Acute lymphoblastic leukemia (ALL) is a hematologic malignancy that is most frequent in pediatric patients, with a median age at diagnosis of 17 years, but that has a second incidence peak in older adults at approximately age 50.1,2 The treatment for ALL consists of multiple chemotherapy phases to achieve and maintain remission followed by hematopoietic stem cell transplantation or maintenance therapy to prevent relapse, depending on the disease risk.3 Examples of treatment regimens for ALL per guidelines from the National Comprehensive Cancer Network include hyper-CVAD, CALGB 8811, and ECOG 1910 in adults and CALGB 10403 (C10403), COG AALL0434, and the DFCI ALL regimen based on the DFCI Protocol 00-01 in adolescents and young adults.3 Maintenance therapy consists of a backbone of daily oral 6-mercaptopurine (6-MP) and weekly oral methotrexate (MTX) with vincristine. In C10403, this is given for 2 or 3 years from the start of the interim maintenance treatment phase. The combination of 6-MP and MTX was first shown to prolong remission in the mid-1900s and has since become standard of care, but is often associated with adverse events, including myelosuppression and hepatotoxicity.4-6
MTX and 6-MP are antimetabolite agents associated with large interpersonal variations in pharmacokinetic properties and total drug exposure.7 The prodrug 6-MP undergoes metabolism via multiple pathways, including NUDT15 and TPMT.7-9 Genetic variations in these enzymes are known contributors to interpersonal variations in adverse events.9 It is recommended to consider pre-emptive TPMT and NUDT15 testing to guide initial dosing,3 but this practice is not standardized. Specifically, empiric dose reductions are recommended if patients are heterozygous or homozygous for a no-function allele because this confers intermediate and poor metabolism of thiopurines, respectively. The folate antimetabolite MTX prevents the formation of reduced folates and purine synthesis by inhibiting dihydrofolate reductase and thymidylate synthetase.7,10 MTX undergoes polyglutamation to increase intracellular retention.7 The polyglutamated form accumulates in red blood cell precursors and may be retained in erythrocytes throughout their life span. Variations in the levels of polyglutamated MTX in erythrocytes and polymorphisms in target enzymes are thought to contribute to inter- and intrapersonal differences in treatment effects.7
Because these agents display unpredictable pharmacokinetic and pharmacodynamic parameters, the recommended doses of 6-MP and MTX in treatment protocols are considered starting doses and must be individually adjusted based on adverse events and the degree of myelosuppression.3,7,8,11 For example, during maintenance treatment, C10403 investigators recommended increasing the dose of 6-MP and/or MTX to target an absolute neutrophil count (ANC) of 750 to 1500 cells/mm3.11 For hematologic adverse events, they recommended holding 6-MP and MTX for grade 4 neutropenia or grade >2 thrombocytopenia and to restart at full dose, unless it was a recurrence. For hepatotoxicity, the C10403 protocol recommended holding MTX for grade 4 aspartate transaminase (AST) and/or alanine transaminase (ALT) elevations or bilirubin >2 mg/dL. The prescribing information for MTX and 6-MP provide minimal guidance; for MTX, withholding or discontinuing treatment is recommended for adverse events, and for 6-MP, treatment adjustment is recommended for excessive hematologic adverse events.10,12
Although treatment protocols, such as C10403 mentioned above, often include guidance on dose modifications, some obscurity within adolescents and young adults exists. Adolescents and young adults can have increased difficulty with adherence, which can be a result of multiple factors, including emotional distress and social support.13,14 In addition, physician compliance with protocol adherence fluctuates and may be impacted by personal experience and observed treatment-related adverse events.15 Some reports show increased adverse events in adolescents and young adults who received pediatric-inspired protocols, however this finding is inconsistent.14,16 Ultimately, compliance to the protocol guidelines, patient adherence, and physician experience with maintenance therapy and dose adjustments vary. This leads to inconsistencies in clinical practice and changes to therapy without increased risks for progression or relapse based on the available literature statement.14,15 A recent study showed superior survival in adolescents and young adults who received pediatric-style chemotherapy instead of hematopoietic stem cell transplantation in first remission, and guidance on how to best dose adjust these patients to balance efficacy and treatment-related adverse events is needed.16 Given these uncertainties, the goal of this study was to describe real-world dose modifications for MTX and 6-MP and to assess the tolerability of maintenance therapy in young adults.
Methods
This retrospective analysis of young adults with newly diagnosed B-cell or T-cell ALL or lymphoblastic lymphoma was conducted at Cleveland Clinic Taussig Cancer Center in Cleveland, OH. The medical records of all patients aged 18 to 39 years who received C10403 induction therapy between January 1, 2015, and January 1, 2020, were reviewed. Patients who did not receive maintenance therapy per C10403 were excluded from the study.
The primary study objective was to characterize the dose adjustments (reductions, interruptions, and escalations) for MTX and 6-MP during maintenance therapy. The secondary study objectives included characterizing the starting doses for MTX and 6-MP, describing the adverse events related to treatment with 6-MP and MTX, and assessing the incidence of relapse.
The patients’ baseline characteristics and laboratory values were collected on day 1 of maintenance therapy. The disease characteristics, including cell lineage, CD20 status, and the presence of extramedullary disease at diagnosis, were collected. Postinduction minimal residual disease (MRD) status, measured via 6-color flow cytometry assay using a threshold of <0.01% bone marrow mononuclear cells, was recorded.18 The risk category at diagnosis was based on cytogenetic features per the C10403 protocol (Appendix Table S1, available Here).11,19
For all dose interruptions, reductions, or escalations, the medication was adjusted and the date, rationale, and new dose were recorded. For dose interruptions, the duration and dose on treatment reinitiation were assessed. The adverse events were assessed per the Common Terminology Criteria for Adverse Events version 5.0.20 Patients were reviewed throughout the duration of maintenance therapy or until disease relapse, as appropriate. For patients receiving ongoing therapy at the time of the data analysis, the data cutoff was on January 18, 2021. Relapse was defined as a reappearance of >5% blasts in the blood, bone marrow, or in any extramedullary site after a complete remission, or CNS relapse (defined as new CNS-3 status or new clinical signs of CNS leukemia).3 Dose intensity during maintenance therapy was calculated by dividing the total dose received per patient by the total duration of maintenance treatment that the patient received using 525 mg/m2 and 20 mg/m2 as the cumulative weekly starting doses for 6-MP and MTX, respectively, per C10403, and reported as a percent.11
Statistical Analysis
The patients’ demographics and outcomes were assessed using descriptive statistics, including median (range) and number (percent).
Results
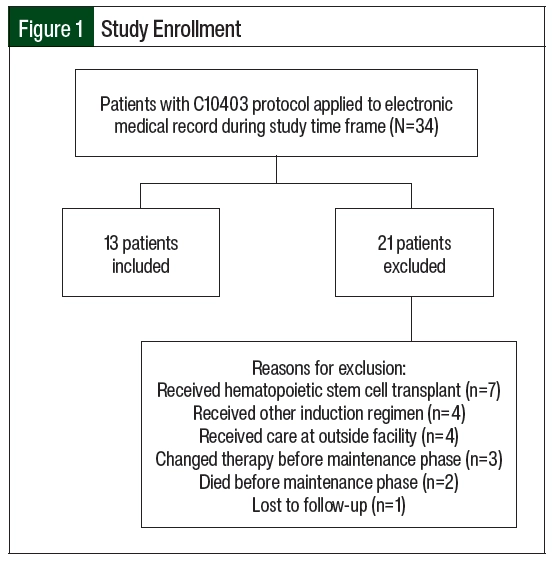
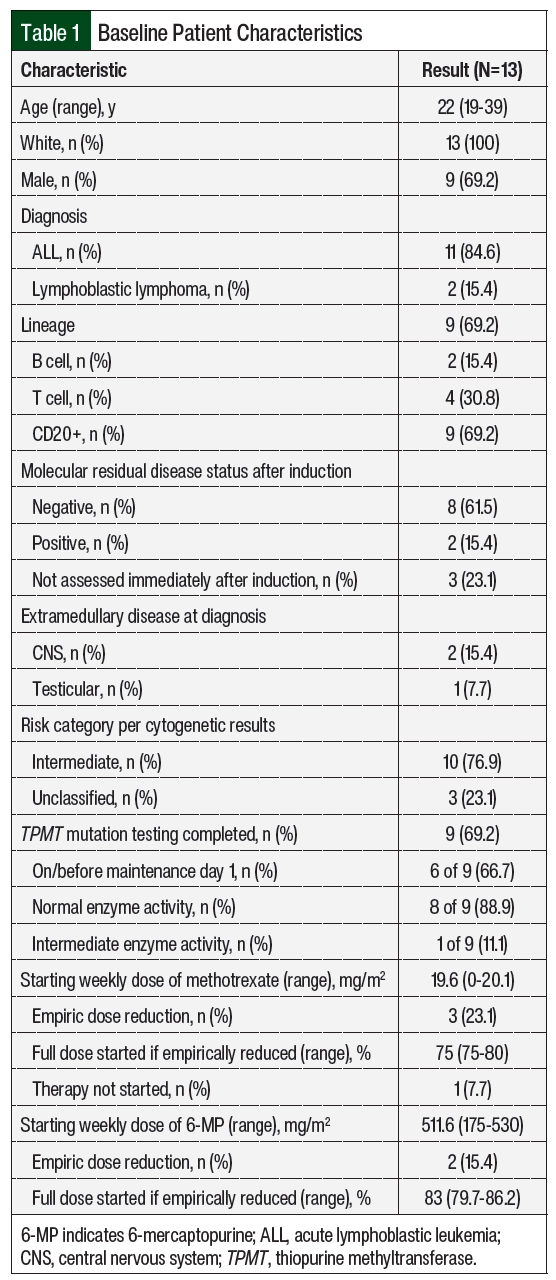
Of the 34 screened patients, 13 were included in the study (Figure 1). The most common reason for study exclusion was proceeding to hematopoietic stem cell transplantation. The patients’ baseline characteristics and patient-specific treatment data are shown in Table 1 and Table 2, respectively. The median age at the start of maintenance therapy was 22 years (range, 19-39 years). The postinduction MRD status was checked in the 10 patients with ALL and was positive in 2 of them. A total of 10 patients had intermediate-risk disease per cytogenetics and 3 patients’ diseases were unclassified. One patient with intermediate-risk disease had trisomy 21 with known Down syndrome. No patients had Philadelphia chromosome–positive disease.
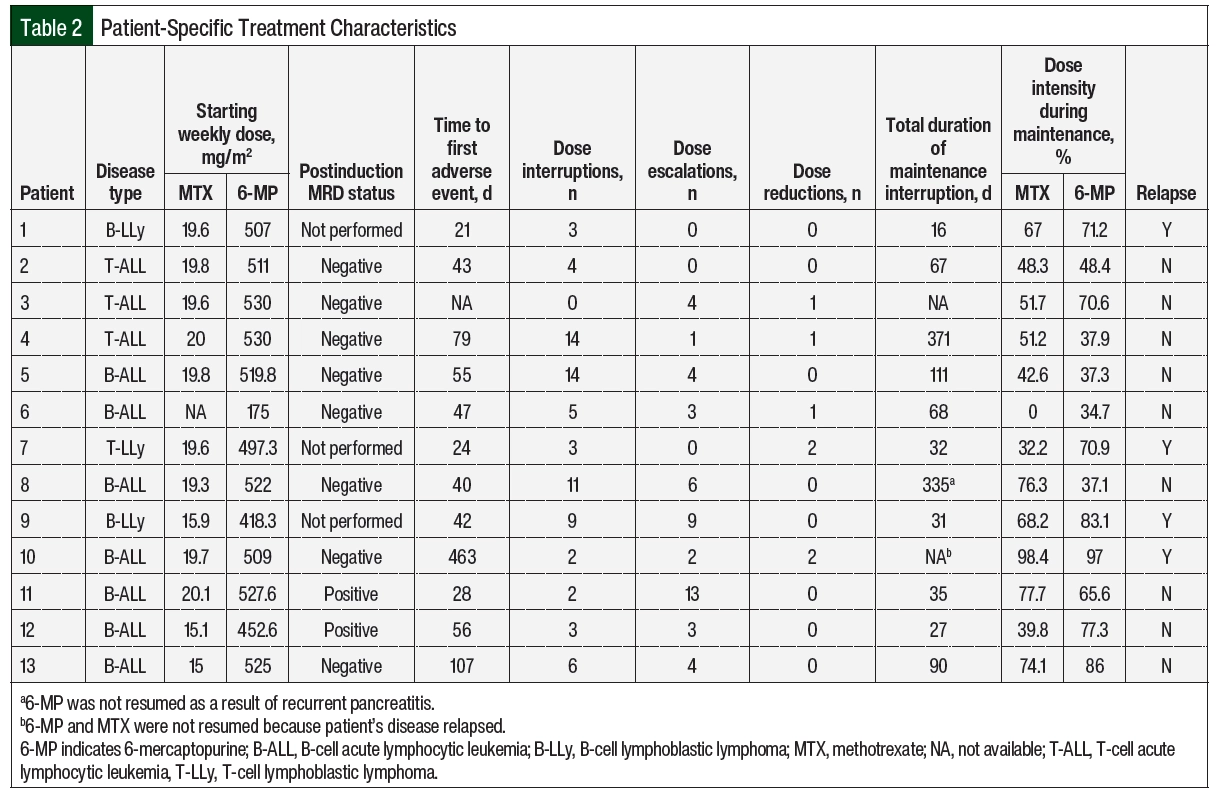
A total of 9 patients were tested for TPMT mutations; 6 of the patients were tested on or before the start of maintenance therapy and 3 were tested during the maintenance treatment. Normal enzyme activity was detected in 8 (89%) of the tested patients. Of the 3 patients tested during maintenance therapy, 1 had the TPMT*1/*3A genotype, conferring intermediate enzyme activity (Patient 5 in Table 2). The median starting weekly doses of 6-MP and MTX were 511.6 mg/m2 (range, 175-530 mg/m2) and 19.6 mg/m2 (range, 0-20.1 mg/m2), respectively. Patient 6 did not receive oral MTX as a result of prolonged bone marrow recovery with previous treatment phases, which is thought to be related to trisomy 21 and the patient’s known Down syndrome, and 6-MP was empirically reduced by 66.7%. Patients 12 and 13 had both agents adjusted for abnormal liver enzymes, and Patient 9 for a healing rectal fistula.
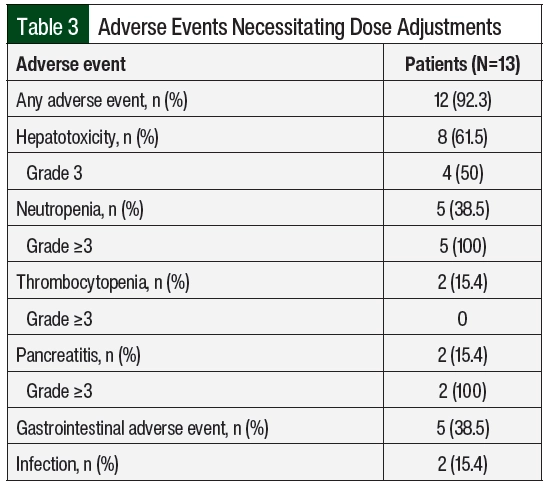
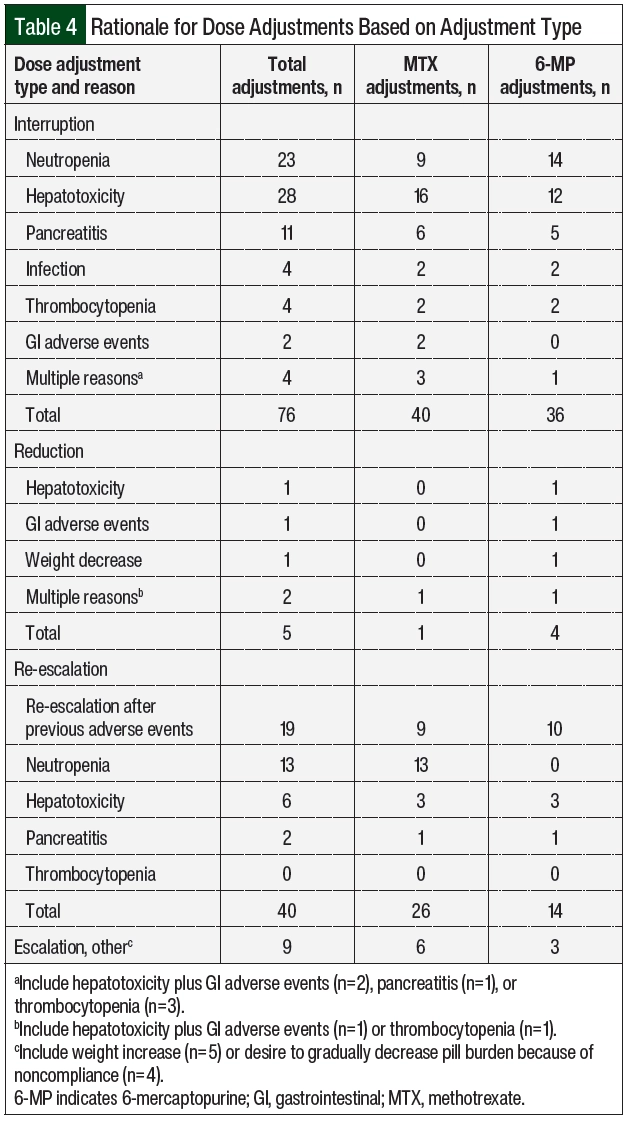
A total of 12 (92%) patients had adverse events necessitating dose adjustments (Table 3), of which hepatotoxicity (61.5%) and neutropenia (38.5%) were the most frequent. The median time to the first adverse events was 43 days from day 1 of maintenance therapy. Patient 10 had no previous adverse events, had neutropenia on day 463, and later had relapsed disease; this patient was not included in the calculation of the time to adverse events. A total of 130 dose adjustments were required, including 76 (58.5%) interruptions, 5 (3.8%) reductions, and 49 (37.7%) escalations.
Table 4 summarizes the rationale for dose modification based on the adjustment type and Table 2 shows the number of adjustments per patient. The reasons for dose interruptions were neutropenia in 23 cases (grade ≥3, 20), hepatotoxicity in 28 cases (grade 3, 11), pancreatitis in 11 cases (grade ≥3, 11), and infection in 4 cases. In all but 11 interruptions, 6-MP and MTX were held at the same time. Dose reductions were rare and mainly resulted from hepatotoxicity or gastrointestinal (GI) adverse events. Of the 49 dose escalations, 40 (81.6%) were re-escalations after treatment with 6-MP and/or MTX was previously interrupted as a result of adverse events and was restarted at a lower dose, mainly neutropenia (n=13) and hepatotoxicity (n=6). There were no escalations above the recommended 525 mg/m2 and 20 mg/m2 dosing for 6-MP and MTX based on neutrophil count. The median duration of the total time off of one or both agents throughout the duration of maintenance was 67 days (Table 2).
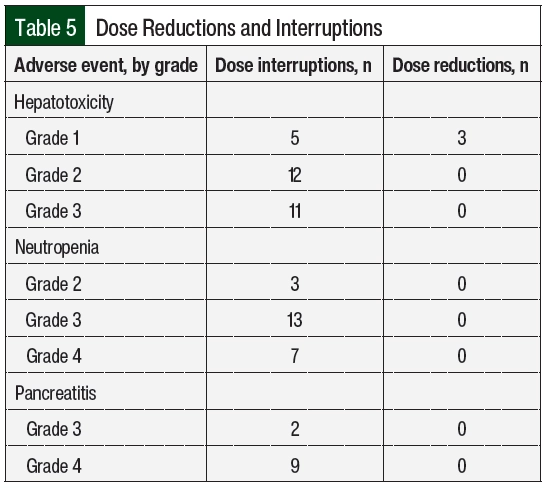
For hepatotoxicity, the most common dose adjustments were dose interruptions, regardless of the severity of the adverse event (Table 5). Treatment with MTX and 6-MP was interrupted in 16 and 12 cases, respectively. In the 3 cases where doses were reduced for grade 1 hepatotoxicity, AST or ALT increased within 2 months and required dose interruption. The durations of interruption were 8, 16, and 20 days for grades 1, 2, and 3 hepatotoxicity, respectively (Appendix Table S2, available Here). At the time the doses were reinitiated, 9 patients had AST or ALT within normal limits, 17 had grade 1 transaminitis, and 1 patient had grade 2 transaminitis. A total of 3 patients had therapy restarted at a reduced dose after the first episode of hepatotoxicity. The doses were restarted at a reduced dose in 15 cases, with a median dose reduction of 30% to 40% (Appendix Table S2) and were subsequently re-escalated in 13 cases. Concomitant hepatotoxic medications were reviewed, including fluconazole (n=8 patients), sulfamethoxazole plus trimethoprim (n=6), cyproheptadine (n=1), and the regular use of acetaminophen (n=1). In addition, 1 patient was receiving doses of pegaspargase that were missed during the previous cycles as a result of adverse events, which was subsequently held during the time of hepatotoxicity. For grade 3 hepatotoxicity, fluconazole was held in 6 cases and sulfamethoxazole plus trimethoprim was held in 2 cases.
In all cases where doses were adjusted for neutropenia, dose interruptions were employed (Table 5). Most interruptions occurred with grade 3 neutropenia. The median interruption durations were 9 and 18 days for grade 3 and grade 4 neutropenia, respectively (Appendix Table S2). A total of 3 interruptions occurred with grade 2 neutropenia for a median of 13 days (range, 13-21.5 days). At the time the doses were reinitiated, 18 cases occurred with ANC >1500 cells/mm3 and 3 cases occurred with grade 3 neutropenia (ANC range, 820-860 cells/mm3). A total of 3 patients had therapy restarted at a reduced dose after the first episode of neutropenia. The doses were restarted at a reduced dose in 15 of the 23 interruptions, with a slightly greater reduction for grade 4 neutropenia than with other grades of neutropenia (Appendix Table S2) and were subsequently re-escalated in 19 cases.
In addition to therapy interruptions, other interventions for neutropenia included evaluating TPMT enzyme activity (n=2), administering granulocyte-colony stimulating factor as a result of extenuating circumstances that limited close follow-up (n=2), and initiating antibacterial prophylaxis with fluoroquinolones when the ANC decreased to <500 cells/mm3. Of note, Patient 5 had intermediate TPMT enzyme activity at the time of the third episode of neutropenia that led to the interruption of both agents. This patient required 13 interruptions because of neutropenia over a 1-year period. Once the intermediate TPMT enzyme activity was identified, the patient continued to receive treatment with MTX and 6-MP with doses reduced by 62.5% and 66.7%, respectively, with no neutropenia recurrence.
Pancreatitis occurred in 2 patients and led to 11 dose interruptions (Table 4) for grade 3 (n=2) and grade 4 (n=9) adverse events (Table 5). In all cases, therapy was held until the resolution of symptoms and the normalization of serum lipase, for a median of 36 days (range, 2-50 days). In 1 patient, 6-MP treatment was not resumed after the fourth recurrence. The doses were reinitiated at a lower dose in 4 of the 9 cases of grade 4 pancreatitis, with a median dose reduction of approximately 50%. The doses were re-escalated after grade 4 pancreatitis 6 times (Appendix Table S2). In 2 cases of 6-MP and MTX being re-escalated to the full dose, pancreatitis recurred after 2 months, leading to therapy interruption.
The less common adverse events that led to dose adjustments included thrombocytopenia, GI adverse events, and infection (Table 4). Grade 2 thrombocytopenia was the cause of 4 interruptions of MTX and 6-MP between 2 different patients. Therapy was held for a median of 13 days (Appendix Table S2). The doses were reduced after the first episode of thrombocytopenia in both patients. On treatment resumption, MTX was reduced in 1 patient by 50% and 6-MP was reduced in the other patient by 33.3%. GI adverse events (nausea, vomiting, and/or diarrhea) were the sole reason for dose adjustments in 3 cases (Table 4). In both cases that included treatment with MTX, therapy was held for 7 days and 16 days and was restarted with a 25% dose reduction. In the 1 case that included 6-MP, the dose was reduced by 33.3%. In all cases, therapy was modified at the same time that supportive care, such as antidiarrheal or antiemetic medications, was started, and the symptoms improved per the documentation. Infection led to interruptions 4 times during the study period among 2 of the patients (Table 4). In both patients, treatment was resumed after the infection resolved, with a total of 32 days and 8 days off therapy for each patient. There was no recurrence of infection after resumption of the treatment.
Overall, 7 (56%) patients completed maintenance during the study period. A total of 3 patients had ongoing treatment at the time of the data cutoff. The median duration of maintenance therapy from day 1 of the interim maintenance treatment was 2.2 years (range, 0.59-3.07 years). A total of 4 (30.8%) patients had disease relapse during the study period (Table 2). Of these patients, 2 had B-cell ALL, 1 had B-cell lymphoblastic lymphoma, and 1 had T-cell lymphoblastic lymphoma. All patients who underwent postinduction MRD testing were negative, and all were intermediate risk based on cytogenetic testing per C10403 at diagnosis.11 In all, 3 of the 4 patients (Patients 1, 7, and 10) relapsed during maintenance at a median of 227 days (range, 97-463), and Patient 9 relapsed 92 days after the completion of therapy. The patient-specific dose intensities of 6-MP and MTX are shown in Table 2. The median dose intensities for 6-MP and MTX were 48.4% (range, 37.1%-86%) and 51.2% (range, 0%-77.7%), respectively, in those who did not relapse and 77.1% (range, 70.9%-97%) and 67.58% (range, 32.2%-98.4%), respectively, in those who did Appendix Figure S1 (available Here).
Discussion
In this study, we assessed 6-MP and MTX dose adjustments during maintenance therapy in young adults with ALL or lymphoblastic lymphoma who received treatment within a 5-year time frame at a large academic institution. All but 1 patient had adverse events leading to a dose adjustment of 6-MP and/or MTX. The most frequent dose adjustments were dose interruptions and subsequent re-escalations after previous adverse events.
Although guideline recommendations and the C10403 protocol support adjusting the doses of 6-MP and MTX based on adverse events and the degree of myelosuppression, some reports suggest that increasing the doses of 6-MP and/or MTX based on the degree of myelosuppression may be less frequent compared with reducing or interrupting treatment because of adverse events.3,11,21,22 Several observational studies have assessed the correlation between low ANC or WBC and a reduced risk for relapse during maintenance therapy with mixed results.23-25 The results of the Nordic Society of Pediatric Hematology and Oncology ALL-92 trial showed that patients who relapsed had significantly higher ANC levels than those who stayed in remission, and those with a median ANC of <2000 cells/mm3 had superior outcomes compared with those with a median ANC above the cutoff.26 However, increasing the dose of these agents beyond the recommended starting dose is often limited by adverse events, as in this analysis where 12 of the 13 patients had adverse events that necessitated dose reductions and/or interruptions that were mainly a result of hepatotoxicity and neutropenia.
Throughout the 5-year time frame in this analysis, there were no dose escalations to maintain a certain degree of myelosuppression. All dose increases were re-escalations towards the target initial doses of 6-MP and MTX after these agents were previously interrupted and restarted at a lower dose as a result of adverse events, aside from 1 patient whose oral chemotherapy doses were gradually titrated to the target initial dose for adherence because of a known history of noncompliance. As previously mentioned, adherence can be challenging in younger patients. A systematic review of literature regarding oral chemotherapy adherence as a maintenance treatment in patients aged ≤39 years showed that nonadherence to oral chemotherapy increases the risk for relapse.27 This review showed lower rates of adherence in older patients; however, only 7 of the 37 studies in the review included adolescents and young adults. One patient with Down syndrome did not receive MTX during maintenance because of the prolonged time to bone marrow recovery with previous treatment cycles. The increased risk for treatment-related adverse events, particularly with MTX, has been well described in this vulnerable population, and dose modifications are often employed.28
The most frequent adverse events leading to dose interruptions in our study were hepatotoxicity and neutropenia. The interruption durations varied depending on the degree of adverse events as previously described. Therapy was resumed at a reduced dose in approximately half of all of the interruptions. The doses were re-escalated towards the recommended target dose, but none of the patients had doses that increased beyond this based on neutrophil count. In the C10403 protocol, dose adjustment recommendations are provided for oral 6-MP and MTX during the maintenance phase based on adverse events. For hematologic adverse events, it is recommended in C10403 to hold 6-MP and MTX only for grade 4 neutropenia or grade ≥2 thrombocytopenia and to resume treatment at full dose once ANC or platelets are ≥750 cells/mm3 and 75,000/µL, respectively.11 If hematologic adverse events recur, C10403 investigators recommended holding treatment as previously stated and resume therapy at 50% of the original dose, with dose increases by 25% every 2 to 4 weeks.11
In this study, all 27 cases of hematologic adverse events were managed with dose interruptions. Of these 27 cases, only 7 involved interruptions for grade 4 neutropenia or grade ≥2 thrombocytopenia; the remaining occurred with less severe adverse events. Of the 6 unique patients whose therapy was interrupted for hematologic adverse events, 5 patients’ doses were reduced after the first episode of a hematologic adverse event, showing some discrepancy from the C10403 protocol recommendations. For hepatotoxicity, the C10403 protocol recommends holding only MTX for grade 4 AST/ALT elevations or bilirubin >2 mg/dL. Elevated AST or ALT was the most common indication for dose interruptions in this study, although none of the patients had grade 4 adverse events. For GI adverse events, C10403 recommends holding MTX for grade 3 diarrhea or vomiting until the resolution of symptoms (to grade <2) and resuming treatment at 50% of the previous dose with re-escalation as tolerated. In our analysis, GI adverse event grading was not routinely documented, but therapy was held for all cases. It is not recommended to hold 6-MP or MTX for pancreatitis in the protocol.
The potential impact of dose intensity on patient outcomes has been previously explored. Because frequent hematologic adverse events necessitating dose reductions and/or interruptions were observed in practice when this combination therapy was first adopted, Pinkel and colleagues sought to determine if lower doses of 6-MP and MTX have similar efficacy with reduced treatment-related morbidity.29 Children with ALL received full- or half-dose 6-MP and MTX, of which the half doses were proved inferior for maintaining remission. The relationship between dose intensity and/or the duration of treatment interruptions and the risk for relapse in pediatric patients has been reported in several publications, with some results showing a trend towards increased rates of relapse in patients who received lower cumulative doses of these oral chemotherapy agents, whereas other studies have not shown a correlation.21,26,30,31 Of note, most of these studies were conducted decades ago. Significant changes in practice and disease monitoring have occurred since then, such as the advent of MRD testing with more sensitive methods, including ClonoSEQ next-generation sequencing technology, which may be used to better guide the need to intensity or de-intensify therapy.32
In the present study, the median duration of maintenance treatment interruption was 67 days. The median dose intensities of 6-MP and MTX were slightly higher in patients who had relapsed disease than in those who did not. In addition, the relapse rate in our population was similar to the rate in C10403.11 These comparisons in our study are limited, however, because of the small sample size.
Limitations
Given its retrospective nature, our study has several limitations. First, the study design was dependent on a chart review of the electronic medical record to review laboratory values, treatment decisions, and the resolution of patient-reported symptoms. Any missing documentation may have impacted the evaluation of treatment modifications. The grading of GI adverse events was not routinely documented, and vincristine and steroid dose modifications were not included in this analysis. Adherence to 6-MP and MTX was unable to be assessed in this retrospective analysis.
There was also variability in the frequency of laboratory follow-up among the study population, and some patients may have had laboratory draws completed externally, which may impact the frequency of the dose adjustments. In addition, TPMT testing before the initiation of 6-MP and metabolite testing to assess adherence to 6-MP was not standardized. Last, because this was a retrospective analysis at a single study center with a primarily White study population, the ability to extrapolate these data to other institutions may be limited given the potential differences in the practice and patient populations.
Conclusion
In this single-center, retrospective study, we assessed the real-world management of 6-MP and MTX in young adults with ALL or lymphoblastic lymphoma during C10403 maintenance. Our results show that adverse events requiring dose adjustments were common in this population. The method of dose adjustments, however, varied from the recommended C10403 dose modifications, reiterating the growing body of literature regarding the uncertainty of optimal monitoring, dose modifications, adherence assessment, and maintenance therapy intensity in this patient population. Future studies with a larger population that assess the relationship between dose intensity and relapse risk in the era of modern disease monitoring are needed.
References
- Jabbour EJ, Faderl S, Kantarjian HM. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2005;80:1517-1527.
- National Cancer Institute SEER Program. Cancer stat facts: acute lymphocytic leukemia. Accessed April 24, 2023. www.seer.cancer.gov/statfacts/html/alyl.html
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Lymphoblastic Leukemia. Version 3.2023. October 9, 2023. Accessed January 30, 2024. www.nccn.org/professionals/physician_gls/pdf/all.pdf
- Frei E III, Karon M, Levin RH, et al. The effectiveness of combinations of antileukemic agents in inducing and maintaining remission in children with acute leukemia. Blood. 1965;26:642-56.
- Toksvang LN, Lee SHR, Yang JJ, et al. Maintenance therapy for acute lymphoblastic leukemia: basic science and clinical translations. Leukemia. 2022;36:1749-1758.
- Ebbesen MS, Nygaard U, Rosthoj S, et al. Hepatotoxicity during maintenance therapy and prognosis in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2017;39:161-166.
- Schmiegelow K, Nielsen SN, Frandsen TL, Nersting J. Mercaptopurine/methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: clinical facts and fiction. J Pediatr Hematol Oncol. 2014;36:503-517.
- Mobargha A. Potential dose-adjustment parameters during 6MP/MTX-therapy for acute lymphoblastic leukemia. EC Paediatrics. 2015;1:62-71.
- Relling MV, Schwab M, Whirl-Carillo M, et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin Pharmacol Ther. 2019;105:1095-1105.
- Methotrexate tablets, for oral use [prescribing information]. Mylan Pharmaceuticals Inc; October 2022. Accessed January 30, 2024. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=70c09984-2b36-424f-8b27-3fd0cd4e833d&type=display
- Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133:1548-1559. Erratum in: Blood. 2019;134:1111.
- Purinethol (mercaptopurine) tablets, for oral use [prescribing information]. Stason Pharmaceuticals, Inc; May 2021. Accessed January 30, 2024. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=bfb03539-907f-4424-9c88-6a03ed9467e6&type=display
- Kondryn HJ, Edmondson CL, Hill J, Eden TOB. Treatment non-adherence in teenage and young adult patients with cancer. Lancet Oncol. 2011;12:100-108.
- Advani AS, Larsen E, Laumann K, et al. Comparison of CALGB 10403 (Alliance) and COG AALL0232 toxicity results in young adults with acute lymphoblastic leukemia. Blood Adv. 2021;5:504-512.
- Kristjánsdóttir ER, Toksvang LN, Schmiegelow K, Rank CU. Prevalence of non-adherence and non-compliance during maintenance therapy in adults with acute lymphoblastic leukemia and their associates with survival. Eur J Haematol. 2022;108:109-117.
- Hough R, Rowntree C, Goulden N, et al. Efficacy and toxicity of a paediatric protocol in teenagers and young adults with Philadelphia chromosome negative acute lymphoblastic leukaemia: results from UKALL 2003. Br J Haemaol. 2016;172:439-451.
- Wieduwilt MJ, Stock W, Advani A, et al. Superior survival with pediatric-style chemotherapy compared to myeloablative allogeneic hematopoietic cell transplantation in older adolescents and young adults with Ph-negative acute lymphoblastic leukemia in first complete remission: analysis from CALGB 10403 and the CIBMTR. Leukemia. 2021;35:2076-2085. Erratum in: Leukemia. 2021;35:2140.
- Wood B, Wu D, Crossley B, et al. Measurable residual disease detection by high-throughput sequencing improves risk stratificiation for pediatric B-ALL. Blood. 2018;131:1350-1359.
- Stock W, Johnson JL, Stone RM, et al. Dose intensification of daunorubicin and cytarabine during treatment of acute lymphoblastic leukemia: results of Cancer and Leukemia Group B study 19802. Cancer. 2013;119:90-98.
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. November 27, 2017. Accessed November 3, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- Peeters M, Koren G, Jakubovicz D, Zipursky A. Physician compliance and relapse rates of acute lymphoblastic leukemia in children. Clin Pharmacol Ther. 1988;43:228-232.
- de Oliveira BM, Macedo Valadares MT, Rezende Silva M, Borato Viana M. Compliance with a protocol for acute lymphoblastic leukemia in childhood. Rev Bras Hematol Hemoter. 2011;33:185-189.
- Schmiegelow K, Pulcynska MK, Seip M. White cell count during maintenance chemotherapy for standard-risk childhood acute lymphoblsatic leukemia: relation to relapse rate. Pediatr Hematol Oncol. 1988;5:259-267.
- Relling MV, Hancock ML, Boyett JM, et al. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999;93:2817-2823.
- Dolan G, Lilleyman JS, Richards SM. Prognostic importance of myelosuppression during maintenance treatment of lymphoblastic leukaemia. Arch Dis Child. 1989;64:1231-1234.
- Schmiegelow K, Björk O, Glomstein A, et al. Intensification of mercaptopurine/methotrexate maintenance chemotherapy may increase the risk of relapse for some children with acute lymphoblastic leukemia. J Clin Oncol. 2003;21:1332-1339.
- Zeng XL, Heneghan MB, Badawy SM. Adherence to oral chemotherapy in acute lymphoblastic leukemia during maintenance therapy in children, adolescents, and young adults: a systematic review. Curr Oncol. 2023;30:720-748.
- Izraeli S, Vora A, Zwaan CM, Whitlock J. How I treat ALL in Down’s syndrome: pathobiology and management. Blood. 2014;123:35-40.
- Pinkel D, Hernandez K, Borella L, et al. Drug dosage and remission duration in childhood lymphocytic leukemia. Cancer. 1971;27:247-256.
- Yeoh A, Collins A, Fox K, et al. Treatment delay and the risk of relapse in pediatric acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2017;34:38-42.
- Schmiegelow K. Prognostic significance of methotrexate and 6-mercaptopurine dosage during maintenance chemotherapy for childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1991;8:301-312.
- Saygin C, Cannova J, Stock W, Muffly L. Measureable residual disease in acute lymphoblastic leukemia: methods and clinical context in adult patients. Haematologica. 2022;107:2783-2793.