There is a scarcity of real-world evidence on the outcomes of biosimilar use, particularly in oncology. In value-based care initiatives, the use of biosimilar drugs can result in cost-savings and efficiency in the delivery of high-value care because the use of biologic drugs in cancer care increases affordability while minimizing the financial toxicity associated with cancer treatment costs.1 The use of biosimilars can enhance patients’ health outcomes when established efficacy, safety, and the need for lowering patient costs are critically assessed by physicians who are deciding whether to prescribe a biosimilar or its reference drug. The entry of biosimilars to the oncology market has driven market competition, leading to cost-savings by decreasing the cost of biologics.1 A biosimilar is a biochemical drug that is comparable with another biologic agent that has already been FDA approved, which is known as the reference drug.2 Bevacizumab-awwb is the first biosimilar to bevacizumab to be approved in the United States. According to Thatcher and colleagues, bevacizumab-awwb has a safety profile that is similar to bevacizumab in patients with advanced nonsquamous non–small cell lung cancer (NSCLC).3
Originator bevacizumab and its biosimilar bevacizumab-awwb are vascular endothelial growth factor (VEGF) inhibitors.4,5 Bevacizumab binds to the VEGF and blocks contact of the VEGF with its receptors (FLT-1 and KDR) on the surface of endothelial cells.4 When VEGF interacts with its receptors, it causes endothelial cell proliferation and the formation of new blood vessels.4 Bevacizumab-awwb and bevacizumab have been compared based on extensive structural and functional drug characterization, animal data, human pharmacokinetic and pharmacodynamic data, and clinical immunogenicity that demonstrate that bevacizumab-awwb is highly similar to bevacizumab and that there are no clinically meaningful differences between the 2 drugs.3,6
Bevacizumab-awwb and bevacizumab are approved by the FDA in combination with other oncologic drugs for the treatment of metastatic colorectal cancer; NSCLC; metastatic renal cell carcinoma; persistent, recurrent, or metastatic cervical cancer; epithelial ovarian, fallopian tube, or primary peritoneal cancer; and recurrent glioblastoma in adults.4,5 Only bevacizumab is approved for the treatment of hepatocellular carcinoma at the time of this study.4,5
The focus of our financial analysis in this study was to demonstrate value in our formulary changes with the adoption of the bevacizumab biosimilar. Reviewing cost-savings through differences in purchase price was an accessible way to determine the financial impact to our institution. Because our institution has a mixed payer population, we could not assume costs based on Medicare Part B’s spending per dose unit. Moreover, data regarding insurer reimbursement or the direct cost to the patients were not readily available for our study. When using bevacizumab-awwb and dose rounding together, significant savings per dose were achieved compared with bevacizumab.7 However, for the purpose of our analysis we mainly focused on the financial impact of the use of bevacizumab-awwb, regardless of dose rounding. Despite our institution’s dose rounding of reference and biosimilar drugs, dose rounding should not change our analysis.
As healthcare costs continue to rise, biosimilars will be the most cost-effective alternative to minimize the financial toxicity associated with oncologic malignancy treatments.8 In a hypothetical financial analysis model for another bevacizumab biosimilar, bevacizumab-bvzr, the 5-year cost-savings were $7,030,924 for a commercial payer and $4,059,257 for Medicare.9 Demonstrating that reference bevacizumab and biosimilar bevacizumab have a similar safety profile in a wide range of patients with cancer has the potential to broaden treatment options, lower payer costs, and improve patient access to a vital oncology drug. The purpose of this study was to compare the safety of bevacizumab with its biosimilar bevacizumab-awwb in various cancer types, focusing on hypertension, proteinuria, severe hemorrhage, and gastrointestinal (GI) perforation. We also aimed to evaluate the cost-savings of treatment with bevacizumab-awwb in a real-world population through an observational, retrospective cohort study.
Methods
In this retrospective chart review study, we collected data to assess the safety outcomes of originator bevacizumab with its biosimilar bevacizumab-awwb at the University of California San Francisco Helen Diller Comprehensive Cancer Center. The study was approved by the University of California San Francisco Institutional Review Board.
The patients were identified based on administered doses of bevacizumab-awwb or reference bevacizumab from June 2019 to May 2021. The study patients were aged ≥18 years and initiated treatment with bevacizumab-awwb or bevacizumab regardless of their cancer type. To be eligible for inclusion in the bevacizumab-awwb arm, the patients could not have received treatment with bevacizumab. Patients were excluded from the trial if they were enrolled in a clinical study or if they switched treatment from reference bevacizumab to bevacizumab-awwb. Patients who switched from treatment with bevacizumab to bevacizumab-awwb were excluded to solely understand the impact of each drug on hypertension, proteinuria, hemorrhage, and GI perforation. By doing this, we aimed to avoid any possible confounding factors that might impact our primary and secondary outcomes from patients switching from bevacizumab to bevacizumab-awwb.
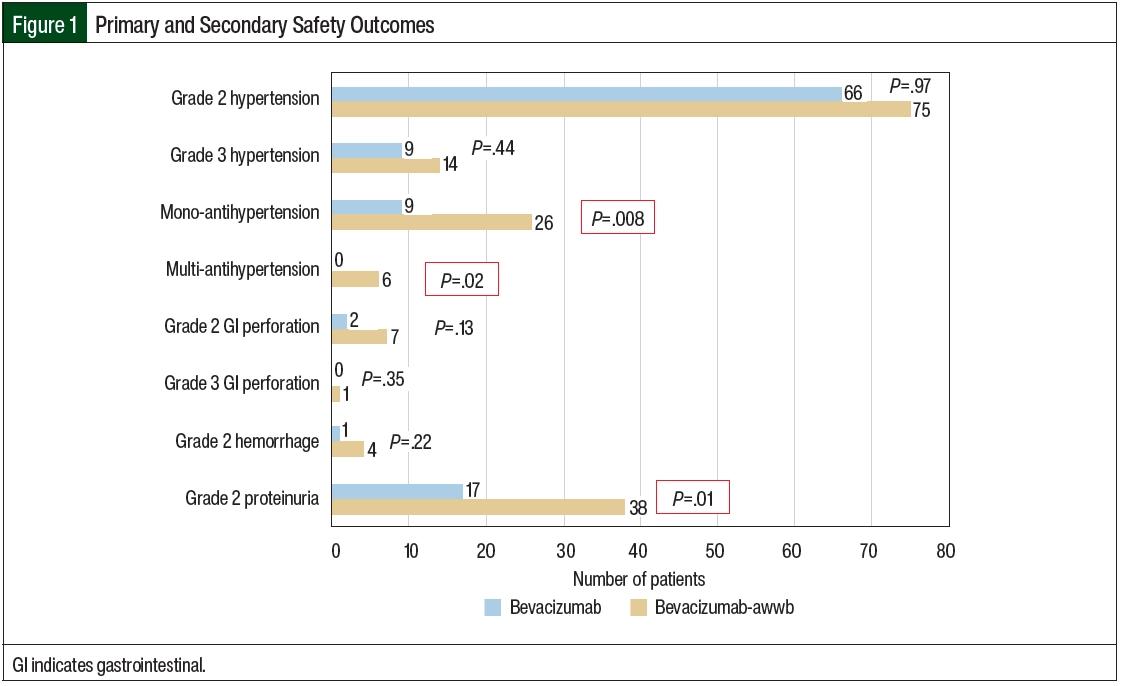
Safety was assessed based on the incidence of new-onset grade ≥2 hypertension, proteinuria, severe hemorrhage, and GI perforation after the initiation of bevacizumab-awwb and bevacizumab according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 (Figure 1; Appendix Table S1, available Here). The primary outcome was safety, with hypertension associated with drug administration being the primary end point. In this study, hypertension was defined as grade ≥2 based on the CTCAE criteria or by the initiation of monotherapy or new combination antihypertensive medications after the initiation of bevacizumab-awwb or bevacizumab. Concomitant antihypertensive medications were assessed at baseline and after each treatment cycle of bevacizumab. The secondary safety outcomes included grade ≥2 proteinuria, severe hemorrhage, and GI perforation.
Laboratory evaluations, including urine protein, were assessed monthly per protocol. The incidence of severe hemorrhage or GI perforation was assessed by reviewing medical records and progress notes. The safety parameters selected were based on severe adverse events that are frequently associated with bevacizumab treatment. The incidence of infusion reactions that can occur with biologics and biosimilar therapies was not included in the secondary outcomes because most patients were receiving other chemotherapy or biologic agents that may have also caused infusion reactions, which would confound the results. Hypertension, proteinuria, and GI perforation are distinctly recognized as common adverse events associated with treatment with bevacizumab-awwb and bevacizumab based on their prescribing information.4,5
A real-world assessment of the financial impact of incorporating oncologic biosimilars in cancer care is essential to maintain high-quality care while minimizing financial burden. An additional secondary outcome was the assessment of cost-savings based on purchase price. The average wholesale acquisition price for bevacizumab-awwb was compared with that of bevacizumab in the cost-savings analysis. Because bevacizumab-awwb and bevacizumab come in 100-mg and 400-mg vials, the overall number of each vial for each dose dispensed was used to calculate the total cost. The costs of care, including administrative costs, related to medication infusion, ancillary medications and procedures, nursing time, and other associated costs were excluded.
Statistical Analysis
Categorical variables were analyzed using chi-square testing of baseline characteristics, as well as the primary and secondary outcomes. Multivariable logistic regression was performed to analyze the odds for hypertension, with the primary measure of interest being the reference drug versus the biosimilar drug administration. In addition, we controlled for the cancer type, weight-based dosing level, total number of doses, and the patients’ weight. The number of doses were categorized in increments of 5 and the patients’ weight was categorized in 20-kg increments. The goodness of fit test was confirmed via Hosmer-Lemeshow testing (P=.44), the model specification was confirmed via link test (_hat, P=.00; _phsq, P=.07), and the model specificity was confirmed via receiver operating characteristic (area under the curve, 0.72). To detect a 15% difference with an 80% power, a calculated sample size of 330 patients was required.10 All statistical analyses were performed using STATA Version 16 (StataCorp; College Station, TX).
Results
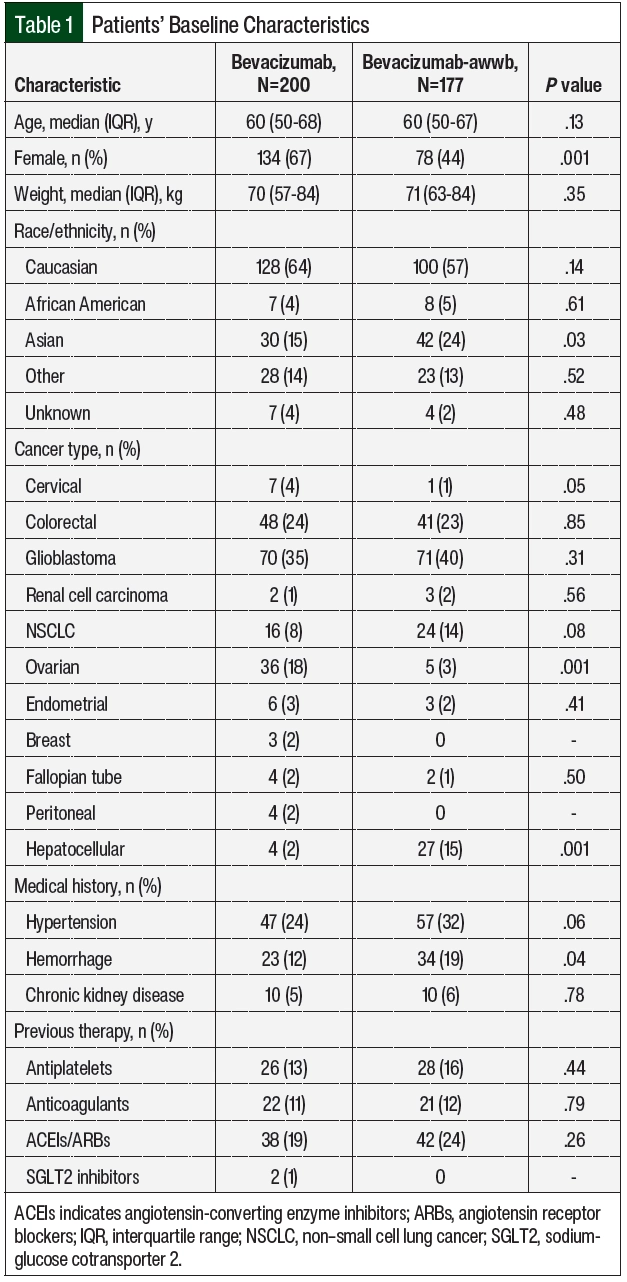
A total of 458 patients received bevacizumab-awwb or bevacizumab between June 1, 2019, and May 31, 2021, at University of California San Francisco Helen Diller Comprehensive Cancer Center. Of the 458 total patients, 377 patients were eligible for study inclusion. There were 177 patients in the bevacizumab-awwb arm, and 200 patients in the bevacizumab arm (Table 1). Of the 81 patients who were excluded from the study, the most common reasons included participation in clinical trials, switching treatment from bevacizumab to bevacizumab-awwb, receiving bevacizumab or bevacizumab-awwb outside of the study’s time frame, or receiving an alternative biosimilar drug. The baseline characteristics were similar between the 2 treatment groups, except the rate of female sex was higher in the bevacizumab group (67% vs 44%, respectively; P=.001) and the percent of Asian patients was higher in the bevacizumab-awwb group (15% vs 24%, respectively; P=.03; Table 1).
A total of 11 cancer types were identified in the study sample, including glioblastoma, NSCLC, colorectal, hepatocellular, ovarian, cervical, endometrial, fallopian tube, peritoneal, breast, and renal carcinoma (Table 1 and Figure 2). Among the bevacizumab-awwb and bevacizumab groups, there were a similar number of patients with each cancer type, except the rate of ovarian cancer was higher in the bevacizumab group (18% vs 3%; P=.001), and the rate of hepatocellular carcinoma was higher in the bevacizumab-awwb group (2% vs 15%; P=.001; Table 1).
Having previous hypertension and chronic kidney disease were similar among both groups, but previous hemorrhage was higher in the bevacizumab-awwb arm than in the bevacizumab arm (19% vs 12%, respectively; P=.04; Table 1). The use of antiplatelet medication, anticoagulation medication, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers was similar among both treatment groups (Table 1).
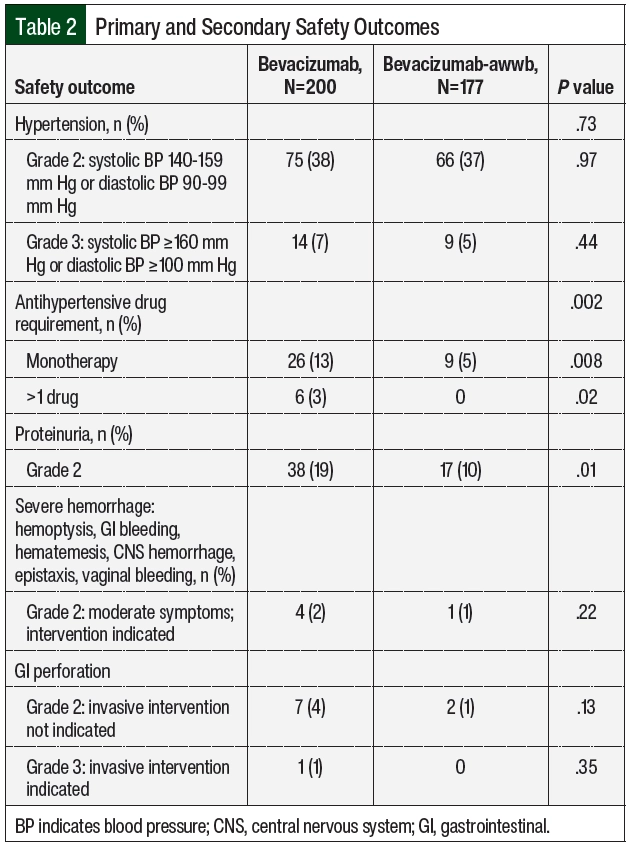
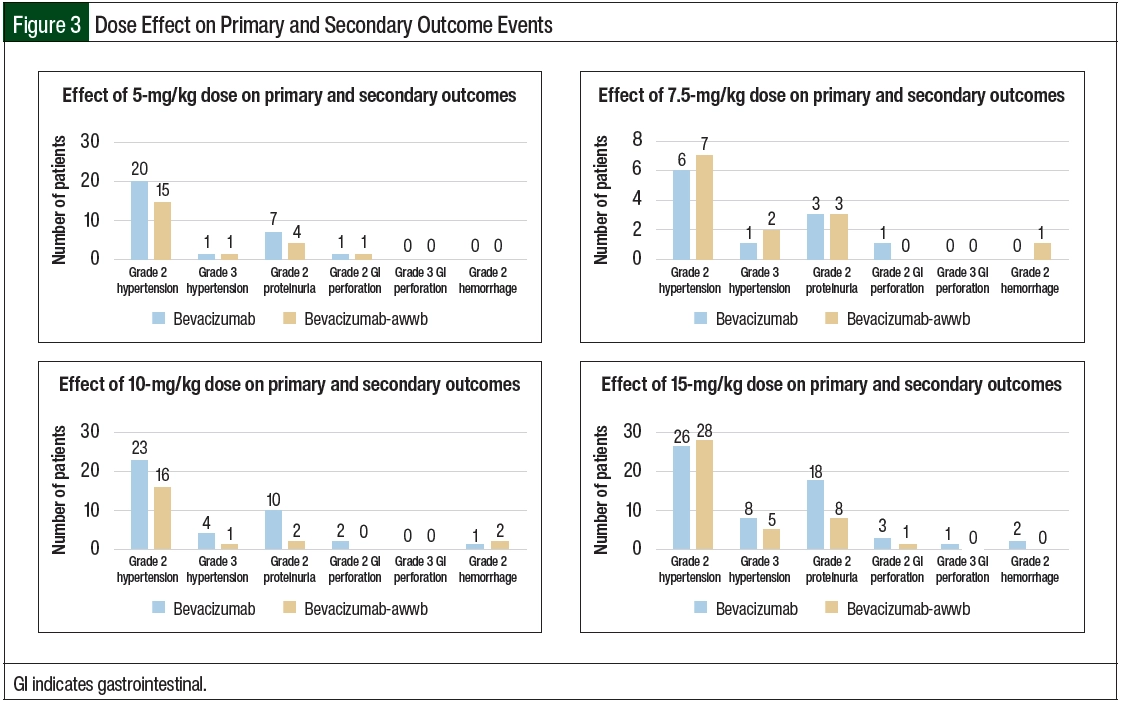
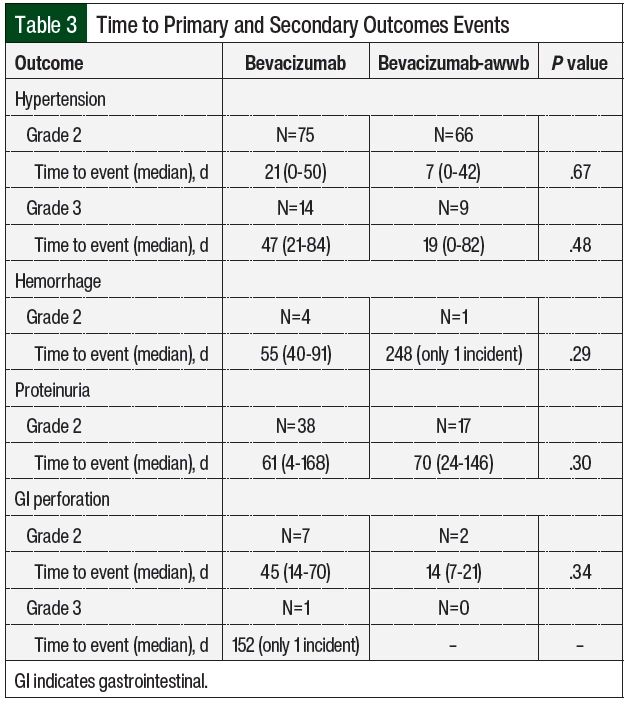
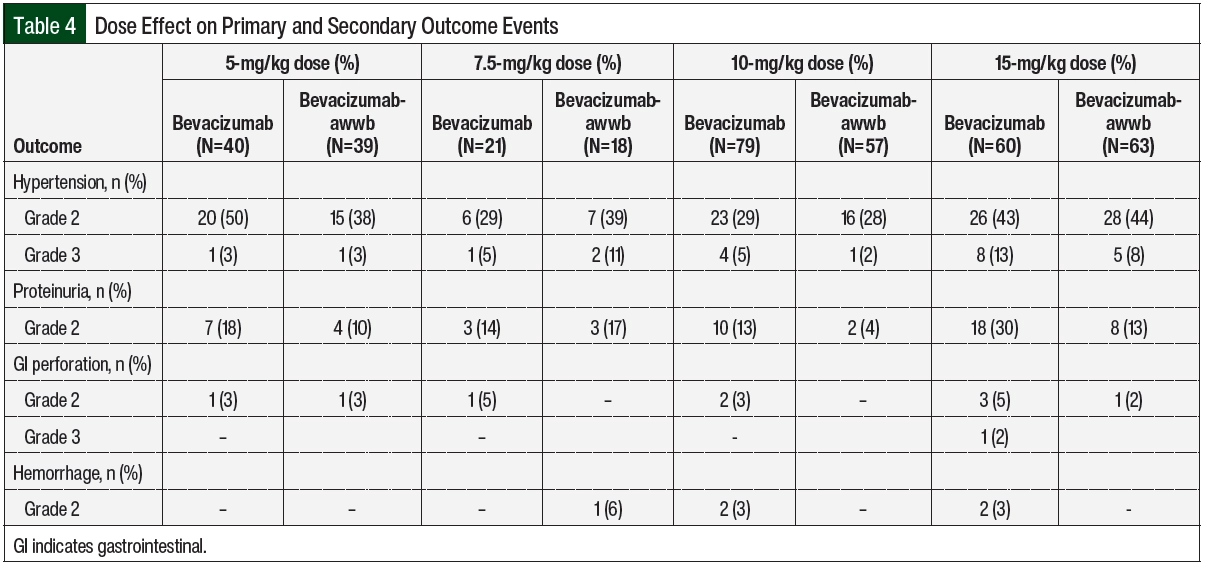
There were no significant differences in grade ≥2 hypertension between the groups (Table 2 and Figure 3). The initiation of antihypertensive monotherapy was higher in the bevacizumab group (13% vs 5%, respectively; P=.008), with treatment with a new combination antihypertensive therapy only in the bevacizumab group (Table 2). The duration of time from treatment initiation to grade ≥2 hypertension was not significantly different between the 2 groups (Table 3). The time to grade ≥2 hypertension was longer in the bevacizumab group than in the bevacizumab-awwb group (21 days vs 7 days for grade 2 hypertension, respectively; P=.67 and 47 days vs 19 days for grade 3 hypertension, respectively; P=.48; Table 3). Grade 2 hypertension was higher in the bevacizumab group than in the bevacizumab-awwb group at the 5-mg/kg (50% vs 38%, respectively) and 10-mg/kg doses (29% vs 28%, respectively; Table 4). Grade 3 hypertension was higher in the bevacizumab group than in the bevacizumab-awwb group at the 10-mg/kg (5% vs 2%, respectively) and 15-mg/kg doses (13% vs 8%, respectively; Table 4 and Figure 3).
Compared with bevacizumab, the adjusted odds ratio (OR) for hypertension in patients who received bevacizumab-awwb was 1.02 (95% confidence interval [CI], 0.63-1.65). In addition, based on multivariable logistic regression predictors of hypertension, ovarian cancer had an adjusted OR of 3.60 (95% CI, 1.36-9.50), whereas NSCLC trended toward significance with an adjusted OR of 2.84 (95% CI, 0.99-8.20). The weight-based dosing was not significantly associated with hypertension; however, the total doses administered were positively correlated. Moreover, compared with patients who weighed <50 kg, weight of >50 kg was associated with an increased odds of hypertension. As a result, it is vital to continue closely monitoring for hypertension in patients who have a prolonged treatment course and in those who weigh >50 kg.
Grade 2 proteinuria occurred more frequently in the bevacizumab group than in the bevacizumab-awwb group (19% vs 10%, respectively; P=.01; Table 2). None of the patients in either group had grade >2 proteinuria. The duration from the initiation of bevacizumab or bevacizumab-awwb to grade 2 proteinuria was not significantly different between both groups (Table 3). The duration to grade 2 proteinuria was shorter in the bevacizumab group than in the bevacizumab-awwb group (61 days vs 70 days, respectively; P=.30; Table 3). Grade 2 proteinuria was higher in the bevacizumab group than in the bevacizumab-awwb group at the 5-mg/kg (18% vs 10%, respectively), 10-mg/kg (13% vs 4%, respectively), and 15-mg/kg doses (30% vs 13%, respectively; Table 4 and Figure 3).
There was no significant difference in grade 2 GI perforation, with 1 incident of grade 3 GI perforation in the bevacizumab group (Table 2). The duration from the initiation of treatment with bevacizumab or bevacizumab-awwb to grade ≥2 GI perforation was not significantly different between the groups (Table 3). The duration to grade 2 GI perforation was shorter in the bevacizumab-awwb group than in the bevacizumab group (45 days vs 14 days, respectively; P=.34; Table 3). The rate of grade 2 GI perforation was higher in the bevacizumab group than in the bevacizumab-awwb group at the 15-mg/kg dose (5% vs 2%, respectively; Table 4).
There was no significant difference in grade 2 hemorrhage between the groups, and grade >2 hemorrhage was not observed in either cohort. The duration from treatment initiation to grade 2 hemorrhage was not significantly different between the groups (Table 3). The duration from treatment initiation to grade 2 hemorrhage was longer in the bevacizumab group than in the bevacizumab-awwb group (55 days vs 248 days, respectively; P=.29; Table 3).
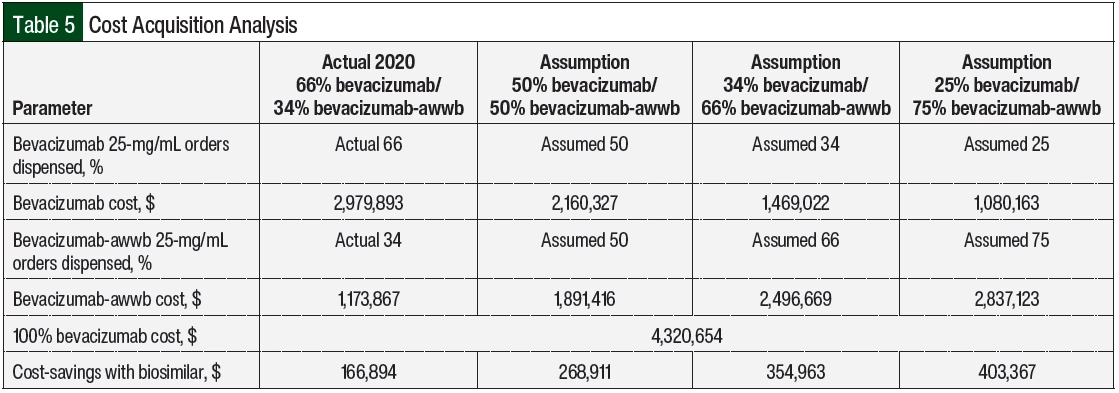
To calculate the financial impact of biosimilar utilization, we analyzed the acquisition costs of bevacizumab-awwb and bevacizumab, with the biosimilar having a financial benefit of lower average wholesale pricing. In 2020, the absolute number of bevacizumab orders dispensed was greater than the orders of bevacizumab-awwb (66% vs 34%, respectively; Table 5). Based on the dispense volume, the acquisition costs were $2,979,893 for bevacizumab versus $1,173,867 for bevacizumab-awwb. Based on the 2020 utilization rate of 34% for bevacizumab-awwb orders, we calculated the total cost saved with the use of bevacizumab-awwb versus bevacizumab to be $166,894.
We further estimated that the institution would save $268,911, $354,963, and $403,367, respectively, if the utilization rates for bevacizumab-awwb increased to 50%, 66%, and 75% (Table 5). The significant cost-savings with the increased use of bevacizumab-awwb is a unique opportunity for the health system to minimize cost while maintaining the quality of cancer care.
Discussion
In the age of value and high cost of cancer care, oncology stewardship incorporating cost-savings while ensuring patient safety is highly valuable to maximize treatment options and lower payer costs. There is a scarcity of real-world evidence on the safety outcomes of bevacizumab-awwb compared with bevacizumab in various patients with cancer. In the phase 3 MAPLE clinical trial, which included 633 adults with nonsquamous NSCLC (309 patients received bevacizumab and 324 received bevacizumab-awwb), bevacizumab-awwb had a similar pharmacokinetics profile, clinical efficacy, and safety that supported its clinical equivalence to bevacizumab.3 The grade ≥3 adverse events with bevacizumab and bevacizumab-awwb in this study were frequently associated with anti-VEGF adverse events. In the patients who received bevacizumab or bevacizumab-awwb, these adverse events included hypertension (5.5% vs 6.8%, respectively), proteinuria (0.3% in both groups), GI perforation (1.3% vs 0.9%, respectively) and pulmonary hemorrhage (1.6% vs 0.6%, respectively).3
Similar to the MAPLE trial, our results showed no significant difference between bevacizumab and bevacizumab-awwb in hypertension, proteinuria, and GI perforation. However, in our study most of the outcome events were grade 2 for hypertension and GI perforation, except for a few incidences of grade 3 hypertension (7% with bevacizumab vs 5% with bevacizumab-awwb; P=.44; Table 2) and an incidence of GI perforation in the bevacizumab group. In our study, only grade 2 proteinuria was observed. Moreover, only grade 2 hemorrhage was observed, which was predominantly epistaxis or hemorrhage related to glioblastoma, unlike in the MAPLE trial in which only pulmonary hemorrhage was reported.3
It is important to note that patients in the MAPLE trial received doses of 15 mg/kg of bevacizumab or bevacizumab-awwb,3 whereas our study included different dosing levels based on the specified cancer type and concomitant chemotherapy regimen received. The higher dosing level received by patients in the MAPLE trial may be correlated with the higher grade of adverse events compared with our study.
In a retrospective study of patients with GI cancer who received bevacizumab (N=42) or bevacizumab-awwb (N=33), the incidences of hypertension and proteinuria were greater in the patients who received bevacizumab (52.4% vs 36.4% for hypertension, respectively; P=.2427 and 35.7% vs 30% for proteinuria; P=.7193, respectively).11 The durations from the initiation of treatment to hypertension and proteinuria were longer with bevacizumab than with bevacizumab-awwb (84 days vs 24.5 days for hypertension; P=.0064; and 213 days vs 53.5 days for proteinuria; P=.0022).11 Similar to our study, the incidences of hypertension and proteinuria were higher in the bevacizumab group than in the bevacizumab-awwb group, with higher proteinuria events in the bevacizumab group being statistically significant (P=.01). Of note, this comparison study in GI cancer reported only the number of incidences and did not report the grading of hypertension and proteinuria,11 which is a limitation to comparing this retrospective study with our study. The times to hypertension and proteinuria events were not statistically significant in our study, but they were shorter and deemed statistically significant between the 2 arms in the aforementioned study.11
In our study, more patients with ovarian, endometrial, or fallopian tube cancer received bevacizumab than bevacizumab-awwb, likely because bevacizumab-awwb was not FDA-approved for these indications during our study’s time frame. Although there was not a significant difference in grade ≥2 hypertension in our study, the initiation of antihypertensive monotherapy and combination therapy were higher in the bevacizumab arm. This observation is likely because more patients in the bevacizumab-awwb arm had preexisting hypertension and were receiving antihypertensive therapy before the initiation of treatment with bevacizumab. Grade 2 proteinuria was lower in the biosimilar arm, likely because less patients received higher doses of 10 mg/kg and 15 mg/kg compared with the bevacizumab group (Table 4). There were no incidences of grade 3 proteinuria, grade 3 hemorrhage, or grade 4 adverse events in either arm.
Based on our study’s time frame, we were interested in understanding the frequency of utilization of bevacizumab-awwb and bevacizumab at University of California San Francisco Health throughout a full calendar year. In 2020, 66% of the dispensed orders were for bevacizumab and 34% were for bevacizumab-awwb. The total cost saved with the utilization rate of 34% for bevacizumab-awwb was $166,894 (Table 5). Based on that understanding, we made assumptions based on the utilization rates for bevacizumab-awwb at 50%, 66%, and 75% and then calculated the cost-savings of switching from bevacizumab to bevacizumab-awwb using the 100% cost of the bevacizumab and subtracting it from the cost of the assumed utilization percent (Appendix Table S2, available Here). If the utilization of bevacizumab-awwb increased to 75% for the year and the volume dispensed stayed the same, there is a potential to save up to $403,367 annually.
Given the increased FDA approval of bevacizumab-awwb for various types of cancer and its comparable safety to bevacizumab, there is a possibility for the biosimilar to exceed 75% utilization in a year. Unfortunately, there are no real-world cost analysis studies that evaluate the financial impact of the use of bevacizumab-awwb as an alternative to bevacizumab with which to compare our findings.
Limitations
Our study has some limitations. The sample size for each type of cancer, the short follow-up period when bevacizumab-awwb was not FDA approved for gynecologic indications, and the nature of its retrospective, single-center design are some of the limitations of this study. Furthermore, it is possible that the documentation was incomplete and that there were variances in the quality of information recorded in the electronic health records.
Conclusion
Bevacizumab-awwb demonstrated a comparable safety profile with bevacizumab, regardless of the primary cancer type when assessing the incidences of grade ≥2 hypertension, proteinuria, hemorrhage, and GI perforation. Moreover, replacing bevacizumab with bevacizumab-awwb can significantly lower the acquisition costs for health systems. Given the current gap in primary literature and the scarcity of real-world evidence on the safety outcomes of bevacizumab-awwb, our results favor expanding the use of bevacizumab-awwb for the treatment of patients with various types of cancer. Treatment with bevacizumab-awwb can expand the therapy options for cancer at lower costs with comparable safety when combined with oncolytic regimens.
Further research is needed with a greater representation for each cancer type and a larger sample size is warranted to generalize this study’s outcomes to provide external validity to our findings. A large-scale study with a longer follow-up period is also needed to assess the safety of bevacizumab-awwb versus other currently approved biosimilars, including bevacizumab-bvzr and bevacizumab-maly.
Acknowledgments
We would like to acknowledge Zlatan Coralic, PharmD, BCPS, and Salem Kamalay, PharmD, BCPS, BCPPS, BCCP, BCGP, Department of Pharmaceutical Services, University of California, San Francisco, for providing statistical analysis support.
Author Disclosure Statement
Dr Alhaja, Dr Tsourounis, Dr Ho, and Dr Fong have no conflicts of interest to report.
References
- Patel KB, Arantes LH Jr, Tang WY, Fung S. The role of biosimilars in value-based oncology care. Cancer Manag Res. 2018;10:4591-4602.
- US Food and Drug Administration. Biosimilars: overview for health care professionals. Accessed February 26, 2024. www.fda.gov/drugs/biosimilars/overview-health-care-professionals
- Thatcher N, Goldschmidt JH, Thomas M, et al. Efficacy and safety of the biosimilar ABP 215 compared with bevacizumab in patients with advanced nonsquamous non-small cell lung cancer (MAPLE): a randomized, double-blind, phase III study. Clin Cancer Res. 2019;25:2088-2095. Erratum in: Clin Cancer Res. 2019;25:3193.
- Avastin (bevacizumab) injection, for intravenous use [prescribing information]. Genentech; September 2022. Accessed February 26, 2024. www.gene.com/download/pdf/avastin_prescribing.pdf
- Mvasi (bevacizumab-awwb) injection, for intravenous use [prescribing information]. Amgen; February 2023. Accessed February 26, 2024. www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/mvasi/mvasi_pi_hcp_english.pdf
- US Food and Drug Administration. 2017. FDA approves first biosimilar for cancer treatment. Accessed May 20, 2022. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-first-biosimilar-cancer-treatment
- Abdelmeseh V, Brown BR, Huynh JP, Zullo AR. Comparative cost savings of biosimilar and dose rounding utilization in oncology care. J Oncol Pharm Pract. 2023;29:1437-1442.
- Saleem T, Qurashi H, Jamali M, et al. Biosimilars as a future, promising solution for financial toxicity: a review with emphasis on bevacizumab. Cureus. 2020;12:e9300.
- Yang J, Liu R, Ektare V, et al. Does biosimilar bevacizumab offer affordable treatment options for cancer patients in the USA? A budget impact analysis from US commercial and Medicare payer perspectives. Appl Health Econ Health Policy. 2021;19:605-618.
- Fleiss JL, Tytun A, Ury HK. A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics. 1980;36:343-346.
- Man Y, Yu H, Mukherjee S, Zalewski Z. Incidence of hypertension and proteinuria in patients treated with bevacizumab versus bevacizumab biosimilar. J Clin Oncol. 2021;39(suppl 3):83.