Kidney cancer is estimated to be diagnosed in 81,800 individuals and will result in an estimated 14,890 deaths in the United States annually.1 Most of these cases of kidney cancer are renal cell carcinoma (RCC).2 Recent data show that the 5-year survival rate for patients with advanced or metastatic RCC is 15.3%, which increased from only 7.3% nearly 20 years ago.1 Improved patient survival in patients with metastatic RCC is largely a result of the use of oral targeted agents. Since 2005, the FDA has approved 8 oral targeted agents for the treatment of advanced or metastatic RCC, including sorafenib, sunitinib, pazopanib, axitinib, cabozantinib, lenvatinib, everolimus, and tivozanib.
Treatment decisions and algorithms for advanced or metastatic RCC have been transformed because of the use of these oral targeted agents, which demonstrate improved outcomes and tolerability compared with earlier cytokine-based therapies.3-9 Although oral therapies for advanced or metastatic RCC are metabolized by the liver, many are still excreted to some extent by the kidneys.10-15 Because of this dependence on renal clearance, many of the oral targeted agents were approved by the FDA based on studies that excluded patients with impaired renal function.3-9 Additional data summarizing the use of oral targeted agents in patients with renal impairment are limited, particularly for newer agents such as lenvatinib, cabozantinib, and tivozanib. Previous studies have estimated that up to 33% of patients with RCC have some degree of renal dysfunction, highlighting the need to optimize oral treatment regimens for patients with advanced or metastatic RCC in the setting of renal impairment.16,17
The landmark trials that led to the approval of these oral targeted agents reported that these medications were well tolerated in the overall population, with an adverse event leading to treatment discontinuation in 4% to 24% of patients.3-9 The tolerance of oral targeted agents in patients with renal impairment or end-stage renal disease (ESRD) who are undergoing dialysis is less documented; however, a few smaller studies have suggested that oral targeted drugs in this setting are also well tolerated.17-20 Czarnecka and colleagues demonstrated the adequate tolerance of sunitinib, sorafenib, and pazopanib in 9 patients with RCC who were undergoing hemodialysis, with only 1 patient discontinuing therapy as a result of an adverse event.19 Gupta and colleagues reported no differences in dose reductions or dose interruptions with sunitinib treatment in patients with RCC and renal insufficiency compared with patients without renal impairment.17 However, patients who received temsirolimus and everolimus had higher rates of dose interruptions and dose reductions in those with renal impairment versus those without renal impairment. Last, in a study by Josephs and colleagues that evaluated the tolerability of sunitinib in 19 patients with renal impairment, only 1 patient discontinued treatment as a result of an adverse event.20 These studies concluded that patients with renal impairment or ESRD while undergoing dialysis could be safely maintained with oral targeted agents if closely monitored, which warrants further investigation of their tolerance in patients with renal impairment.17-20
A retrospective review by McKernan and colleagues evaluated the safety and efficacy of oral targeted agents in 55 patients with advanced or metastatic RCC and baseline renal impairment or ESRD while undergoing dialysis.21 Some patients received more than 1 oral therapy after disease progression or unacceptable adverse events subsequent to receiving a previous line of therapy. Patients who received more than 1 line of treatment with an oral agent were included, and each treatment that they received was documented as a unique treatment episode. All of the study patients had an adverse event of any grade, and 56.8% of treatment episodes ended in treatment discontinuation as a result of an adverse event.21 These data demonstrate slightly worse tolerability of oral targeted agents compared with previous literature that evaluated the use of oral targeted agents in patients with advanced or metastatic RCC and renal impairment.17-20
Because of a lack of data on the tolerability and efficacy of oral targeted agents in the treatment of advanced or metastatic RCC in patients with renal impairment, providers have little guidance on treatment options. The purpose of this study is to expand on the study by McKernan and colleagues and to evaluate the tolerability and efficacy of oral targeted agents in a control cohort of patients without renal impairment.21 These results will allow comparisons to be made in a larger patient population and will help determine the impact that renal function has on the tolerability of oral targeted agents, which will ultimately offer direction for the management of patients with advanced or metastatic RCC.
Methods
This single-center, retrospective chart review evaluated the impact of renal function on the efficacy and safety of oral targeted agents for patients with advanced or metastatic RCC at Froedtert & the Medical College of Wisconsin. McKernan and colleagues previously collected and published data within the same institution that assessed the safety and efficacy of oral targeted agents for advanced or metastatic RCC in patients with baseline renal impairment.21 The results of this study served as a control cohort of patients who received treatment within the same health system and expanded on the data by McKernan and colleagues.21 An internal control was selected primarily in an attempt to increase the validity of the data and to improve the ability to make valuable comparisons between the groups who received treatment at the same institution. This study was approved by an institutional review board through the Medical College of Wisconsin.
Patients were eligible for the study if they had a histologically confirmed diagnosis of advanced or metastatic RCC, had creatinine clearance (CrCl) of ≥60 mL/min, and received treatment within the Froedtert health system between June 2008 and April 2020 with 1 of the 7 oral targeted agents that were approved by the FDA during that time frame, including sunitinib, everolimus, sorafenib, axitinib, pazopanib, lenvatinib, and cabozantinib. In addition, the patients had to be aged ≥18 years and have adverse events and efficacy assessments of the oral therapies documented and available in their electronic health record (EHR). Any patients who received treatment as part of an ongoing clinical trial were excluded. The CrCl cutoff of 60 mL/min was chosen according to the Kidney Disease Improving Global Outcomes 2012 clinical practice guidelines. CrCl was calculated with the Cockcroft-Gault equation using the patient’s actual body weight and serum creatinine that were most recently documented before the beginning of treatment. Patients who received treatment with more than 1 oral targeted agent throughout the course of their disease were included in the analysis, and each treatment that the patient received was documented as a unique treatment episode.
The primary end point in this study was the incidence of treatment discontinuation resulting from an adverse event. The secondary safety end points include the incidence of any-grade adverse events, the incidence of grade ≥3 adverse events, the incidence of dose adjustments resulting from adverse events, and the incidence of treatment interruptions resulting from adverse events. The secondary efficacy end points include progression-free survival (PFS) and overall survival (OS). PFS was defined as the time from treatment initiation to the time of disease progression or death from any cause. OS was defined as the time from treatment initiation to the time of death from any cause. The RECIST V1.1 criteria were used to define the response to treatment. After the data were collected for the cohort of patients with advanced or metastatic RCC without renal impairment (Cohort 2), all of the above outcomes were analyzed and compared with the previous cohort of patients from McKernan and colleagues with advanced or metastatic RCC and renal impairment (Cohort 1).
All adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5. The grading of adverse events was completed by the provider and was documented in the EHR. When adverse event grading was not available using chart review, the primary investigator completed the grading using information contained in the provider notes.
Statistical Analysis
Descriptive statistics were used to describe the patients’ characteristics and their disease, treatments, and adverse events. Kaplan-Meier estimates were used to analyze PFS and OS. Last, a logistic regression analysis was used to compare the patients with and without renal impairment in terms of the incidence of adverse events leading to the discontinuation of treatment after adjusting for other factors associated with the outcome. Regression enabled us to evaluate the effect that various medications had on the incidence of adverse events and to compare that effect between the 2 patient groups as defined by renal impairment.
The data from Cohort 1 as analyzed by McKernan and colleagues included 55 patients and 74 unique treatment encounters.21 With an estimated difference in the primary end point between the cohorts of 20%, 275 total unique treatment encounters were needed for Cohort 2 to achieve 87% power.
Results
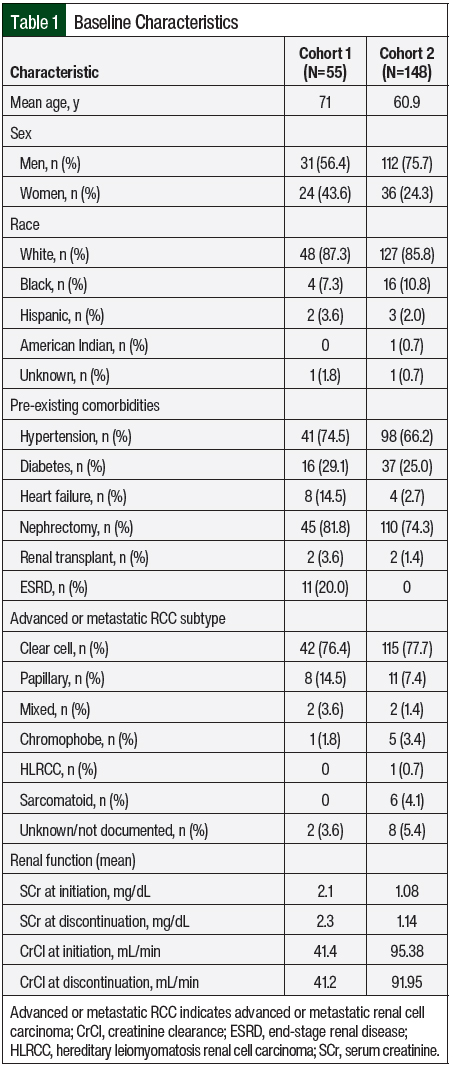
Between June 2008 and April 2020, 148 patients comprising 268 unique treatment episodes met the inclusion criteria and were included in the analysis. At least 1 treatment episode with each of the 7 oral targeted agents included in this study was evaluated. The baseline patient characteristics were summarized based on the total number of patients included in the analysis (Table 1). In all, 75.7% of the patients were men, and the average age was 60.9 years. The majority of patients were White (85.8%), and many patients had pre-existing comorbid conditions, such as hypertension (66.2%) and diabetes (25%). A nephrectomy was performed in 74.3% of patients, and 1.4% of patients had a previous renal transplant. The most common RCC histologic subtypes were clear cell (77.7%) and papillary (7.4%). At the time of treatment initiation, the mean serum creatinine and CrCl were 1.08 mg/dL and 95.38 mL/min, respectively. Similar to Cohort 1, renal function stayed fairly constant throughout the duration of treatment.
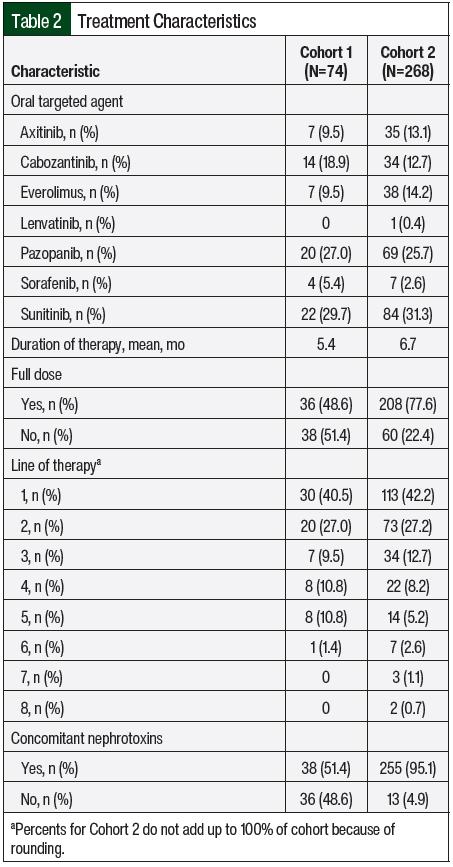
The treatment characteristics were summarized based on each unique treatment episode (Table 2). Sunitinib was received the most frequently (31.3%), followed by pazopanib (25.7%), everolimus (14.2%), axitinib (13.1%), cabozantinib (12.7%), sorafenib (2.6%), and lenvatinib (0.4%). There was combination treatment with an oral targeted agent plus an immune checkpoint inhibitor in 1 treatment episode across both groups. The treatment episode was in Cohort 2, and the patient received axitinib plus pembrolizumab. The patients’ treatments were initiated at the target dose 77.6% of the time, and 42.2% of treatment episodes were the first line of therapy for advanced or metastatic RCC. The mean duration of therapy was 6.7 months.
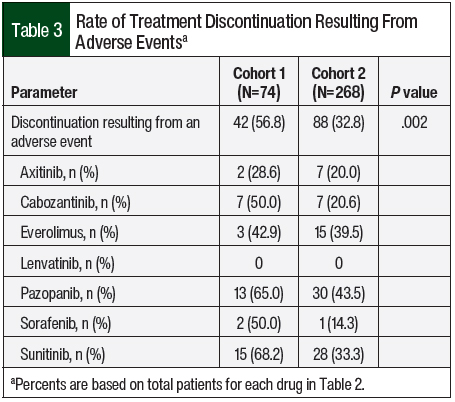
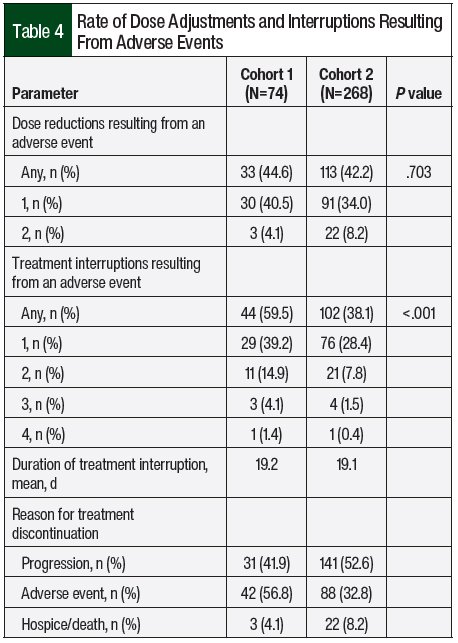
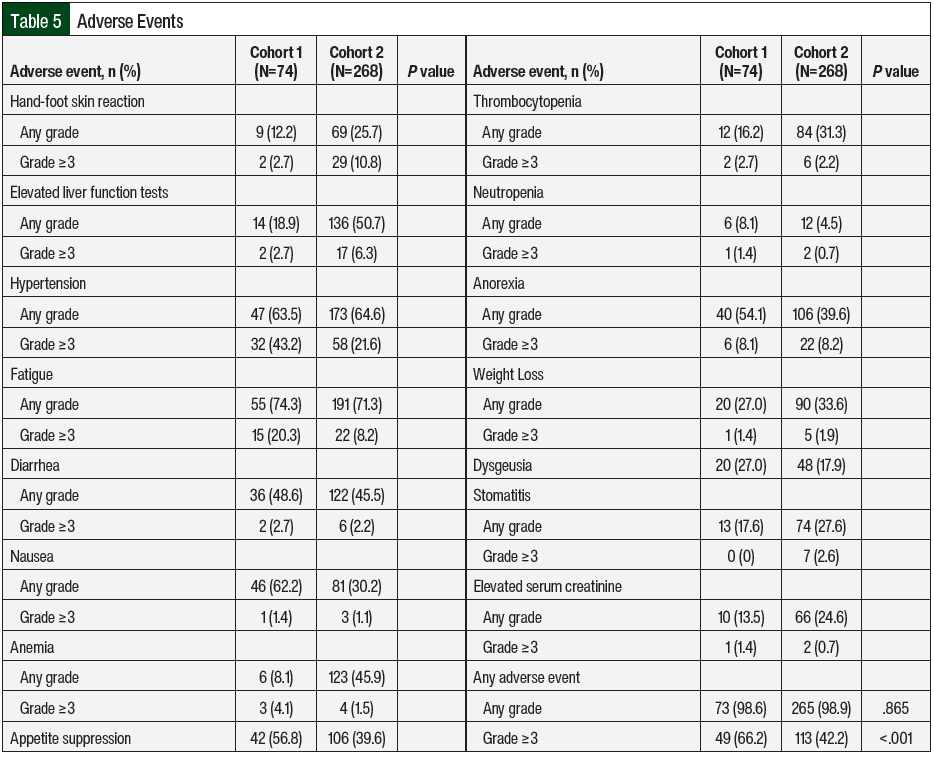
The primary end point of treatment discontinuation as a result of an adverse event occurred in 32.8% of treatment episodes in Cohort 2, which was a significant improvement compared with Cohort 1 (56.8%; P=.002; Table 3). In Cohort 2, pazopanib had the highest rate of treatment discontinuation as a result of an adverse event (43.5%), followed by everolimus (39.5%) and sunitinib (33.3%). The most common adverse events that resulted in treatment discontinuation were elevated liver function tests (LFTs; 18.2%), fatigue (12.5%), diarrhea (11.4%), pneumonitis (8%), mucositis (8%), and hand-foot skin reaction (6.8%). Table 4 outlines the incidence of dose adjustments and treatment interruptions resulting from adverse events. In Cohort 2, 42.2% of the patients required a dose reduction because of an adverse event, with 8.2% of patients requiring 2 dose adjustments. Treatment interruptions resulting from an adverse event occurred in 38.1% of the treatment episodes. The treatment interruptions resulting from an adverse event were significantly improved in Cohort 2 compared with Cohort 1 (38.1% vs 59.5%, respectively; P<.001).
Nearly all treatment episodes included an adverse event of any grade, with grade ≥3 adverse events occurring in 42.2% of the episodes in Cohort 2. The rates of any-grade adverse events were similar between Cohorts 1 and 2 (98.6% vs 98.9%, respectively; P=.865; Table 5); however, the rate of grade ≥3 adverse events was significantly lower in Cohort 2 than in Cohort 1 (42.2% vs 66.2%, respectively; P<.001). The adverse events and respective incidence and severity are shown in Table 5. Some of the most common any-grade adverse events in Cohort 2 were fatigue (71.3%), hypertension (64.6%), and LFT elevations (50.7%). Hypertension (21.6%), hand-foot skin reaction (10.8%), anorexia (8.2%), and fatigue (8.2%) were the most common grade ≥3 adverse events. Of note, there were no grade 5 adverse events in either cohort.
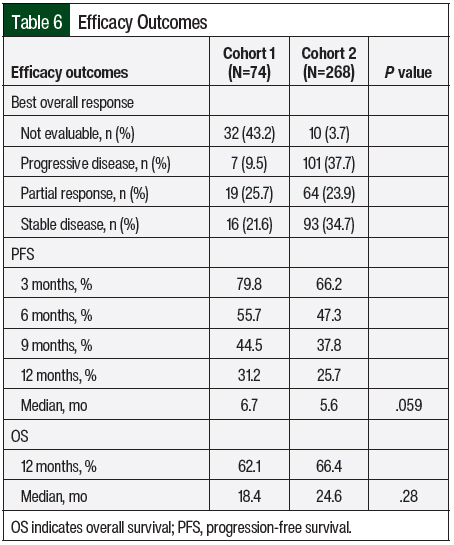
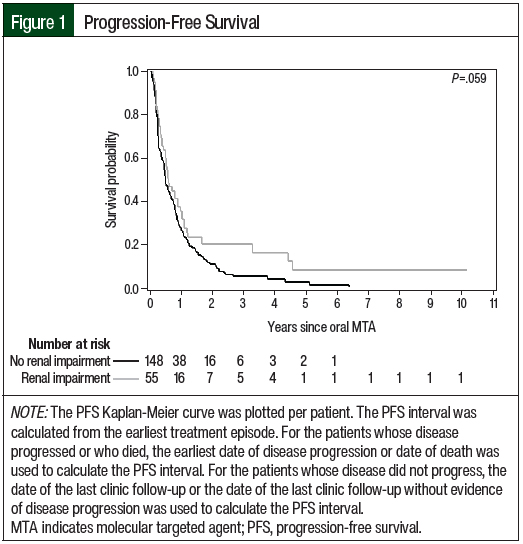
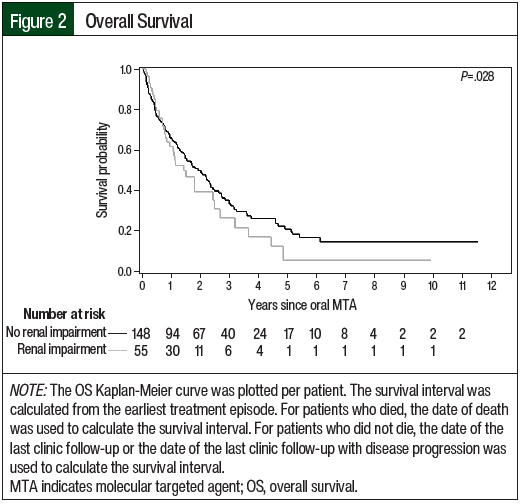
The efficacy outcomes data are summarized in Table 6. According to the RECIST criteria, the treatment responses in Cohort 2 were partial response (23.9%), stable disease (35.1%), and progressive disease (37.7%). The responses in Cohort 1 included partial response (25.7%), stable disease (21.6%), and progressive disease (9.5%). Of note, 43.2% of the treatment episodes in Cohort 1 did not have treatment evaluation imaging performed, and the best overall response was not evaluable. In Cohort 2, treatment scans were not available for evaluation in 3.7% of treatment episodes. The median PFS favored Cohort 1 versus Cohort 2 (6.7 months vs 5.6 months, respectively; P=.059), and the median OS favored Cohort 2 versus Cohort 1 (24.6 months vs 18.4 months, respectively; P=.28); however, neither of these differences were significant. The Kaplan-Meier curves for PFS and OS are shown in Figure 1 and Figure 2, respectively.
Discussion
The data from this study revealed several differences in tolerability between the cohorts, which supports the prediction that decreased renal function may result in impaired tolerability of oral targeted agents in patients with advanced or metastatic RCC.
The renal function of the patients in Cohort 2 more closely resembled the patients included in the majority of the previous landmark studies, most of which only included patients with a creatinine clearance of ≥30 mL/min.3-5,8 Although the rate of treatment discontinuation resulting from an adverse event was lower in Cohort 2 than in Cohort 1, the rate was still higher than anticipated compared with previous literature.3-9 The results of Choueiri and colleagues’ CABOSUN study, which compared treatment with cabozantinib and sunitinib in patients with advanced or metastatic RCC, showed an overall discontinuation rate of 20% as a result of an adverse event.3 Similarly, the results of a study by Motzer and colleagues that evaluated treatment with pazopanib versus with sunitinib in patients with advanced or metastatic RCC showed an average 22% discontinuation rate from adverse events in both groups combined.5
Cohorts 1 and 2 were similar in the rates of dose reductions resulting from adverse events, and these rates closely align with those of previous studies.3,5 The results of the CABOSUN study showed adverse events leading to dose reductions in an approximate 41% of the patients who received cabozantinib or sunitinib, which is consistent with both of the cohorts in our study.3 Likewise, the study by Motzer and colleagues that compared treatment with pazopanib and sunitinib showed dose reductions resulting from adverse events in approximately 48% of the overall patient population.5 Conversely, Cohort 2 in our study had a significantly decreased rate of dose interruptions as a result of adverse events compared with Cohort 1. In the study by Motzer and colleagues, approximately 47% of the overall population had a dose interruption as a result of an adverse event, which was lower than the rate in Cohort 1 in our study.5
Nearly all of the treatment episodes in Cohorts 1 and 2 had any-grade adverse events, which closely reflects observations from previous studies.3,5 Studies by Choueiri and colleagues and Motzer and colleagues demonstrated that any-grade adverse events occurred in 99% of patients.3,5 In all, 68% and 58% of patients in the studies by Choueiri and colleagues and Motzer and colleagues, respectively, had grade 3 or 4 adverse events.3,5 Interestingly, these rates of severe adverse events more closely align with the rates in Cohort 1 of our study. The rates of specific side effects were similar among all the study populations, with fatigue, hypertension, and diarrhea being the most common. Of note, Cohort 2 had a much higher rate of elevated LFTs than Cohort 1; however, this increased rate of LFTs elevations closely mimics the rates in previous studies.3,5 In the study by Choueiri and colleagues, approximately 62% and 32% of the patients receiving cabozantinib and sunitinib, respectively, had LFT elevations.3 Motzer and colleagues reported LFT elevations in 61% and 60% of patients receiving pazopanib and sunitinib, respectively.5
Although there are similarities in Cohorts 1 and 2, these data suggest that the tolerability of oral targeted agents for the treatment of advanced or metastatic RCC is at least partially influenced by renal function. These data demonstrate the potential for the increased incidence of grade 3 and 4 adverse events, treatment interruptions as a result of adverse events, and treatment discontinuations as a result of adverse events in patients with impaired renal function. Healthcare providers must use caution when managing patients with advanced or metastatic RCC and renal impairment with oral targeted agents.
Another difference between the cohorts in our study is in the use of concomitant nephrotoxins. Interestingly, many more patients in Cohort 2 received concurrent nephrotoxins compared with Cohort 1. The most probable explanation for this difference is the use of contrast for imaging procedures. Contrast is a known nephrotoxin, and the majority of patients in Cohort 2 underwent imaging procedures that used contrast dye to evaluate their disease status. Cohort 1 likely included more patients who stopped the medication before obtaining disease evaluation scans or who had imaging performed without the use of contrast because of their baseline renal dysfunction and concern that the contrast may cause it to worsen.
In general, the efficacy results of the patients in both cohorts in our study are worse than in previous studies.3,5 In the study by Choueiri and colleagues, the median PFS for patients receiving cabozantinib and sunitinib were 8.2 months and 5.6 months, respectively.3 The study by Motzer and colleagues showed similar results, with median PFS of 8.4 months and 9.5 months for pazopanib and sunitinib, respectively.5 In the study by Choueiri and colleagues, the patients who received sunitinib had a similar median PFS to both cohorts in our study; however, the other groups, including a different population of patients receiving sunitinib in the study by Motzer and colleagues, had a higher median PFS.3,5
A similar trend was noted among the groups with median OS as well. Choureiri and colleagues reported a median OS of 30.3 months with cabozantinib treatment and 21.8 months with sunitinib.3 In the study by Motzer and colleagues, the median OS was 28.4 months with pazopanib and 29.3 months with sunitinib.5
Limitations
The primary end point in our study was the rate of treatment discontinuation resulting from adverse events, and the primary focus as a whole pertains to adverse events and the tolerability of oral targeted agents in patients with advanced or metastatic RCC. Although our study was not powered appropriately to detect differences related to efficacy, PFS and OS data were included for completeness and to allow observations to be made.
This study was a retrospective chart review spanning back to 2008. The data collected were limited to the documentation available in the EHR, which is prone to variation among healthcare providers, especially pertaining to subjective data. In addition, the data for each cohort were collected by a single independent reviewer, and the independent reviewer was different for the 2 cohorts. Although several steps were put in place to standardize the data collection process and to eliminate inconsistencies, the interpretation of some data, particularly the grading of adverse events when not explicitly available, could vary based on the reviewer.
Our study evaluated patients who received any FDA-approved oral targeted agent for the treatment of advanced or metastatic RCC at the time of therapy. Because of this, irregularity exists in the number of patients who received each oral targeted agent in our study. This variability impeded our ability to compare the outcomes among the oral therapies within each cohort, as well as our ability to compare the outcomes among the patients who received the same oral targeted agent in separate cohorts.
Another factor that limits our ability to make comparisons to previous landmark studies relates to our inclusion criteria, particularly performance status and the line of treatment. Many of the previous studies required patients to have an Eastern Cooperative Oncology Group performance status score of 0 to 2, whereas our study did not have any inclusion restrictions based on performance status. Similarly, most of the previous studies evaluated the use of oral targeted agents in patients with no previous systemic treatment. Conversely, only approximately 40% of the treatment episodes in Cohorts 1 and 2 were the first line of treatment.
In a study by Sternberg and colleagues that examined treatment with pazopanib versus placebo in patients with advanced or metastatic RCC, they noted a difference in the treatment discontinuation rates as a result of adverse events in patients who received cytokine-based therapy compared with treatment-naïve patients (19% vs 12%, respectively).22 The baseline performance status and extent of previous treatment could certainly impact the tolerability and efficacy of oral targeted therapies.
Because our study followed clinical practice and was not part of a clinical trial, the treatment decisions were left up to the provider. Most clinical trials will provide clear guidance for when it is appropriate to dose reduce, interrupt treatment, and discontinue treatment as a result of adverse events. In practice, some providers may have different thresholds to alter therapy as a result of adverse events. This lack of regulation and consistency impairs our ability to compare these data to historical cohorts.
Last, the variability in assessing a patient’s response to treatment is also a limitation in our study and impairs our ability to interpret the efficacy results. Patients undergo imaging to assess disease status and treatment response, most often after 2 or 3 months of therapy, but the exact frequency in which scans are obtained can vary based on the provider. With Cohorts 1 and 2 receiving treatment at the same medical center by the same set of providers, this provider variation is partially accounted for. However, it still limits the ability to compare the results from other studies and institutions. In addition, the frequency of routine scans to assess treatment response can fluctuate based on each patient’s specific course of treatment. If a patient stops treatment after 2 months because of an adverse event and switches to a different therapy without getting new baseline scans, the apparent PFS of the initial treatment may be falsely prolonged because of the lack of evaluation. This is a likely confounding variable given that nearly 12 times as many treatment episodes in Cohort 1 did not have imaging to evaluate disease response compared with Cohort 2.
Conclusion
Oral targeted therapy for advanced or metastatic RCC is associated with a variety of adverse events, with many patients reporting at least 1 adverse event of any grade. Although these oral agents exhibit primarily hepatic metabolism, tolerability concerns remain for patients with renal impairment. Because of this concern, more patients with renal impairment in our study began treatment at a reduced dose. Despite this approach, patients with renal impairment demonstrated a higher rate of grade ≥3 adverse events, treatment interruptions as a result of an adverse event, and treatment discontinuation resulting from an adverse event. The median PFS rates were similar between the groups; however, the OS rates were numerically improved in the cohort of patients without renal impairment. The improved efficacy and safety outcomes in the patients without renal impairment emphasize the role renal function plays in the tolerability of oral targeted agents.
Optimizing dosing and monitoring strategies for these oral targeted therapies in patients with advanced or metastatic RCC and renal impairment could lead to improved patient outcomes. Further investigation of the specific treatment selections and dosing is necessary to supplement the growing body of knowledge regarding the use of oral targeted agents in patients with renal impairment.
Acknowledgements
This project team would like to acknowledge the statisticians who assisted in this study, Ruta Brazauskas, PhD, and Bi Qing Teng, MS. We also like to acknowledge Matthew Bemis, PharmD, for his contributions toward the data collection.
Author Disclosure Statement
Dr Mayer, Dr Carroll, Dr Rhoades, and Dr Zook have no conflicts of interest to report.
References
- National Cancer Institute SEER Program. Cancer stat facts: kidney and renal pelvis cancer. https://seer.cancer.gov/statfacts/html/kidrp.html. Accessed September 15, 2023.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Kidney Cancer. Version 1.2024. June 21, 2023. www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed September 15, 2023.
- Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35:591-597.
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473-1482. Errata in: Lancet Oncol. 2016;17:e270; Lancet Oncol. 2018;19:e509.
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722-731.
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931-1939.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125-134.
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115-124.
- Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21:95-104.
- Cabometyx (cabozantinib) tablets, for oral use [prescribing information]. Exelixis; September 2023. Accessed September 22, 2023.
- Lenvima (lenvatinib) capsules, for oral use [prescribing information]. Eisai; November 2022. Accessed September 22, 2023.
- Inlyta (axitinib) tablets, for oral administration [prescribing information]. Pfizer; September 2022. Accessed September 22, 2023.
- Nexavar (sorafenib) tablets, for oral use [prescribing information]. Bayer HealthCare Pharmaceuticals; August 2023. Accessed September 22, 2023.
- Sutent (sunitinib malate) capsules, for oral use [prescribing information]. Pfizer; August 2021. Accessed September 22, 2023.
- Fotivda (tivozanib) capsules, for oral use [prescribing information]. AVEO Pharmaceuticals; March 2021. Accessed September 22, 2023.
- Miller DC, Schonlau M, Litwin MS, et al. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2008;112:511-520.
- Gupta S, Parsa V, Heilbrun LK, et al. Safety and efficacy of molecularly targeted agents in patients with metastatic kidney cancer with renal dysfunction. Anticancer Drugs. 2011;22:794-800.
- Shetty AV, Matrana MR, Atkinson BJ, et al. Outcomes of patients with metastatic renal cell carcinoma and end-stage renal disease receiving dialysis and targeted therapies: a single institution experience. Clin Genitourin Cancer. 2014;12:348-353.
- Czarnecka AM, Kawecki M, Lian F, et al. Feasibility, efficacy and safety of tyrosine kinase inhibitor treatment in hemodialyzed patients with renal cell cancer: 10 years of experience. Future Oncol. 2015;11:2267-2282.
- Josephs D, Hutson TE, Cowey CL, et al. Efficacy and toxicity of sunitinib in patients with metastatic renal cell carcinoma with severe renal impairment or on haemodialysis. BJU Int. 2011;108:1279-1283.
- McKernan J, Schmidt L, Rhoades M. Tolerance of oral targeted therapies in patients with metastatic renal-cell carcinoma and renal impairment. J Hematol Oncol Pharm. 2021;11(5):270-276.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.