Structurally related to cyclophosphamide, ifosfamide is an alkylating chemotherapeutic agent used to treat a variety of solid tumors and hematologic malignancies.1 Ifosfamide is a prodrug that is metabolized by the cytochrome P450 3A4 (CYP3A4) enzyme to its active (ifosfamide mustard and 4-hydroxy-ifosfamide) and neurotoxic metabolites. Neurotoxicity is a wellknown adverse effect of ifosfamide occurring in up to 60% of patients.2
The signs and symptoms of neurotoxicity may include confusion, drowsiness, seizures, blurred vision, auditory or visual paranoid hallucinations, extrapyramidal symptoms, and urinary incontinence. Female sex, hepatic or renal dysfunction, and low albumin have been associated with increased ifosfamide neurotoxicity. The risk of neurotoxicity may also increase when ifosfamide is administered with other drugs metabolized through the CYP3A4. Dechloroethylation via CYP3A4 results in the release of potential neurotoxic metabolites. Thus CYP3A4 inducers have the potential to increase formation of both active and neurotoxic metabolites. Conversely, CYP3A4 inhibitors may impede with the activation of ifosfamide.
Ifosfamide-based chemotherapy is the standard of care for treatment of soft-tissue sarcomas. It is one of the most active single agents used to treat sarcoma, usually given in conjunction with doxorubicin and occasionally a platinum analog.3 Ifosfamide is a key component in the treatment for metastatic sarcoma and as adjuvant therapy in surgically resected soft-tissue sarcomas. These regimens, including mesna, doxorubicin, and ifosfamide (MAI), can be very emetogenic.
The National Comprehensive Cancer Network guidelines identify ifosfamide-based chemotherapy combinations used to treat sarcoma as moderate to high in emetogenic potential.4 Dose-limiting nausea and vomiting are common, adversely affecting patients’ ability to tolerate further chemotherapy and overall quality of life. Dexamethasone and a 5-hydroxytryptamine-3 (5-HT3) antagonist are considered standard of care for nausea and vomiting prevention in moderatehigh regimens, with a strong consideration given for the addition of aprepitant.4
Aprepitant is approved by the US Food and Drug Administration for use in combination with corticosteroids and 5-HT3 receptor antagonists in moderately to highly emetogenic chemotherapy regimens. In clinical practice and clinical trials, aprepitant has proved to benefit patients by preventing acute and delayed nausea and vomiting.5
Concomitant use of aprepitant with ifosfamide is controversial because of a perceived potential drug interaction. Aprepitant is known to inhibit and induce CYP3A4, making it a potentially significant problem in patients receiving medications metabolized by CYP3A4.6 Dexamethasone can also induce CYP3A4. In combination, these 2 agents contribute to the pharmacokinetic variability of ifosfamide and its active metabolites.
A 2007 case report first reported increases of 66.7% and 37.3% in the neurotoxic 2 and 3 dechloroethylifosfamide metabolites 2 hours after aprepitant administration.7 Another recent case report described a patient receiving ifosfamide-based chemotherapy who exhibited symptoms of neurotoxicity after adding aprepitant to the antiemetic regimen.6 Howell and colleagues noted an increased trend of neurotoxicity with the addition of aprepitant to ifosfamide-containing regimens in sarcoma,8 whereas a subsequent letter to the editor reported no change in neurotoxicity rates with the combination.9
The debate about the significance of the drug interaction between aprepitant and ifosfamide is important, because nausea/vomiting and neurotoxicity directly impact patients’ tolerance of the therapy designed to improve their prognosis. The primary objective of this study was to determine whether concomitant aprepitant was associated with an increased risk of neurotoxicity in patients with sarcoma receiving high-dose ifosfamidebased chemotherapy. In addition, we considered other patient and treatment factors that may influence ifosfamide- induced neurotoxicity.
Methods
A retrospective study was conducted on all patients (the total number by the end of the study was 65) diagnosed with any histologic subtype of sarcoma who received ifosfamide-based chemotherapy at Roswell Park Cancer Institute (RPCI) between January 2005 and July 2009. Patients were identified through the RPCI pharmacy system. Patients could receive any number of chemotherapy regimens, as long as the treatment included ifosfamide.
The treatment regimens had various schedules of 5-HT3 antagonists based on physician preference and the drug formulary at the time of treatment. All patients were assessed on whether they received a concomitant corticosteroid (ie, dexamethasone) or aprepitant for nausea and vomiting prophylaxis.
Data on ifosfamide dose, regimen, cycle number, infusion length (hours), and cumulative dose at the time of the cycle were collected. Demographics including age, sex, chemotherapy regimen received, diagnosis, height, actual body weight, ideal body weight, body surface area, albumin, aspartate aminotransferase, alanine aminotransferase (ALT), total bilirubin, hemoglobin, serum creatinine, and calculated creatinine clearance based on ideal body weight at time of admission were collected.
The written notes from nurses, mid-level practitioners, and physicians in each patient case report form were reviewed to identify neurotoxic events. There had to be clear documentation by the provider that neurotoxicity was suspected or probable, but other potential confounding variables could not be ruled out. Treatment of neurotoxicity with methylene blue or by discontinuing ifosfamide was also recorded.
Our primary research concern was the effect of aprepitant use on the incidence of neurotoxicity. All 65 patients in this study received a high dose of ifosfamide. Other independent variables of interest included body weight, infusion schedule (continuous vs 5 hours or less), ideal body weight, and baseline measures of albumin, liver function tests, and hemoglobin. Preliminary analysis showed the natural log transformation of ALT to be better suited for statistical modeling.
The primary neurotoxicity model was fit, using multivariable logistic regression to control for potential confounding variables. The full model was specified to include the independent variables discussed above and the plausible interaction of infusion schedule. The full model was reduced using manual backward select with a P value threshold of .05, starting with the interaction term, and constrained to retain the aprepitant main effect. For the final model, goodness of fit was assessed using the Hosmer-Lemeshow test. Similar methods were used to investigate the probability of receiving aprepitant.
In our sample, all 65 patients received high doses of ifosfamide. Approximately 25% of the patients experienced neurotoxicity. The primary null hypothesis assumed no association between aprepitant use and toxicity rate. If the true neurotoxicity rate among aprepitant users in this population is 42% (corresponding to an odds ratio of 2.2), logistic regression modeling with a 2-sided significance level of 0.05 would correctly reject the null hypothesis in 80% of similarly conducted experiments. When the variability in the toxicity rate explained by additional covariates offsets the incremental loss in degree(s) of freedom, a lower minimum detectable odds ratio would be obtained. Some power is lost if other covariates in the model are correlated with aprepitant use.
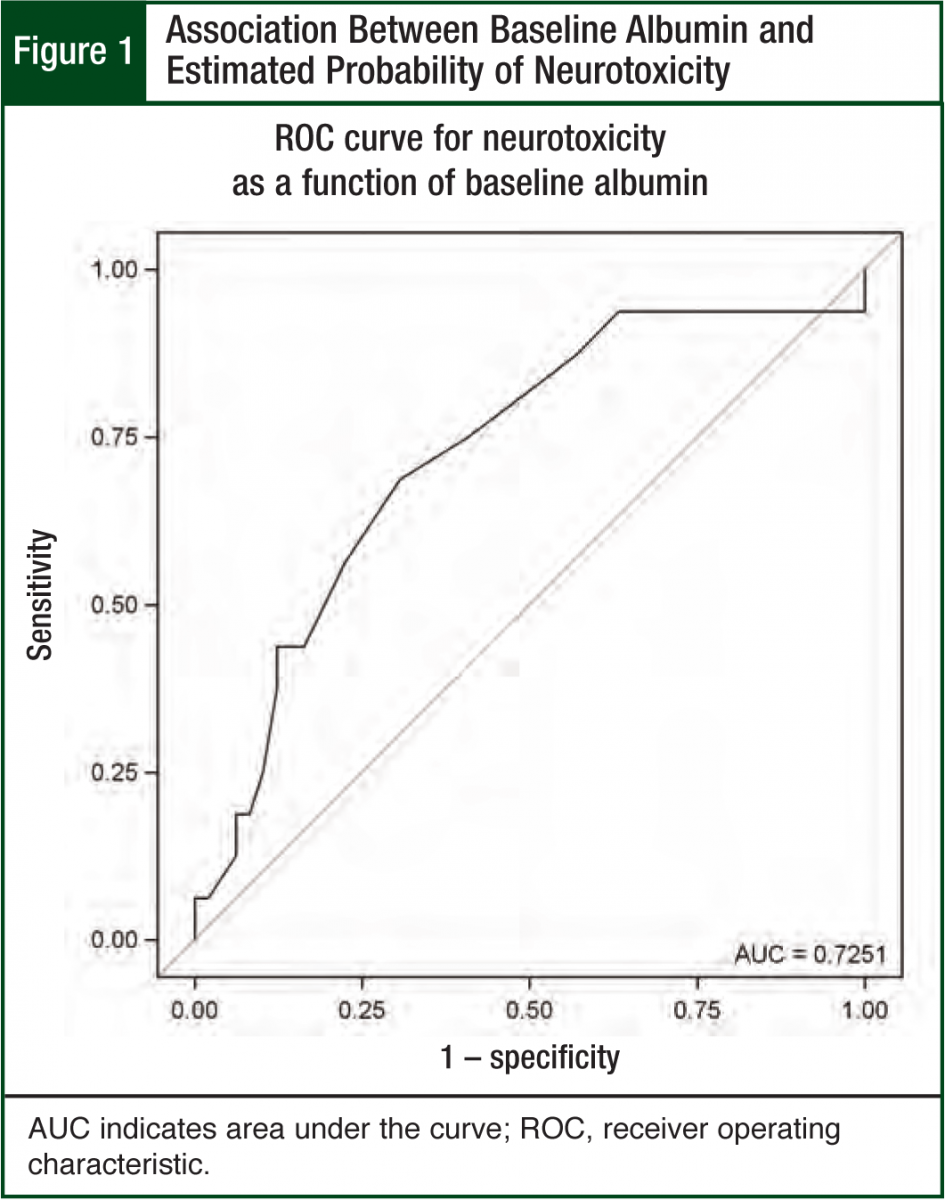
To be useful, predictions for the probability of neurotoxicity generated from the final model should accurately separate patients who experienced neurotoxicity from those who did not. A statistically significant factor in a logistic regression model may not be useful in predicting neurotoxicity in an individual patient. The predictive ability of the final logistic regression model was assessed in 2 ways. First, the receiver operating characteristic (ROC) curve plots the sensitivity as a function of (1–specificity) for all possible predicted probabilities est imated from the final model.10 This plot summarizes the predictive power of the model, with larger gaps between the ROC curve and the 45-degree reference line indicating greater predictive power. Second, the concordance index estimates the probability of concordance between the predicted probability and the observed neurotoxicity outcomes. The concordance index is the area under the ROC curve (AUC). An AUC of 0.5 suggests the model predictions are no better than random guessing. AUC values >0.8 are generally considered useful for predicting neurotoxicity in individual patients.
These primary results were supplemented with univariate descriptions of associations between baseline patient or disease characteristics and aprepitant use or presence of neurotoxicity. Statistical significance of these associations were assessed using Wilcoxon rank sum test (for continuous covariates) and the Pearson chi square test (for categorical covariates). P <.05 was deemed statistically significant. No adjustments were made to this threshold to control the effects of multiple testing on the overall type I error rate. Confidence intervals (CIs) of 95% were presented as the plausible range of values for the true (unknown) population parameter that is supported by the data. All analyses were completed with SAS version 9.2 (SAS Institute, Cary, NC).
Results
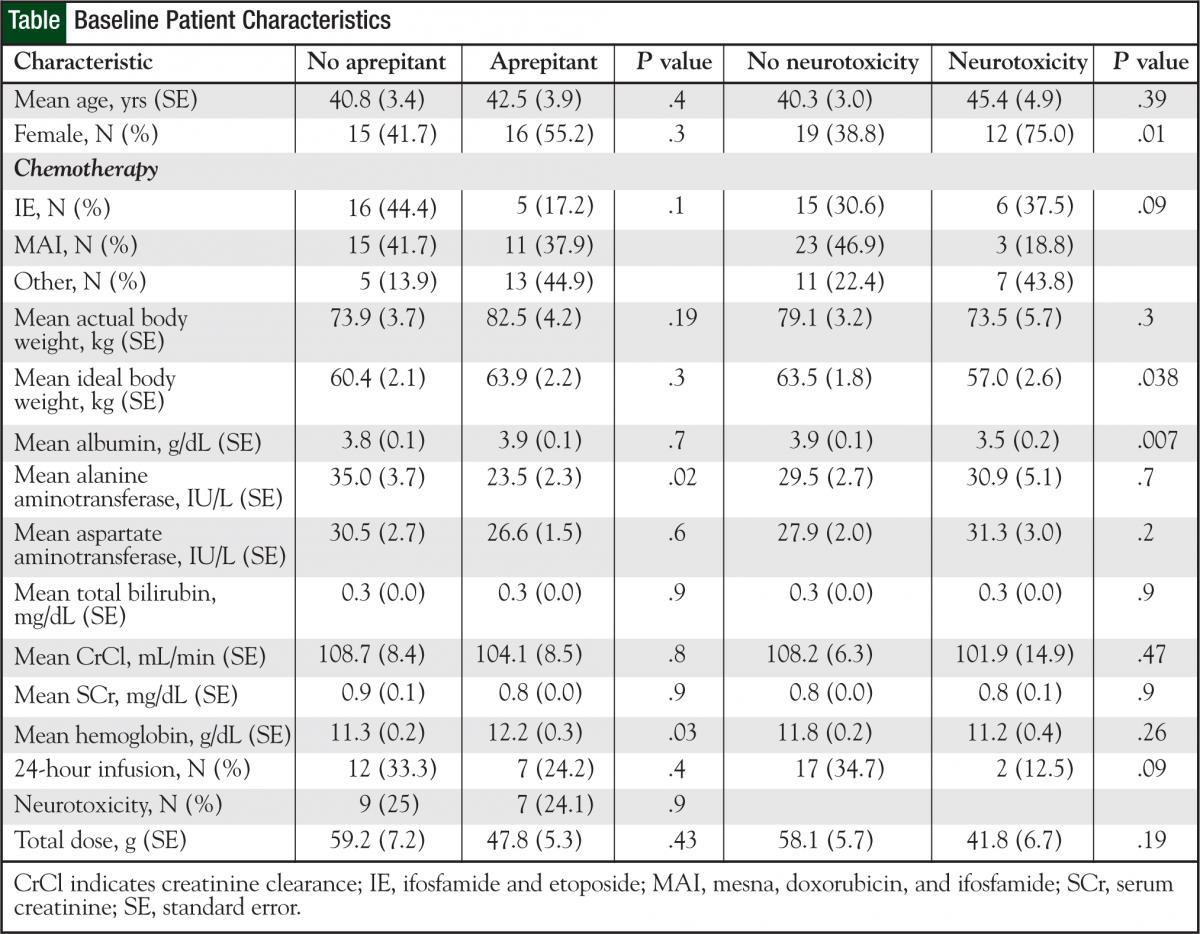
The 65 patients received a total of 308 cycles of ifosfamide-based chemotherapy. The Table (page 19) shows patient characteristics. Twenty-nine (45%) patients received aprepitant during ifosfamide-based chemotherapy.
Of the 65 patients, 16 (25%) had a neurotoxicity event during ifosfamide chemotherapy as documented by the attending physician. Of the 16 patients, 7 (43.8%) received aprepitant during ifosfamide-based chemotherapy.
Of the 222 cycles without aprepitant, 17 (7.6%) involved documented neurotoxicity compared with 5 (5.8%) of the 86 cycles with aprepitant, a not significant difference (P = .57).
Subgroup comparisons were performed on patients who experienced a neurotoxic event versus those who did not (Table). Patients with documented neurotoxicity during ifosfamide therapy tended to be female, have a lower albumin level and ideal body weight, and received ifosfamide infusions over 5 hours or less. Five of the 16 patients who had neurotoxicity were treated with methylene blue. Three patients who had previous ifosfamiderelated neurotoxicity received methylene blue as prophylaxis on the subsequent cycle.
Univariate results suggest a significant association between neurotoxicity, sex (P = .01, women more likely), low albumin (P = .007), and ideal body weight (P = .038). Neither univariate analysis (P = .936) nor the multivariate model (P = .86) detected a significant association between aprepitant use and the probability of neurotoxicity. Low albumin was the only significant variable predicting for neurotoxicity in the multivariate model (P = .025).
The adjusted odds ratio for neurotoxicity in patients treated with aprepitant versus those treated without it was 1.15 (95% CI, 0.2-5.5), and it was not significant (P = .9). This estimate was adjusted for sex, infusion schedule, and other covariates that may influence the association between aprepitant use and neurotoxicity.
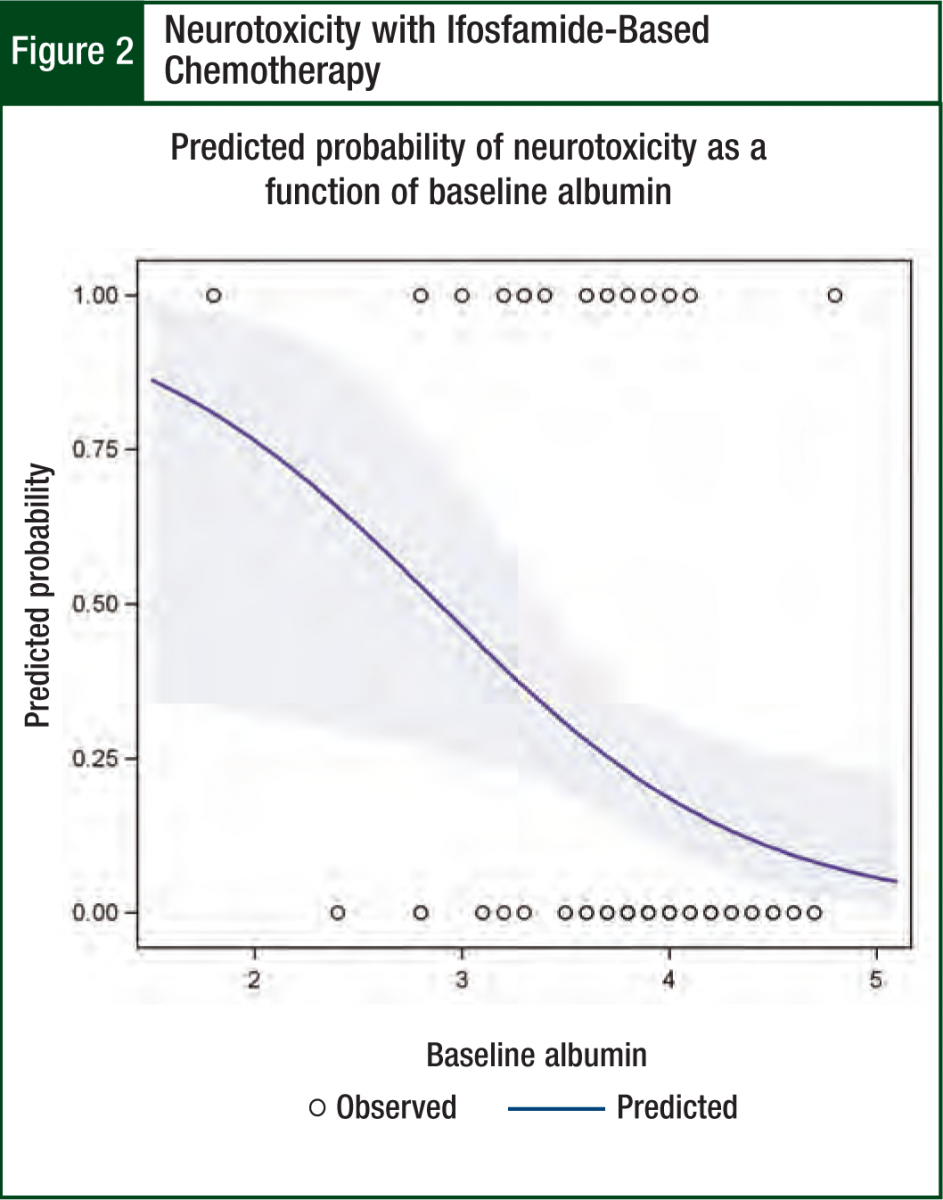
Albumin was the only significant predictor (odds ratio = 0.2; P = .03; 95% CI, 0.06-0.8) in the multivariable neurotoxicity model (Hosmer-Lemeshow chi square = 3.6, P = .82). The modeled association between albumin and neurotoxicity is illustrated in Figures 1 and 2. Figure 1 shows the protective effect of higher albumin levels on the predicted probability of neurotoxicity. The ROC curve indicates how accurately albumin levels can be used to categorize patients by neurotoxicity status. The AUC of 0.73 suggests a moderate degree of accuracy for predicting neurotoxicity in individual patients (Figure 1).
Discussion
Of the 65 patients in our study who received ifosfamide-based chemotherapy for sarcoma, 16 experienced (25%) a neurotoxic event. This number is within the range described in previous literature reports.2 We found no evidence to show that aprepitant increased the rate of neurotoxicity with high-dose ifosfamide therapy. Less than half of the patients (7 of 16) with a neurotoxic event received aprepitant during ifosfamide-based chemotherapy. There was no significant difference in the number of cycles of chemotherapy with or without aprepitant and neurotoxicity rates (P = .57). However, consistent with previous studies,8,9,11 low albumin was a significant indicator of increased risk for neurotoxicity.
Durand and colleagues were first to report a positive association between aprepitant and the development of neurotoxicity during ifosfamide treatment.7 They described a case of a 57-year-old woman who experienced neurotoxicity while receiving ifosfamide to treat osteosarcoma. Their pharmacokinetic results revealed a significant increase in the formation of neurotoxic metabolites from ifosfamide when used concomitantly with aprepitant. A subsequent case report described development of neurotoxicity when aprepitant was added to ifosfamide-based chemotherapy.6
Howell and colleagues’ retrospective study characterized the occurrence of neurotoxicity in patients treated with ifosfamide and concurrent aprepitant.8 All 45 patients included in their study received a 2- to 4-day regimen of MAI. Eighteen percent (8 of 45) of these patients developed neurotoxicity during therapy. Their results revealed a trend toward increased neurotoxicity (P = .176) with the use of concomitant aprepitant.
Similar to our results, they noted a significant difference in low albumin in patients who developed neurotoxicity. Subsequently, in a letter to the editor, Ho and Yuen share their retrospective experience with aprepitant-associated ifosfamide neurotoxicity.9 Twelve percent (7 of 54) of the patients they reviewed experienced neurotoxicity.
Not surprising, and similar to our report and that of Howell and colleagues, the cases of neurotoxicity had significantly lower albumin levels than patients without neurotoxicity (P = .0021). But the described albumin levels (mean, 1.9) in neurotoxicity cases were clinically lower than ours (mean, 3.5; standard error, 0.2) and those of Howell and colleagues (mean, 2.88); 47% of their neurotoxicity cases and 59% of the controls received concomitant aprepitant.8 They found no significant association with aprepitant and the development of neurotoxicity.8
The methodologies, number of patients, and baseline characteristics were similar between all the studies. Our report is in direct agreement with Ho and Yuen9 in that aprepitant did not increase neurotoxicity with concomitant ifosfamide-based chemotherapy. This contrasts the results from Howell and colleagues, who found a trend toward increased neurotoxicity with the addition of aprepitant to ifosfamide.8
Putting our work in context, the drug-drug interaction models may be more complex than initially envisioned. Ifosfamide has been described as a prodrug that requires enzymatic activation by the liver via CYP3A4 to both active (4-hydroxy-ifosfamide) and neurotoxic metabolites (chloroacetaldehyde and 2- and 3-dechloroethylifosfamide).12 It is plausible that drugs that induce or inhibit CYP3A4 can have a significant impact on ifosfamide pharmacokinetics. Dexamethasone, a known CYP3A4 inducer, has been shown to increase chloroacetaldehyde peak concentrations by 1.5-fold and the AUC time curve by 1.3-fold.13 Phenytoin has also been shown to increase dechloroethylation in pediatric patients receiving ifosfamide.14
But it must be noted that ifosfamide can induce its own metabolism within the first 24 hours, thereby complicating interpretation of the pharmacokinetic results.12,15 Also, no definitive evidence exists on whether CYP3A4 is the only relevant enzyme contributing to ifosfamide metabolism in vivo. In vitro studies document multiple liver microenzymes contributing to ifosfamide metabolism.12
Another important point, which has yet to be documented or described in the literature, to our knowledge, is the potential impact of aprepitant on the efficacy of ifosfamide. Ifosfamide is activated via hydroxylation in the liver by CYP3A4 to its active form. Therefore, inhibitors or inducers of CYP3A4 have the potential to affect formation of active metabolites.
As Ho and Yuen note, both aprepitant and ifosfamide are highly protein-bound drugs.9 They postulate that low albumin levels can lead to increased free unbound aprepitant and ifosfamide, thereby increasing the formation of all ifosfamide active and inactive metabolites. Although this explanation is plausible, there are no pharmaco kinetic data available to refute or to support this claim.
Patients in our study who had documented neurotoxicity during ifosfamide therapy tended to be female, have a lower ideal body weight, and received ifosfamide infusions over less than 5 hours. It has been previously reported that females exhibit more neurotoxicity with ifosfamide than males.16 But the increased neurotoxicity rates with a lower ideal body weight and shorter ifosfamide infusion time were somewhat surprising. It has been previously reported in small groups of patients that longer infusion times of ifosfamide may lower toxicity and metabolites.17,18 But subsequent pharmacokinetic assessments comparing prolonged infusions did not show any difference in pharmacokinetic parameters.19,20
The results of our study are based on a convenience sample derived from retrospective chart reviews from a single institution. The use of sequential cases tends to reduce biases inherent in this approach. However the possibility of bias induced by important, perhaps unmeasured, factors remains a weakness common to all retrospective studies.
Conclusion
Neurotoxicity is a potentially dose-limiting side effect occurring with ifosfamide-based chemotherapy, often necessitating at least temporary cessation of therapy. This study documents that aprepitant was not associated with increased risk of neurotoxicity in RPCI patients receiving ifosfamide-based chemotherapy. Our finding that low albumin levels were a risk factor for ifosfamide neurotoxicity is consistent with previously published studies and may be helpful in identifying patients at higher risk for neurotoxicity.
Based on these data, the medical sarcoma service at our institute has made aprepitant part of the standard order set for patients with normal or near-normal albumin levels receiving ifosfamide-based chemotherapy deemed to be moderately high to highly emetogenic (ie, MAI). In patients with lower than normal albumin levels, we advocate ifosfamide dose reduction. Definitive resolution of this question will require pharmacokinetic study of both the active ifosfamide metabolites and the neurotoxic metabolites simultaneously.
Author Disclosure Statement
Drs Jarkowski, Miller, Blustein, and Wong, and Ms Hecke have reported no actual or potential conflicts of interest.
References
- Zhang J, Tian Q, Zhou SF. Clinical pharmacology of cyclophosphamide and ifosfamide. Curr Drug Ther. 2006;1:55-84.
- Patel PN. Methylene blue for management of ifosfamide-induced encephalopathy. Ann Pharmacother. 2006;40:299-303.
- Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol. 1993;11:1276-1285.
- Ettinger DS, Armstrong DK, Barbour S, et al. Antiemesis. Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2009;7:572-595.
- Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112-4119.
- Jarkowski A 3rd. Possible contribution of aprepitant to ifosfamide-induced neurotoxicity. Am J Health Syst Pharm. 2008;65:2229-2231.
- Durand JP, Gourmel B, Mir O, Goldwasser F. Antiemetic neurokinin-1 antagonist aprepitant and ifosfamide-induced encephalopathy. Ann Oncol. 2007;18:808-809.
- Howell JE, Szabatura AH, Hatfield Seung A, Nesbit SA. Characterization of the occurrence of ifosfamide-induced neurotoxicity with concomitant aprepitant. J Oncol Pharm Pract. 2008;14:157-162.
- Ho H, Yuen C. Letter to the editor. J Oncol Pharm Pract. 2010;16:137-138.
- Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654-657.
- David KA, Picus J. Evaluating risk factors for the development of ifosfamide encephalopathy. Am J Clin Oncol. 2005;28:277-280.
- Kerbusch T, de Kraker J, Keizer HJ, et al. Clinical pharmacokinetics and pharmacodynamics of ifosfamide and its metabolites. Clin Pharmacokinet. 2001;40:41-62.
- Brüggemann SK, Pfäffle S, Peters SO, Wagner T. Influence of short-term use of dexamethasone on the pharmacokinetics of ifosfamide in patients. Drug Metab Dispos. 2007;35:1721-1724.
- Ducharme MP, Bernstein ML, Granvil CP, et al. Phenytoin-induced alteration in the N-dechloroethylation of ifosfamide stereoisomers. Cancer Chemother Pharmacol. 1997;40:531-533.
- Lind MJ, Margison JM, Cerny T, et al. Comparative pharmacokinetics and alkylating activity of fractionated intravenous and oral ifosfamide in patients with bronchogenic carcinoma. Cancer Res. 1989;49:753-757.
- Sweiss KI, Beri R, Shord SS. Encephalopathy after high-dose ifosfamide: a retrospective cohort study and review of the literature. Drug Saf. 2008;31:989-996.
- Kerbusch T, Mathôt RA, Keizer HJ, et al. Influence of dose and infusion duration on pharmacokinetics of ifosfamide and metabolites. Drug Metab Dispos. 2001;29:967-975.
- Cerny T, Castiglione M, Brunner K, et al. Ifosfamide by continuous infusion to prevent encephalopathy. Lancet. 1990;335:175.
- Brain EG, Rezai K, Weill S, et al. Variations in schedules of ifosfamide administration: a better understanding of its implications on pharmacokinetics through a randomized cross-over study. Cancer Chemother Pharmacol. 2007;60:375-381.
- Singer JM, Hartley JM, Brennan C. The pharmacokinetics and metabolism of ifosfamide during bolus and infusional administration: a randomized cross-over study. Br J Cancer. 1998;77:978-984.