Advances in the treatment of acute myeloid leukemia (AML) have led to new therapies and optimized treatment regimens; however, some of these treatments are associated with the life-threatening complication of differentiation syndrome. Dedicated guidelines in the management of differentiation syndrome have not been established. The purpose of this review is to summarize the background of and risk factors for differentiation syndrome, the prophylaxis for and treatment of differentiation syndrome, and special considerations for acute promyelocytic leukemia (APL; a subtype of AML), late-onset differentiation syndrome, and supportive-care measures that other reviews may not have addressed comprehensively.
SYMPTOM OVERVIEW
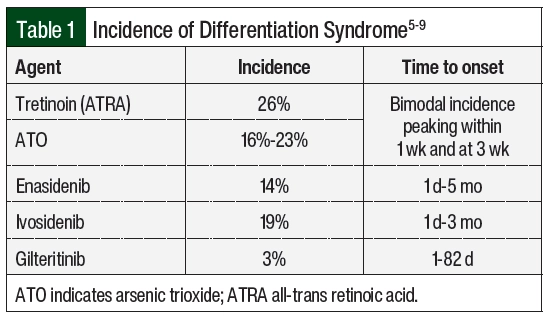
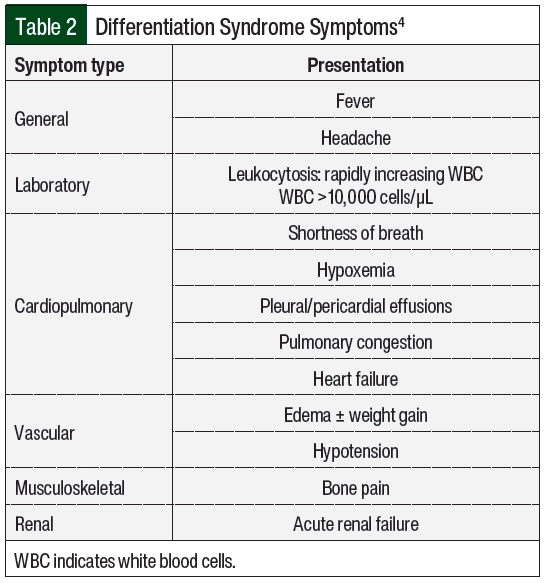
Differentiation syndrome is a life-threatening complication associated with differentiating agents that are historically used for the treatment of APL, the M3 subtype of AML, based on the French-American-British classification systems, or AML with certain genetic abnormalities, including the PML-RARA fusion gene based on the World Health Organization classification.1,2 Tretinoin (all-trans retinoic acid [ATRA]) and arsenic trioxide (ATO) in the treatment of APL are associated with an incidence rate of differentiation syndrome of 25% in the United States and Europe.3 Differentiation syndrome was formerly known as retinoic acid syndrome because it occurred in patients with APL who received treatment with ATRA,4 but differentiation syndrome also occurs with other therapies.5-9 These therapies include the isocitrate dehydrogenase (IDH) inhibitors enasidenib and ivosidenib, as well as the FMS-related tyrosine kinase 3 (FLT3) inhibitor gilteritinib. The incidence of differentiation syndrome of known differentiating agents is summarized in Table 1.5-9 The incidence of differentiation syndrome in APL is reduced when differentiating agents are given in combination with chemotherapy.10 The symptoms of differentiation syndrome are shown in Table 2 and include fever of unknown origin, edema that may result in weight gain, pulmonary infiltration with dyspnea, pleural and pericardial effusions, hypotension, and renal failure.4 The grading of differentiation syndrome has not been standardized; thus, clinical discretion may be used, taking into consideration the number of symptoms and the patient’s disease status.11 Common Terminology Criteria for Adverse Events version 5.0 may be used for individual symptoms.12
Differentiation syndrome may be fatal if treatment is not initiated promptly. Mortality associated with differentiation syndrome has been reported to be as high as 30% in historic trials; however, earlier recognition and the initiation of corticosteroids have improved the mortality rate to approximately 1% in more recent trials.13-16 This reduction in mortality may also be attributed to many protocols including empiric corticosteroids as differentiation syndrome prophylaxis.
ETIOLOGY
The genetic driver of APL is t(15;17), which results in the fusion of the genes PML on chromosome 15 and RARA on chromosome 17.17 This fusion gives rise to the PML-RARA oncogene. PML and RARA are essential to myeloid stem-cell differentiation and proliferation.17 The PML-RARA fusion results in the loss of function of both proteins. Promyelocytes remain in an immature state because of the inability to differentiate.18 The backbone of APL treatment consists of ATRA and ATO, which inhibit portions of the PML-RARA oncogene to prevent the production of the protein and enable cell differentiation. ATRA inhibits RARA and induces terminal differentiation, whereas ATO inhibits PML and induces partial differentiation.3 Myeloid differentiation can occur rapidly in the presence of ATRA and ATO. It is this rapid maturation that promotes tissue and interstitial infiltration of leukocytes along with cytokine release, which both contribute to the symptoms of differentiation syndrome.18
In patients with IDH mutation–positive AML, leukemic cells remain in an immature state. IDH mutation promotes the conversion of intracellular alpha-ketoglutarate to 2-hydroxyglutarate (2-HG). The accumulation of 2-HG can inhibit cellular differentiation.19 Exposure to the IDH inhibitors enasidenib (IDH2) and ivosidenib (IDH1) reduces 2-HG production and can result in rapid differentiation of cells. Expression of the genes for CCAAT/enhancer-binding protein alpha (CEBPA) and transcription factor PU.1 is needed for normal myeloid differentiation.20-22 In patients with FLT3 mutation–positive AML, the expression of these genes is suppressed, which results in undifferentiated myeloid blasts. The use of FLT3 inhibitors restores the expression of CEBPA and PU.1.20-22 Differentiation syndrome has been reported with the FLT3 inhibitor gilteritinib, but it has not been associated with other FLT3 inhibitors.23
The main risk factor for differentiation syndrome specific to APL is a leukocyte count of >10,000 cells/µL.17 In patients with AML (excluding APL), those with a leukocyte count of >10,000 cells/µL have a higher risk for differentiation syndrome.17
In drug development, a novel drug class in clinical trials has exhibited the risk for differentiation syndrome. SNDX-5613 and KO-539 are menin inhibitors that have demonstrated differentiation syndrome in phase 1 clinical data.24-26 SNDX-5613 is being studied in the phase 1/2 AUGMENT-101 trial for the treatment of relapsed or refractory AML with MLL gene rearrangement or NPM1 mutations, which has a 14% incidence of differentiation syndrome.24,25 KO-539 is under investigation in the phase 1/2 KOMET-001 trial for the treatment of relapsed or refractory AML.26 The FDA placed a hold on phase 1b of KOMET-001 in December 2021 after the death of a patient that resulted from differentiation syndrome, which was later lifted in January 2022 with agreement to pursue risk mitigation strategies.27
PROPHYLAXIS AND CYTOREDUCTION
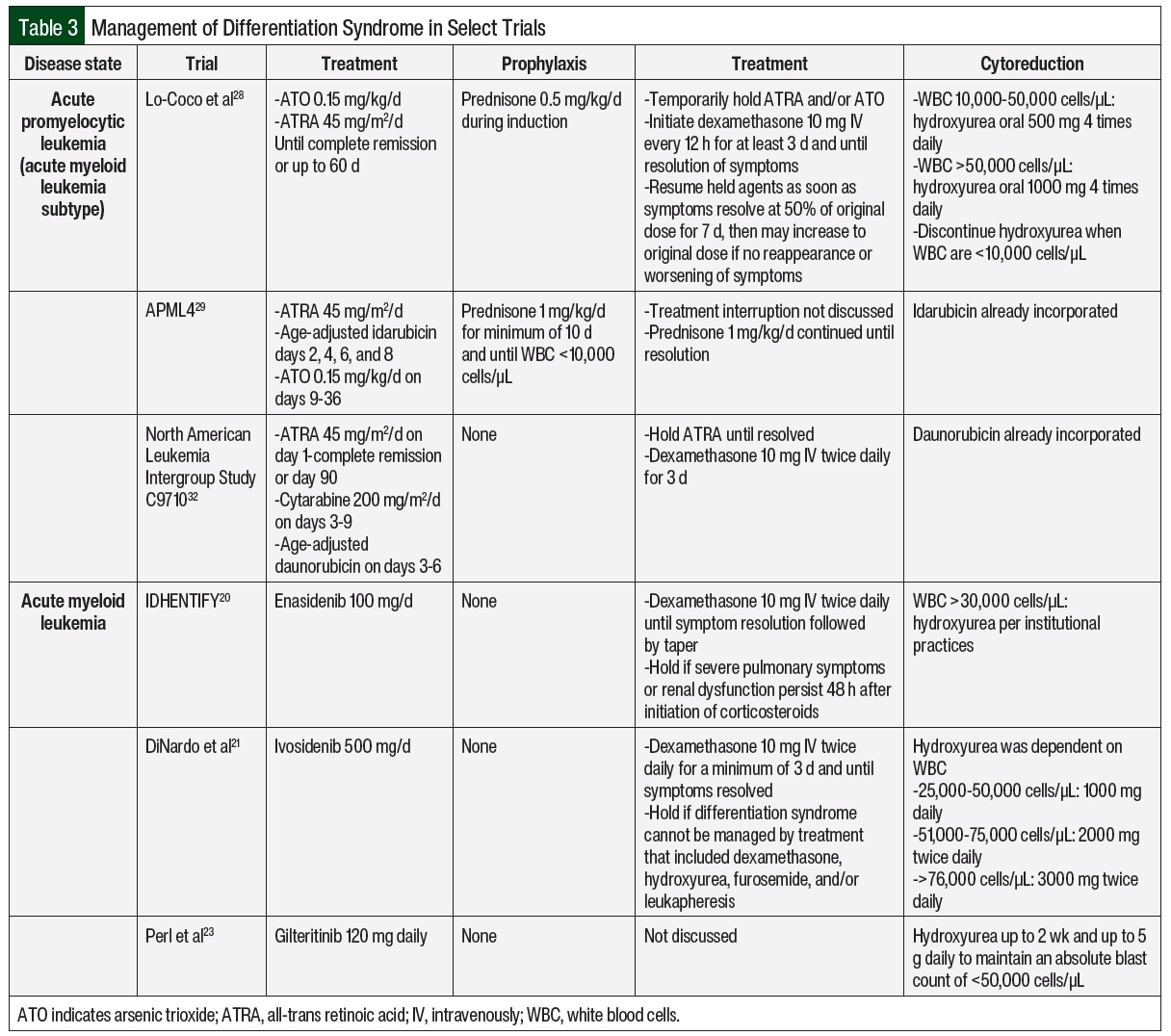
The incorporation of differentiation syndrome prophylaxis into APL chemotherapy regimens has contributed to decreased incidence and mortality related to differentiation syndrome.11 Prednisone 0.5 mg/kg daily has been used in patients with low-risk APL who are undergoing induction therapy with ATRA and ATO and then tapered appropriately at the completion of induction therapy.28 Another strategy has been used in patients with high-risk APL with the treatment induction of ATRA, ATO, and idarubicin with prednisone 1 mg/kg daily for a minimum of 10 days until the white blood cell (WBC) count declines to <10,000 cells/µL.29 If corticosteroid prophylaxis is not included in the treatment protocol, it should be strongly considered for patients with APL and a WBC count of >10,000 cells/µL.17 The prophylaxis of differentiation syndrome is not well defined for small molecule inhibitors for the treatment of AML because clinical trials did not use corticosteroids as prophylaxis. In clinical practice, the prophylaxis of differentiation syndrome consists only of cytoreductive measures for high-risk patients as determined by treatment protocol (some examples can be found in Table 3) or institutional standard.
The use of prolonged corticosteroids as prophylaxis for differentiation syndrome may pose an infection risk in patients who often present with prolonged neutropenia.3,30 The corticosteroid-related adverse events to monitor include hypertension, hyperglycemia, insomnia, and gastrointestinal bleeding.30 Monitoring should include blood pressure and glucose readings along with any new patient-reported symptoms.30 In an acute-care setting, this can occur at least daily. In an ambulatory setting, frequency can be determined based on additional patient factors. Closer monitoring should be done in patients with conditions that may be exacerbated by corticosteroid use, such as diabetes, cardiovascular disease, and a history of gastrointestinal bleeding or perforation.30 Stress ulcer prophylaxis with a proton pump inhibitor should be considered while corticosteroids are administered, especially in patients with additional risk factors.30 If a prolonged course of corticosteroids is required, prophylaxis against Pneumocystis jirovecii pneumonia (PJP) should be considered.31 The agents may include the combination of sulfamethoxazole plus trimethoprim or monotherapy with dapsone, atovaquone, or pentamidine.31 Hematology/oncology pharmacists can recommend to providers that patients are prescribed these supportive-care agents while they are receiving corticosteroids, along with counseling patients on adverse events to monitor for at home. The pharmacist may provide insight into supportive-care agents with considerations made for potential drug–drug interactions with acid-suppressing agents or the risk for QTc prolongation with antimicrobial prophylaxis in patients with neutropenia. In addition, patients requiring PJP prophylaxis who have a sulfa allergy, the pharmacist may provide recommendations based on adverse-event profile, cost, or medication compliance in the selection of alternative PJP active agents.
With advancements in electronic medical records, hematology/oncology treatment plans can be templated to include standardized order sets with supportive-care and monitoring parameters. The hematology/oncology pharmacist can drive the development of the standardized protocol to include prophylaxis and monitoring parameters based on literature and institutional standards. For example, a standardized treatment plan for APL can include the prophylactic corticosteroids according to the study protocol, laboratory monitoring at the desired frequency, and communications to nursing staff on the signs and symptoms of differentiation syndrome that should indicate provider notification to allow for the patient to be promptly evaluated. If differentiation syndrome is suspected, a decision to initiate treatment and possibly hold the offending agent(s) based on severity can be made in a timely manner, which may reduce morbidity.
Cytoreduction should be considered in high-risk patients as a way to mitigate differentiation syndrome.17 The selection and dosing of cytoreductive agents are often protocol- and patient-specific, such as high WBC counts, comorbidities, infections, or cytopenias. In patients with APL and leukocytosis, the preferred treatment regimens include chemotherapy, such as anthracyclines or gemtuzumab ozogamicin. A cytoreductive agent may be included in the chemotherapy regimen, but it may be initiated empirically if the WBC count rises to >10,000 cells/µL in the absence of clinical differentiation syndrome. Hydroxyurea is often preferred for leukocytosis during treatment.17 Examples of cytoreductive strategies from various protocols are shown in Table 3.17,20,21,23,28,29,32 In AML treatments (excluding APL), cytoreduction should be considered in the setting of leukocytosis per treatment protocol (examples in Table 3) or institutional standard. Leukapheresis may also be considered in patients with AML-associated leukocytosis but is not routinely recommended in patients with APL because of the possible risk for worsening coagulopathy.17,18 Enasidenib, ivosidenib, and gilteritinib have also been studied in combination with azacitidine, which reduces the risk for differentiation syndrome. The incidences of differentiation syndrome were 10%, 14%, and not reported for azacitidine combined with enasidenib, ivosidenib, and gilteritinib, respectively, which are numerically lower than the incidences with these drugs as monotherapy, as noted in Table 1.34-36 There is currently no treatment recommendation for prophylactic corticosteroids in patients with AML who are receiving small molecule inhibitors.
TREATMENT
There are various resources for management recommendations of differentiation syndrome in major guidelines, such as those from the National Comprehensive Cancer Network (NCCN), American Society of Hematology (ASH), landmark studies, and the prescribing information from the FDA. Summarized below and in Table 3 are some recommendations for the treatment of differentiation syndrome.
In general, the management of differentiation syndrome should not vary significantly, regardless of the causative agent. Early identification and the initiation of high-dose corticosteroids are essential to the management of differentiation syndrome.4 Corticosteroids mediate the effects of differentiation syndrome by the inhibition of cytokines.4 A high suspicion for differentiation syndrome should be maintained following treatment initiation.17 Dexamethasone 10 mg administered intravenously twice daily for at least 3 to 5 days is recommended when differentiation syndrome is suspected. This treatment should continue until the patient’s symptoms resolve and then should be tapered over the next 2 weeks.17
The rapid tapering or discontinuation of corticosteroids may result in a rebound of differentiation syndrome symptoms. However, the severity of differentiation syndrome and patient comorbidities might dictate treatment tapering over a shorter or longer period based on clinical judgment. The differentiating agent(s) should be held in cases of severe or refractory differentiation syndrome symptoms. The severe symptoms might include hypoxia requiring mechanical ventilation or renal failure associated with differentiation syndrome or the presence of several differentiation syndrome–associated symptoms, whereas refractory symptoms may refer to those that do not improve after 48 hours since the corticosteroids were initiated.17,20
In general, inpatient management of differentiation syndrome is warranted to allow for close monitoring in the setting of decompensation. Patients with suspected differentiation syndrome in the absence of severe symptoms who are clinically stable may be considered for outpatient management. The approach and dosing of oral dexamethasone would be unchanged, but patients should have close and frequent follow-up with a low threshold for admission at the first sign of decompensation. The decision for outpatient management should be made carefully with discussion between the provider and the patient of the potential risks. When ATRA and ATO are held, they may be resumed at 50% of the original dose on resolution of symptoms.28 If tolerated with no recurrence of differentiation syndrome, ATRA and ATO may be escalated to the full dose after 7 days.28 There are no dose adjustments required for differentiation syndrome among any of the small molecule inhibitors.7-9 If a small molecule inhibitor is held, it may be resumed once differentiation syndrome–associated symptoms have resolved to grade ≤2.7-9
Consideration should be given to the time to the onset of differentiation syndrome, especially with small molecule inhibitors for which differentiation syndrome may occur as long as 6 months from the initiation of treatment.7-9 This may pose challenges in the outpatient setting, where patients are monitored less closely. Differentiation syndrome should remain on the differential for patients presenting with differentiation syndrome–associated symptoms. Postmarketing case reports have also suggested atypical differentiation syndrome manifestations such as thyroiditis and rash.37,38 Although these symptoms are nonspecific, consideration should be given to initiate corticosteroids promptly while undergoing additional workup. Patients with severe symptoms should be admitted for further management and work up. The resolution of symptoms after the initiation of treatment with corticosteroids may confirm a diagnosis of differentiation syndrome. When tapering corticosteroids, caution should be taken not to taper too quickly because small molecule inhibitors have half-lives of several days, which may require a longer taper to avoid rebound symptoms.7-9
Although a standardized approach for the treatment of differentiation syndrome is yet to be established, healthcare workers may consider the following recommendations for clinical practice based on our review of available literature:
- Initiate cytoreduction for leukocytosis >10,000 cells/µL during treatment for APL and protocol-directed thresholds in AML, typically with hydroxyurea.
- Once differentiation syndrome is suspected, prompt initiation of dexamethasone 10 mg intravenously twice daily is essential and should be continued for ≥3 days or until symptom resolution.
- A slow taper of the corticosteroid may be considered to prevent rebound differentiation syndrome based on clinical judgment.
- Consider risk versus benefit of prolonged corticosteroids in the setting of severe differentiation syndrome symptoms.
- Ensure patient is receiving appropriate supportive-care medications (eg, proton pump inhibitor), if appropriate, while on corticosteroids.
- Hold differentiating agent in patients with severe differentiation-syndrome symptoms (eg, hypoxic respiratory failure, severe renal dysfunction) or symptoms that persist for 48 hours.
- If differentiating agent is held, resume once symptoms resolve to grade ≤2. ATRA and ATO should be resumed at 50% of the dose for 7 days and increased to 100% of the dose if differentiation syndrome does not recur; the small molecule inhibitors may be resumed without dose modification.
CONCLUSION
Differentiation syndrome is a medical emergency that requires prompt management and close hemodynamic monitoring. Differentiation syndrome is most frequently associated with the treatment of the AML subtype APL, with ATRA and ATO, but it has also been observed with other AML treatments, including enasidenib, ivosidenib, and, less so, gilteritinib. Because the risk for differentiation syndrome may persist for weeks to months in clinical practice, healthcare providers should include differentiation syndrome on their differential for patient workup.
The identification and diagnosis of differentiation syndrome may be difficult in clinical practice because the symptoms of differentiation syndrome may be present in other acute conditions.
The management of differentiation syndrome differs across recommendations from the NCCN, ASH, clinical trial protocols, and drug prescribing information, which complicates the determination of a standardized treatment approach. Overall, differentiation syndrome requires the vigilant recognition of symptoms and urgent intervention with high-dose corticosteroids to prevent morbidity and mortality with considerations made to avoid compromising the efficacy of treatment for APL or AML.
Hematology/oncology pharmacists may assist in the recognition and management of differentiation syndrome. The role of the pharmacist is important in the management and monitoring of patients who are at risk for differentiation syndrome. Pharmacists may provide dosing recommendations for anticancer therapies in patients with differentiation syndrome or other adverse events. Pharmacists can ensure that patients receive the appropriate supportive care, which may be especially vital in patients with acute leukemias who received oral oncolytic agents because the presentation of differentiation syndrome may be delayed or unexpected. In this setting, the pharmacist can empower the patient to be aware of symptoms that need further evaluation to reduce the morbidity associated with delayed differentiation syndrome. In the outpatient setting, pharmacists can also encourage adherence to supportive care medications to avoid potentially preventable adverse events.
Author Disclosure Statement
Dr Fazal is a consultant to Blueprint Medicines, Bristol Myers Squibb, CTI BioPharma, Incyte, Gilead Sciences, GlaxoSmithKline, Karyopharm Therapeutics, Novartis, Omeros, Sanofi, Servier Pharmaceuticals, Taiho Pharmaceutical, and Takeda Pharmaceuticals, and is on the Speakers Bureau for Blueprint Medicines, Bristol Myers Squibb, CTI BioPharma, Gilead Sciences, GlaxoSmithKline, Incyte, Janssen Pharmaceuticals, Karyopharm Therapeutics, Novartis, Omeros, Pfizer, PharmaEssentia, Sanofi, Servier Pharmaceuticals, Stemline Therapeutics, Taiho Pharmaceutical, and Takeda Pharmaceuticals. Dr Mirabile and Dr Ursu have no conflicts of interest to report.
REFERENCES
- Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620-625.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
- Mi J-Q, Li J-M, Shen Z-X, et al. How to manage acute promyelocytic leukemia. Leukemia. 2012;26:1743-1751.
- Sanz MA, Montesinos P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood. 2014;123:2777-2782.
- Tretinoin capsules, for oral use [prescribing information]. Par Pharmaceutical; 2023.
- Trisenox (arsenic trioxide) injection, for intravenous use [prescribing information]. Teva Pharmaceuticals USA; 2022.
- Tibsovo (ivosidenib) tablets, for oral use [prescribing information]. Servier Pharmaceuticals; 2023.
- Idhifa (enasidenib) tablets, for oral use [prescribing information]. Bristol-Myers Squibb; 2022.
- Xospata (gilteritinib) tablets, for oral use [prescribing information]. Astellas Pharma; 2022.
- Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504-510.
- Montesinos P, Sanz MA. The differentiation syndrome in patients with acute promyelocytic leukemia: experience of the pethema group and review of the literature. Mediterr J Hematol Infect Dis. 2011;3(1):e2011059.
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. November 27, 2017. Accessed December 22, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP. The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med. 1992;117(4):292-296.
- Vahdat L, Maslak P, Miller WJ, et al. Early mortality and the retinoic acid syndrome in acute promyelocytic leukemia: impact of leukocytosis, low-dose chemotherapy, PMN/RAR-alpha isoform, and CD13 expression in patients treated with all-trans retinoic acid. Blood. 1994;84(11):3843-3849.
- Sanz MA. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the pethema group. Blood. 2003;103(4):1237-1243.
- Sanz MA, Montesinos P, Rayón C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137-5146.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Acute Myeloid Leukemia. Version 6.2023. October 24, 2023. Accessed December 22, 2023. www.nccn.org/professionals/physician_gls/pdf/aml.pdf
- Tallman MS, Altman JK. How I treat acute promyelocytic leukemia. Blood. 2009;114:5126-5135.
- Du X, Hu H. The roles of 2-hydroxyglutarate. Front Cell Dev Biol. 2021;9:651317.
- Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722-731.
- DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378:2386-2398.
- Zheng R, Friedman AD, Levis M, et al. Internal tandem duplication mutation of FLT3 blocks myeloid differentiation through suppression of C/EBPα expression. Blood. 2004;103:1883-1890.
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381:1728-1740.
- Stein EM, Aldos I, DiPersio JF, et al. Safety and efficacy of menin inhibition in patients with MLL-rearranged and NPM1 mutant acute leukemia: a phase 1, first-in-human study of SNDX-5613 (AUGMENT 101). Presented at: American Society of Hematology Annual Meeting & Exposition 2021; December 11-14, 2021. Abstract 699.
- McGeehan J. A first-in-class Menin-MLL1 antagonist for the treatment of MLL-r and NPM1 mutant leukemias. Presented at: 2020 AACR Virtual Annual Meeting I; April 27-28, 2020. Abstract DDT01-01.
- Wang ES, Altman JK, Pettit KM, et al. Preliminary data on a phase 1/2a first in human study of the menin-KMT2A (MLL) inhibitor KO-539 in patients with relapsed or refractory acute myeloid leukemia. Blood. 2020;136(Supple1):7-8.
- Tucker N. FDA lifts partial clinical hold from KOMET-001 in R/R AML. Targeted Oncol. January 24, 2022. Accessed December 22, 2023. www.targetedonc.com/view/fda-lifts-partial-clinical-hold-from-komet-001-in-r-r-aml
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111-121.
- Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120:1570-1580.
- Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30.
- Limper AH, Knox KS, Sarosi GA, et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96-128.
- Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116:3751-3757.
- Pollyea DA, Tallman MS, de Botton S, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019;33:2575-2584.
- Montesinos P, Recher C, Vives S, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386:1519-1531.
- DiNardo CD, Schuh AC, Stein EM, et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021;22:1597-1608.
- Wang ES, Montesinos P, Minden MD, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140:1845-1857.
- Annamaraju P, Gopishetty S, Goparaju N, et al. Thyroiditis: a rare manifestation of enasidenib-induced differentiation syndrome. Case Rep Oncol. 2020;13:583-587.
- Kallen ME, Koka R, Singh Z, et al. Teenage mutant neutrophilic precursors: leukemia cutis with IDH2 mutation on enasidenib therapy. Leuk Res. 2020;96:106406.