebo (14.3 vs 6.0 months). Subgroup analyses demonstrated that the antiproliferative effects of octreotide LAR were most beneficial in newly diagnosed gastrointestinal NET patients with low hepatic tumor volume and resected primary tumors.19 Furthermore, octreotide LAR was well-tolerated, with diarrhea and flatulence reported as the most common adverse events. For practitioners, PROMID represents the first placebo-controlled trial to demonstrate inhibition of tumor growth with a somatostatin analog in this patient population.
After publication of the PROMID study results, the National Comprehensive Cancer Network (NCCN) clinical practice guidelines for gastrointestinal NETs were updated to reflect the recommendation of octreotide therapy, 150 μg to 250 μg subcutaneously (SC) three times daily or 20 mg to 30 mg IM every 4 weeks, in patients with no symptoms of carcinoid syndrome and with unresectable tumors as an alternative to clinical trial or repeated imaging every 3 to 6 months until disease progression or symptomatic presentation.36
Other somatostatin analogs also have elicited interest in the management of carcinoid tumors. Faiss and colleagues conducted a randomized, open-label trial of lanreotide (1 mg SC three times daily) versus interferon (IFN) alfa (5 X 106 units SC three times weekly) alone and in combination in 80 treatment-naïve patients with documented metastatic GEP NETs.3 After 12 months of follow-up, tumor progression had occurred in 14 of 25 patients receiving lanreotide, 15 of 27 patients receiving IFN, and 14 of 28 patients treated with the combination. The authors concluded that, although the response rate was lower than previously reported in nonrandomized studies, lanreotide was comparable in efficacy to IFN alfa, which was considered the standard of care at the time of study design. Although these results failed to establish lanreotide as first-line therapy in this patient population, it remains a somatostatin analog of interest. An ongoing phase 3 clinical trial (ClinicalTrials.gov Identification No. NCT00353496) is evaluating the activity of a new autogel formulation of lanreotide versus placebo in patients with pancreatic or intestinal NETs.
Pasireotide is a novel somatostatin analog that binds to SSTR1, SSTR2, SSTR3, and SSTR5 better than octreotide. It has shown efficacy in controlling symptoms of carcinoid syndrome in a phase 2 clinical trial. Patients with pathologically confirmed metastatic carcinoid tumor with symptoms (flushing and diarrhea) refractory to management with octreotide LAR were treated with pasireotide titrated to a maximum daily dose of 1200 μg SC twice daily. Partial responses (PRs) were reported in nine of 44 patients (20%) and a complete response was observed in two patients (5%) with regard to symptom control.14 To date, pasireotide has not been evaluated for antiproliferative activity in patients with carcinoid tumors.
Other strategies have evaluated the use of radiolabeled somatostatin analogs in the management of patients with carcinoid tumors and PNETs. Somatostatin scintigraphy has been used with clinical success since the early 1990s to localize previously undetected or metastatic lesions, and it remains an NCCN recommendation for visualization of these tumors.37 Because many NETs highly express somatostatin receptors, radiolabeled compounds intended for scintigraphy have been evaluated for therapeutic efficacy. The most promising of these compounds, 90Y-DOTA0,Tyr3 and 177Lu-DOTA0,Tyr3, have demonstrated overall response rates in clinical trials ranging from 24% to 33% for patients with GEP NETs.38 Adverse events included hematologic toxicity, nausea/vomiting, abdominal discomfort, and, less commonly, renal insufficiency.
Interferon Alfa
IFN-based therapy has been an option in the management of carcinoid tumors since the publication of a pivotal study by Oberg and colleagues in 1983.39 This early research evaluated 3 million international units of leukocyte IFN daily for 1 month followed by 6 million international units daily for an additional 2 months in patients with midgut carcinoid tumors and carcinoid syndrome. Although this was a small study enrolling only nine patients, control of symptoms and reduction in urinary 5-hydroxyindoleacetic acid levels was achieved in six patients; however, no effect on tumor growth was reported. This trial stimulated an increase in research and development seeking a better understanding of the mechanism of action of IFNs and their role in NETs. It is now believed that IFNs have not only direct antiproliferative effects on malignant cells but also function within the cell cycle resulting in G1 and G0 arrest, affect cellular differentiation, and inhibit angiogenesis via interactions with tyrosine kinase 2 and Janus kinase 1, among other molecular targets.7,40,41
Having demonstrated efficacy with IFN in patients with metastatic GEP NETs in the German trial conducted by Faiss and colleagues alone and in combination with the somatostatin analog lanreotide as described previously, subsequent efforts have focused on improving the tolerability and clinical delivery of IFN-based therapy. These adverse events, which include fatigue, a flulike condition with constitutional symptoms (ie, fever, chills, malaise), myelosuppression, and elevations in hepatic enzymes, require diligent monitoring.3 In addition, patient adherence and discontinuation of IFN therapy are major challenges in ensuring sustained treatment of patients with carcinoid tumors.16
Drawing on experiences in the management of patients with chronic hepatitis C, where improvement in tolerability was noted, Pavel and colleagues evaluated pegylated IFN alfa in patients with GEP NETs. Patients progressing on octreotide LAR therapy were converted to pegylated IFN alfa-2b 50 μg to 100 μg weekly on the basis of body weight, laboratory analysis (including hepatic function and leukocyte count), and subjective reports of symptoms. Stable disease was noted in 13 of 17 patients, and symptomatic improvement was observed in seven of 10 patients. Tolerability of therapy was reported in 15 of 17 patients, with fatigue and weakness as the most common adverse events.16 Because more significant strides in tumor response and symptom management have been reported with other NET treatments, the most recent iteration of the NCCN clinical practice guidelines for NETs recommend use of IFN-based therapy only in patients with metastatic disease in whom no other treatment modalities are considered feasible.36
Mammalian Target of Rapamycin Inhibitors
The mammalian target of rapamycin (mTOR) is an intracellular serine/threonine kinase responsible for the regulation of cellular growth, cell signaling, and metabolism. Cell proliferation mediated by mTOR has been described via the expression of the insulin-like growth factor (IGF)-1 and the IGF-1 receptor by low- to intermediate- grade NETs.42 Thus, inhibition of this pathway was speculated to be a viable mechanism for tumor control in patients with NETs and has resulted in suppression of growth of these malignancies.43
Everolimus is an oral rapamycin derivative originally developed in the 1990s as an immunosuppressant for patients having undergone transplantation. It subsequently has been approved for patients with renal cell carcinoma and is used in the unlabeled setting for patients with PNETs. The first clinical trial evaluating the efficacy of everolimus alone or in combination with octreotide LAR was conducted by Yao and colleagues at The M. D. Anderson Cancer Center. This open-label phase 2 trial treated patients with low-grade NETs (30 carcinoid; 30 islet cell) with octreotide LAR 30 mg every 28 days and everolimus 5 mg or 10 mg daily.21 The overall response rates, 17% for carcinoid and 27% for islet cell patients, and median progression-free survival (PFS), 63 weeks and 50 weeks, respectively, demonstrate efficacy for the combination of octreotide and everolimus.21 Although superior PFS was noted for the cohort of patients receiving 10 mg of everolimus, more grade 3/4 toxicities were reported in this group, requiring dose reductions including aphthous ulcers (9%), leukopenia (6%), thrombocytopenia (6%), hypophosphatemia (16%), and pneumonitis (3%).21
A confirmatory open-label phase 2 trial sought to further assess the efficacy of everolimus in patients with metastatic PNETs following failure of cytotoxic chemotherapy. Patients were randomized to receive everolimus 10 mg daily (n = 115) or everolimus 10 mg daily in combination with octreotide LAR ≤30 mg monthly (n = 45). PR or disease stabilization occurred more frequently in patients receiving combination therapy. This was confirmed further by an extended PFS of 16.7 months in the combination arm compared with 9.7 months in the single-agent everolimus group.23
Adverse toxicities in both studies involving patients with NETs have been similar to those reported previously with everolimus. These include stomatitis, rash, diarrhea, fatigue, nausea, headache, and abdominal pain.21,23 Grade 1/2 pneumonitis was observed in 6% of patients receiving everolimus and 13% of patients receiving everolimus and octreotide. All cases of pneumonitis were considered reversible and were treated by treatment interruption and subsequent dose reduction.
Another phase 3 study of everolimus in patients with advanced low- to intermediate-grade PNETs was recently completed. The RADIANT-3 double-blind trial randomized patients to receive everolimus 10 mg daily (n = 207) or placebo (n = 203). The primary end point, PFS, was extended from 4.6 months to 11 months (P <.0001) in patients treated with everolimus.24,44 Although overall the response rate was low (5% PR for patients receiving everolimus, 2% PR for placebo), 73% of patients treated with everolimus achieved stable disease compared with 51% of patients receiving placebo.24 At the time of publication, median overall survival had not been met. However, 73% of patients initially randomized to the placebo arm have crossed over to receive everolimus, thus potentially complicating this analysis. Significant grade 3/4 adverse events in patients treated with everolimus included stomatitis (7%), anemia (6%), hyperglycemia (5%), and thrombocytopenia (4%).
Currently, NCCN clinical practice guidelines consider everolimus a category 2B recommendation for patients with advanced PNETs.36 To date, the use of everolimus often has been limited to patients with advanced PNETs, frequently after failure of systemic chemotherapy. With the completion of the RADIANT-3 trial, everolimus may become the standard of care in patients with advanced PNETs, pending approval of this indication by the US Food and Drug Administration.
Vascular Targeted Therapies
NETs have been reported to be highly vascularized malignancies with common overexpression of vascular endothelial growth factor (VEGF), and VEGF receptors that may be linked to tumor growth and progression.45 For this reason, interest has focused on the potential use of angiogenesis inhibitors, such as sunitinib and bevacizumab, in the clinical management of these diseases.
Sunitinib
Sunitinib is an oral multitargeted tyrosine kinase inhibitor with activity against VEGF receptors type 1 and 2, platelet-derived growth factor receptors alpha and beta, stem-cell factor receptor, FMS-like tyrosine kinase-3, glial cell line–derived neurotrophic factor, and c-kit.46,47
A phase 2 study of sunitinib 50 mg daily for the first 4 weeks of a 6-week cycle (2 weeks off treatment) in 109 patients (41 carcinoid; 66 PNET) demonstrated a 16.7% objective response rate in patients with PNETs and 2.4% objective response rate in patients with carcinoid tumors. In addition, stable disease was observed in 68% of patients with PNETs and in 83% of patients with carcinoid tumors.13 Median time to progression was 7.7 months in PNET patients and 10.2 months in carcinoid patients. The most common grade 3 and 4 toxicities attributed to sunitinib were hematologic (primarily neutropenia), fatigue, hypertension, nausea/vomiting, and diarrhea. The clinical efficacy demonstrated by sunitinib in this study was evaluated further in a confirmatory phase 3 trial.
The phase 3 comparison of sunitinib 37.5 mg daily versus placebo was conducted in 171 patients with advanced PNETs and was stopped by the independent data monitoring committee at the interim analysis after preliminary results indicated significant differences in efficacy as well as serious adverse events, including death, favoring patients randomized to sunitinib.18,48 Those patients receiving sunitinib achieved a median PFS of 11.4 months versus 5.5 months for those patients receiving placebo (P <.001). Two patients treated with sunitinib achieved a complete response and six additional patients had a PR (objective response rate: 9.3% sunitinib, 0% placebo; P = .007). A greater number of significant adverse events (grade 3 and 4) were experienced by patients treated with sunitinib, including neutropenia (12%), hypertension (10%), and hand-foot syndrome (6%).18,48 In addition, there were nine deaths in the sunitinib arm (1 study-related death, cardiac failure) and 21 deaths in the placebo arm (1 study-related, dehydration). However, because patients experiencing disease progression on placebo could cross over to sunitinib, median survival could not be calculated for either treatment arm.
Overall, sunitinib has shown reasonable efficacy in the management of patients with advanced PNETs in the setting of clinical trials. It has been well-tolerated and, although grade 3/4 toxicities including hand-foot syndrome have not been significant in the clinical trials (1.9% to 6%), practitioner experience with sunitinib in other malignancies may be limiting its use in this patient population at this time.13,18,48
Bevacizumab
Bevacizumab is a humanized anti-VEGF antibody that has been studied extensively in virtually all solid malignancies. 49 A phase 2 study enrolling 44 patients with metastatic carcinoid tumors and currently managed with stable doses of octreotide LAR compared bevacizumab 15 mg/kg intravenously (IV) every 3 weeks to pegylated IFN alfa-2b (in combination with the prestudy dose of octreotide LAR) for 18 weeks or until disease progression. 22 After this prespecified time point, patients were further treated with both bevacizumab and pegylated IFN until disease progression. Overall response rates (18% PR, 77% stable disease vs 0% PR, 68% stable disease) favored bevacizumab, and median PFS after 18 weeks of therapy was lengthened for patients treated with bevacizumab (95% vs 68%).22 Hypertension and fatigue were the most commonly reported grade 3/4 adverse events in patients receiving bevacizumab, whereas granulocytopenia and fatigue were reported in those patients treated with pegylated IFN.22
Ongoing phase 2/3 clinical trials are evaluating the role of bevacizumab in the management of patients with NETs in combination with octreotide and pegylated IFN (ClinicalTrials.gov Identification No. NCT00569127), temsirolimus (ClinicalTrials.gov Identification No. NCT01010126), pertuzumab (ClinicalTrials.gov Identifi - cation No. NCT01121939), and everolimus and octreotide (ClinicalTrials.gov Identification No. NCT01229943). However, at this time, available data do not support the addition of bevacizumab to any treatment regimen for patients with NETs.
Cytotoxic Chemotherapy
Although NETs are frequently considered to be relatively resistant to chemotherapy, cytotoxic drug combinations have long been regarded as a cornerstone in the management of patients with these types of tumors.50 One of the most commonly used regimens, combining 5- fluorouracil (5-FU) with streptozocin, resulted in a measurable increase in objective response rate (69% vs 45%; P = .05) and median overall survival (2.2 years vs 1.4 years; P = .004) when compared with the combination of streptozocin and the alkylating agent chlorozotocin in patients with PNETs.15 The impressive results obtained from this Eastern Cooperative Oncology Group (ECOG) study created hope that PNETs might be more chemotherapy-sensitive than previously believed. However, other trials attempting to verify the response rates and survival benefit of this chemotherapy combination have failed to validate these findings.51
A more contemporary phase 2/3 study conducted by ECOG did demonstrate a measurable increase in overall survival (24.3 vs 15.7 months; P = .0267) in 249 patients with metastatic carcinoid tumors treated with streptozocin and 5-FU in comparison with those receiving doxorubicin and 5-FU. This benefit is consistent with what had been previously reported. It is important to note that there were no differences between the regimens with regard to response rate (15.9% vs 16%) or PFS (4.5 vs 5.3 months).20 These observations led the authors to speculate that surrogate markers for tumor response, such as PFS and response rate, may be inadequate for the determination of activity in some neuroendocrine tumors, including those carcinoid tumor patients enrolled in this study.20 In addition, severe or life-threatening adverse events were reported in 70% of patients treated with streptozocin and 5-FU (hematologic toxicities, 20%; vomiting, 17%; diarrhea, 7%; renal failure, 2%) and 63% of patients receiving doxorubicin and 5- FU (hematologic toxicities, 24%; skin, 6%; mucosal toxicity, 6%).
With existing data in conflict regarding the potential benefit to patients with PNETs treated with doublet combinations of streptozocin, doxorubicin, and 5-FU, an additional trial combined these three drugs in patients with locally advanced and metastatic pancreatic endocrine carcinomas. The median response rate in the 84 patients treated was 39%. PFS at 2 years was 41% and overall survival at 2 years was 74%.11 Patients reasonably tolerated this regimen, with 22.6% experiencing grade 3/4 toxicities (leukopenia/neutropenia, 10.7%; mucositis, 4.7%; fatigue, 4.7%).
A newer strategy of applying cytotoxic chemotherapy in the management of patients with metastatic NETs has used dacarbazine and temozolomide alone and in combination with other chemotherapy drugs. The ECOG E6282 study evaluating single-agent dacarbazine 850 mg/m2 IV every 28 days in 50 patients with unresectable PNETs reported a response rate of 33% of patients and a median survival rate of 19.3 months.17 These results demonstrated the potential efficacy of single-agent dacarbazine therapy for patients with advanced NETs.
Temozolomide, an oral alkylating agent and alternative to dacarbazine, was evaluated in combination with thalidomide in 29 patients with metastatic NETs in a phase 2 study. Thalidomide was selected specifically for treatment in this setting because of its presumed antiangiogenic activity via inhibition of the VEGF and basic fibroblast growth factor pathways.12,52 This oral combination regimen (temozolomide 150 mg/m2 for 7 days every other week; thalidomide 50-400 mg daily) resulted in a 25% overall response rate among all patients. When evaluated separately, however, the response rate was 45% among patients with PNETs compared with 7% among patients with carcinoid tumors. Grade 3/4 toxicities occurred with less frequency than reported for other chemotherapy options used to treat NETs and included lymphopenia, diarrhea, and infection.12 Again, these studies appear to favor the chemotherapeutic sensitivity of PNETs as compared with carcinoid tumors.
In addition to systemic treatment options, localized strategies should be considered for patients with liveronly metastasis. These therapies include transarterial chemoembolization (TACE) and radiofrequency ablation (RFA), both of which are intended to gain locoregional control and improve patient outcomes. The goal of TACE is to disrupt the hepatic blood supply to cause tumor ischemia and necrosis, thus controlling growth. TACE procedures provide focused treatment using particulate embolization, often with polyvinyl alcohol particles, with or without the administration of local chemotherapy. If chemotherapy is used, many protocols combine cisplatin, doxorubicin, and mitomycin C and deliver this cytotoxic therapy via catheterization of the hepatic artery.53
These procedures are not without risk with patients subject to carcinoid crisis, liver abscess, biliary obstruction, hepatic artery dissection, and renal failure.53 However, for those patients who gain benefit, median PFS, in one case series, was 10 months with 33 months overall survival.54 In contrast, RFA administers high-frequency alternating electrical current to ablate or destroy small-volume metastases.
This technique primarily is limited to patients with tumors smaller than 3 cm.55 Median survival in one study was 29 months with 94% of patients experiencing symptom improvement.55
Many cytotoxic therapies have been investigated as potential options in the management of NETs but have resulted in low response rates, poor overall survival, or unacceptable levels of toxicity. Because of these less than desirable outcomes, cytotoxic chemotherapy rarely should be considered as first-line management in treatment- naïve patients diagnosed with NETs. Rather, chemotherapy should be reserved for those patients who have failed previous therapies and in whom other treatment modalities are not feasible. Locoregional management, however, should be considered for any patient with unresectable liver metastases and otherwise overall good performance status and life expectancy.
Conclusions
Although surgical resection of primary tumors and isolated metastases remains the mainstay of treatment for patients with carcinoid tumors and PNETs, advances in biological and targeted therapies have increased interest in these rare tumors and has provided new options for the clinical management of these patients. The evidence for cytotoxic chemotherapy remains controversial with patients experiencing significant toxicities while achieving less than desirable responses, thus relegating the role of these therapeutic options to the palliative care setting. However, for as ineffective as the application of chemotherapy may be, the publication of the PROMID study has rejuvenated interest in the use of the somatostatin analogs, specifically octreotide LAR, in the routine management of patients with metastatic carcinoid tumors.
In our practice, patients with metastatic carcinoid tumors are initiated on octreotide LAR 30 mg IM every 4 weeks, often within 1 month of tumor resection. Practitioners should ensure tolerance to octreotide therapy before converting patients to the LAR formulation. However, no specific protocol exists in the literature or from the manufacturer as to how patients should be converted. For this reason, we have adopted dosing octreotide immediate-release 50 μg SC twice daily for 2 weeks before a patient’s first LAR administration.
For patients with metastatic PNETs, many patients in our practice are treated with streptozocin and fluorouracil following surgical resection and converted to everolimus upon disease progression. This strategy has been well-tolerated, and anecdotal observations suggest that patients have achieved a median overall survival comparable with that reported in the literature.
Given the heterogeneity of carcinoid tumors and PNETs and the lack of standardization in choice of therapy, practitioner experience is very important in the management of these patients. Considerable interest, however, has existed in recent years in generating research to better understand these rare malignancies. More complete elucidation of the biochemical and molecular pathways involved in tumor growth and progression of these malignancies has allowed for the introduction of specific therapies to take advantage of these cellular processes. Recent studies of mTOR inhibitors, angiogenesis inhibitors, and other novel compounds have demonstrated increases in efficacy while minimizing toxicity to patients and have potentially unveiled the future management of NETs.
Disclosure
Dr Stricker did not report any potential financial conflicts of interest.
References
- Appetecchia M, Baldelli R. Somatostatin analogues in the treatment of gastro enteropancreatic neuroendocrine tumours, current aspects and new perspectives. J Exp Clin Cancer Res. 2010;29:19.
- Klimstra D, Modlin I, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-712.
- Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors—The International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689-2696.
- Eriksson B. New drugs in neuroendocrine tumors: rising of new therapeutic philosophies? Curr Opin Oncol. 2010;22:381-386.
- Soga J, Yakuwa Y, Osaka M. Carcinoid syndrome: a statistical evaluation of 748 reported cases. J Exp Clin Cancer Res. 1999;18:133-141.
- Solcia E, Kloppel G, Sobin L, et al. Histological Typing of Endocrine Tumours. 2nd ed. Berlin: Springer; 2000.
- Oberg K. Diagnosis and treatment of carcinoid tumors. Expert Rev Anticancer Ther. 2003;3:863-877.
- Scherubl H, Cadiot G, Jensen R, et al. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010;42:664-671.
- Yao J, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072.
- Modlin I, Lye K, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Kouvaraki M, Ajani J, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762-4771.
- Kulke M, Stuart K, Enzinger P, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401-406.
- Kulke M, Lenz HJ, Meropol N, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403-3410.
- Kvols L, Wiedenmann B, Oberg K, et al; for the SOM230 Carcinoid Study Group. Safety and efficacy of pasireotide (SOM230) in patients with metastatic carcinoid tumors refractory or resistant to octreotide LAR: results of a phase II study. J Clin Oncol. 2006;24(18S):Abstract 4082.
- Moertel C, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin- fluorouracil, or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519-523.
- Pavel M, Baum U, Hahn E, et al. Efficacy and tolerability of pegylated IFN-α in patients with neuroendocrine gastroenteropancreatic carcinomas. J Interferon Cytokine Res. 2006;26:8-13.
- Ramanathan R, Cnaan A, Hahn R, et al. Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma. Study of the Eastern Cooperative Oncology Group-E6282. Ann Oncol. 2001;12:1139-1143.
- Raymond E, Dahan L, Raoul J, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513.
- Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, doubleblind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663.
- Sun W, Lipsitz S, Catalano P, et al. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol. 2005;23:4897-4904.
- Yao J, Phan A, Chang D, et al. Efficacy of RAD001 (Everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311-4318.
- Yao J, Phan A, Hoff P, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26:1316- 1323.
- Yao J, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69-76.
- Yao J, Shah M, Ito T, et al; for the RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523.
- Hellman P, Lundstrom T, Ohrvall U, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg. 2002;26:991-997.
- Norton J. Surgical management of carcinoid tumors: role of debulking and surgery for patients with advanced disease. Digestion. 1994;55(supp 3):98-103.
- Oberg K. Neuroendocrine gastrointestinal tumors—a condensed overview of diagnosis and treatment. Ann Oncol. 1999;10(suppl 2):S3-S8.
- Caplin M, Buscombe J, Hilson A, et al. Carcinoid tumour. Lancet. 1998;352:799-805.
- Kulke M, Mayer R. Carcinoid tumors. N Engl J Med. 1999;340:858-868.
- Bousquet C, Puente E, Buscail L, et al. Antiproliferative effect of somatostatin analogs. Chemotherapy. 2001;47(suppl 2):30-39.
- Kvols L, Moertel C, O’Connell M, et al. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315:663-666.
- Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16:2963- 2970.
- Fazio N, Cinieri S, Lorizzo K, et al. Biological targeted therapies in patients with advanced enteropancreatic neuroendocrine carcinomas. Cancer Treat Rev. 2010;36(suppl 3):S87-S94.
- Evers B, Parekh D, Townsend C Jr, Thompson J. Somatostatin and analogues in the treatment of cancer. Ann Surg. 1991;213:190-198.
- Grozinsky-Glasberg S, Shimon I, Korbonits M, Grossman AB. Somatostatin analogues in the control of neuroendocrine tumours: efficacy and mechanisms. Endocr Relat Cancer. 2008;15:701-720.
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology Neuroendocrine Tumors. V.2.2010. www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed February 25, 2011.
- Lamberts S, Bakker W, Reubi J, Krenning E. Somatostatin-receptor imaging in the localization of endocrine tumors. N Engl J Med. 1990;323:1246-1249.
- Kwekkeboom D, Kam B, van Essen M, et al. Somatostatin receptor-based imagining and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53-R73.
- Oberg K, Funa K, Alm G. Effects of leukocyte interferon on clinical symptoms and hormone levels in patients with mid-gut carcinoid tumors and carcinoid syndrome. N Engl J Med. 1983;309:129-133.
- Fazio N, de Braud F, Delle Fave G, Oberg K. Interferon-alpha and somatostatin analog in patients with gastroenteropancreatic neuroendocrine carcinoma: single agent or combination? Ann Oncol. 2007;18:13-19.
- Pestka S, Langer J, Zoon K, Samuel C. Interferons and their actions. Annu Rev Biochem. 1987;56:727-777.
- von Wichert G, Jehle P, Hoeflich A, et al. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60:4573-4581.
- Moreno A, Akcakanat A, Munsell M, et al. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr Relat Cancer. 2008;15:257-266.
- Yao J, Shah M, Ito T, et al. A randomized, double-blind, placebo-controlled, multicenter phase III trial of everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET) (RADIANT-3). Ann Oncol. 2010;21(suppl 8): Abstract LBA9.
- La Rosa S, Uccella S, Finzi G, et al. Localization of vascular endothelial growth factor and its receptors in digestive endocrine tumors: correlation with microvessel density and clinicopathologic features. Hum Pathol. 2003;34:18-27.
- Papaetis G, Syrigos K. Sunitinib: a multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs. 2009;23:377-389.
- Faivre S Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734-745.
- Niccoli P, Raoul J, Bang Y, et al. Updated safety and efficacy results for the phase III trial of sunitinib (SU) versus placebo (PBO) for treatment of pancreatic neuroendocrine tumors (NET). J Clin Oncol. 2010;28(15S):Abstract 4000.
- Bevacizumab. Anti-VEGF monoclonal antibody, Avastin, rhumab-VEGF. Drugs R D. 2002;3:28-30.
- Turner N, Strauss S, Sarker D, et al. Chemotherapy with 5-fluorouracil, cisplatin, and streptozocin for neuroendocrine tumours. Br J Cancer. 2010;102:1106-1112.
- Cheng P, Saltz L. Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer. 1999;86:944-948.
- D’Amato R, Loughnan M, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082-4085.
- Kiely J, Rilling W, Touzios J, et al. Chemoembolization in patients at high risk: results and complications. J Vasc Interv Radiol. 2006;17:47-53.
- Bloomston M, Al-Saif O, Klemanski D, et al. Hepatic artery chemoembolization in 122 patients with metastatic carcinoid tumor: lessons learned. J Gastrointest Surg. 2007;11:264-271.
- Reddy S, Clary B. Neuroendocrine liver metastases. Surg Clin North Am. 2010;90:853-861.