Adverse events resulting from chemotherapy are numerous, but one of the most common and serious is neutropenia. The results of neutropenia can have devastating clinical and financial consequences for patients. The rate of febrile neutropenia–related mortality is between 2% and 21% among hospitalized patients, with hospitalization costs totaling $2.3 billion for adults in 2012.1 In addition, chemotherapy dose reductions and delays are associated with higher mortality risk and decreased 5-year absolute survival in patients with early-stage breast cancer.2 Patients who are older (aged >65 years) and with comorbidities have the highest risk for complications.3 To help mitigate this potentially life-threatening complication and the associated costs, growth factor is recommended in patients who have at least a 20% risk for febrile neutropenia based on patient- and treatment-specific factors.3
Filgrastim and pegfilgrastim are 2 recombinant growth factors approved by the FDA to decrease the incidence of infection, as manifested by febrile neutropenia, in patients receiving myelosuppressive chemotherapy.4,5 These agents are dosed subcutaneously, at least 24 hours after the patient has received chemotherapy. Filgrastim is administered daily for up to 2 weeks or until a desired absolute neutrophil count is reached.4 Pegfilgrastim is a pegylated, long-acting formulation of filgrastim that was designed for one-time administration 24 hours after receiving each chemotherapy cycle.5 In 2018, pegfilgrastim-jmdb, the first of now several pegfilgrastim biosimilars, was approved by the FDA with the goal of promoting competition with pegfilgrastim and reducing drug costs for patients.6 Since the introduction of several pegfilgrastim biosimilars to the market, the pegfilgrastim drug that is used relies on factors such as institutional formularies, cost, and payer mandates.
Although the one-time administration of pegfilgrastim has reduced the need for injection visits compared with filgrastim, returning for treatment the day after chemotherapy for this injection is not always feasible or convenient for patients. This is especially true for patients who travel long distances for their cancer treatment. Recently, the COVID-19 pandemic has highlighted the desire to administer pegfilgrastim on the same day as chemotherapy because this strategy is more convenient for patients and could mitigate an additional exposure to COVID-19.
Administering pegfilgrastim on the same day as chemotherapy is not recommended in the prescribing information, which specifically states that pegfilgrastim should not be administered until at least 24 hours after chemotherapy. There is a theoretical risk that a same-day dosing strategy for pegfilgrastim may exacerbate neutropenia through the stimulation of progenitor cells while cytotoxic chemotherapy is still in high concentrations in the body.7 One possible solution to the convenience problem in lieu of same-day administration is the use of an on-body autoinjector formulation. The on-body injector device allows the patient to get the pegfilgrastim injection at home, without having to come back to a clinic the next day.5 The injector system is placed on the patient before leaving the clinic on the day he or she receives chemotherapy, and the device automatically injects pegfilgrastim into the patient’s skin approximately 27 hours later.5 There is a potential for the on-body injector device to fail, which has been reported in up to 6.9% of patients in 1 study.8 If the autoinjector device fails, the patient needs to urgently return to the clinic to receive a manual injection of pegfilgrastim or they may even need daily filgrastim injections if pegfilgrastim would then be administered too close to the next cycle of chemotherapy.8,9 A patient could have life-threatening febrile neutropenia if the pegfilgrastim injections are missed. The use of the on-body injector system is limited further by payer coverage mandates, with many payers now requiring the use of lower-cost pegfilgrastim biosimilars, for which there is no on-body injector option.7
Current studies examining the use of same-day pegfilgrastim vary in their design, and the evidence supporting this strategy is minimal.10-12 Many studies analyze healthcare claims data or examine a variety of disease states together rather than specific malignancies or chemotherapy regimens.10-12
The purpose of this study is to examine the difference between same-day pegfilgrastim administration and next-day pegfilgrastim administration on the incidence of a 4-part composite of neutropenia-related events (NREs). Our secondary outcomes were to clarify the differences between these groups in hospitalization for febrile neutropenia, the length of dose delays of chemotherapy for neutropenia, mortality, and the incidence of COVID-19.
Methods
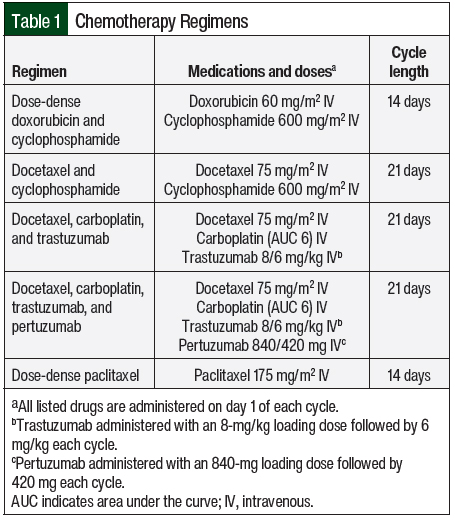
We conducted a retrospective, single-center, matched-cohort chart review of electronic medical records (EMRs) to evaluate the same-day versus next-day administration of pegfilgrastim in patients with breast cancer who received at least 1 cycle of curative-intent chemotherapy between January 1, 2019, and October 31, 2020, at The University of Kansas Health System (TUKHS). A total of 5 different chemotherapy regimens were selected for this study, each of which had a ≥20% risk for febrile neutropenia if growth factor support is not used (Table 1). Patients must have received their entire chemotherapy regimen and pegfilgrastim injection at our institution and within 3 calendar days after the last dose of chemotherapy was received. Brand-name and biosimilar pegfilgrastim were allowed to be administered. Patients were excluded from the study if they switched between same-day and next-day administration of pegfilgrastim within the study period, had empiric dose reductions to their chemotherapy plan on their first cycle of chemotherapy, or had already had breast cancer for which chemotherapy was administered.
Patients who received same-day pegfilgrastim, which was defined as pegfilgrastim administered during the same appointment as chemotherapy, were matched 2:1 by age and chemotherapy regimen with patients who received next-day pegfilgrastim. During our initial data collection from the EMR, data on the patients who received same-day pegfilgrastim and met the inclusion criteria were gathered first. At that point, data were collected on the patients who received next-day pegfilgrastim to allow for 2:1 matching by age and treatment regimen. The exclusion criteria were applied during the chart review process.
The primary end point was a composite of 4 NREs that could have occurred during any cycle of chemotherapy in the study period. The 4 NREs for this study were the chemotherapy cycle was delayed as a result of neutropenia, the dose of chemotherapy was reduced because of neutropenia, planned chemotherapy cycles were cancelled secondary to neutropenia, and the patient was admitted to the hospital with a suspected or confirmed diagnosis of febrile neutropenia. For dose delays, dose reductions, and cancellations, neutropenia was documented on the day of the planned chemotherapy or in laboratory results up to 2 days before chemotherapy was administered.
If a patient had multiple NREs throughout their chemotherapy regimen, this was only counted once in the primary outcome. These NREs were chosen because they represented objective and common clinical outcomes related to neutropenia and had meaningful impacts on a patient’s treatment course. Progress notes in the inpatient and outpatient settings, along with documentation in the chemotherapy plan itself by providers, were used to determine if patients met the criteria for an NRE.
For the study’s secondary outcomes, each NRE was evaluated for associations with the pegfilgrastim dosing strategy along with the chemotherapy regimen. In addition, data were collected on the length of dose delays for neutropenia, mortality during chemotherapy, and the incidence of COVID-19.
Firth’s penalized likelihood logistic regression model was used for the primary outcome to identify the effects of same-day versus next-day administration of pegfilgrastim. Statistical analysis was not performed on any of the secondary outcomes.
Results
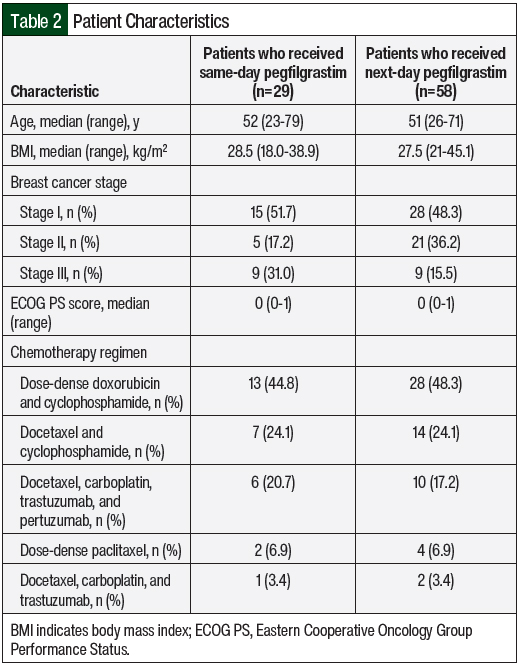
There were 29 patients who received same-day pegfilgrastim and 58 patients who received next-day pegfilgrastim in the final analysis for this study. The baseline characteristics were similar between the 2 groups with respect to age, performance status, and body mass index (Table 2). There were more patients with stage II breast cancer in the next-day pegfilgrastim cohort (36.2%) than in the same-day pegfilgrastim cohort (17.2%), and there were more patients with stage III breast cancer in the same-day pegfilgrastim cohort (31%) than in the next-day pegfilgrastim cohort (15.5%). The chemotherapy regimens with the highest use in the same-day and next-day pegfilgrastim groups were dose-dense doxorubicin and cyclophosphamide (44.8% vs 48.3%, respectively); docetaxel and cyclophosphamide (24.1% for both); and docetaxel, carboplatin, trastuzumab, and pertuzumab (20.7% vs 17.2%, respectively).
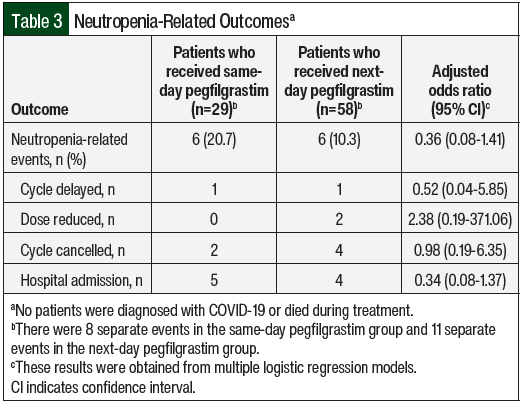
A total of 6 patients in each of the same-day (20.7%) and next-day (10.3%) pegfilgrastim groups had an NRE during their chemotherapy course (adjusted odds ratio, 0.36; 95% confidence interval, 0.08-1.41; Table 3). A total of 8 separate events occurred in the same-day pegfilgrastim group and 11 separate events in the next-day pegfilgrastim group. The most common occurrence was hospital admission for suspected or confirmed febrile neutropenia. Patients who had the same type of event occur more than once were only counted for 1 incidence in the study. For example, if a patient had multiple dose delays, they were only counted 1 time for that type of NRE. Two cycles of chemotherapy were cancelled in the same-day pegfilgrastim cohort and 4 cycles were cancelled in the next-day pegfilgrastim cohort. One chemotherapy cycle was delayed as a result of neutropenia in each group for 7 days for each instance. Overall, the differences between which NRE was more likely to occur in the 2 groups were not significant.
All 6 patients with NREs in the next-day pegfilgrastim cohort received dose-dense doxorubicin and cyclophosphamide, whereas 5 patients in the same-day pegfilgrastim cohort received dose-dense doxorubicin and cyclophosphamide and 1 patient received docetaxel and carboplatin. There were no instances of treatment-related mortality or COVID-19 in either group.
Discussion
The administration of pegfilgrastim on the same day as chemotherapy is not a standard-of-care practice, primarily because of the indication in the prescribing information and a theoretical concern that treatment with pegfilgrastim could increase the risk for neutropenia and compromise patients’ outcomes.5 In our study, there were no differences that confirmed or refuted the theoretical concern for increased bone marrow adverse events as a result of same-day pegfilgrastim administration in patients with breast cancer.
We believe that this is the first study of its kind that specifically evaluates a composite of clinical outcomes with same-day versus next-day pegfilgrastim use in patients with breast cancer. Previous studies have specifically examined the use of pegfilgrastim in patients with a variety of malignancies and have examined only cycle-1 neutropenia as the primary outcome.10-12 Although Burris et al note that neutropenia was 1.2 days longer in the same-day pegfilgrastim group versus the next-day pegfilgrastim group, this may not correlate with more clinically relevant outcomes such as febrile neutropenia. Burris and colleagues also note that same-day pegfilgrastim was statistically noninferior to next-day pegfilgrastim.10
In other studies, the incidence of febrile neutropenia was retrospectively sourced from private and Medicare healthcare claims data in patients with a variety of malignancies, and these studies did specifically report on breast cancer.8,13
Our data show that not all curative-intent regimens are equal in terms of risk for NREs, with all but 1 NRE occurring after treatment with dose-dense doxorubicin and cyclophosphamide. Although all of the chemotherapy regimens in this study are classified as being high risk for causing neutropenia, dose-dense doxorubicin and cyclophosphamide may pose the highest risk for NREs. Future studies should consider the heterogeneity of regimens when studying this clinical question to determine the role that specific chemotherapy regimens have in relation to neutropenia with the same-day administration of pegfilgrastim.
Limitations
Our study has several limitations. First, we are limited by the study’s retrospective nature and the small sample size of patients who received same-day pegfilgrastim. In addition, many of the patients who received same-day pegfilgrastim were from a single provider at 1 cancer center location compared with the other providers in the health system who exclusively prescribe next-day pegfilgrastim. The 2:1 matching strategy did exclude many potential patients who received next-day pegfilgrastim from the analysis because the number of patients who received same-day pegfilgrastim was small. A larger, prospective, randomized trial would help alleviate this limitation and would expand the number of patients who received same-day pegfilgrastim.
Our results relied heavily on the accuracy and information entered into the EMR. There may have been patients who were admitted to an outside hospital for neutropenic fever or who had other complications that were not captured in this study. In addition, dose reductions or delays may have been multifactorial and not only related to neutropenia. As a result, some events may not have been properly recorded.
This study does not address the external validity of these results in patients with other malignancies or patients receiving chemotherapy regimens other than those used in this study. Last, our composite outcome broadly categorizes events that are related to neutropenia, but it may not encompass all possible complications related to the same-day administration of pegfilgrastim.
Conclusion
The results of this retrospective review do not show that the administration of same-day pegfilgrastim results in a significant increase of NREs in patients receiving curative-intent chemotherapy for breast cancer compared with the standard next-day dosing strategy for pegfilgrastim. Larger interventional studies are needed to validate these results to expand the use of same-day pegfilgrastim.
Based on the results of this review, the practice of considering same-day pegfilgrastim on a case-by-case basis will likely continue at TUKHS until evidence dictates otherwise, because it is convenient for patients and is not detrimental to the patients’ outcomes. TUKHS plans to continue to collect data on patients who receive same-day pegfilgrastim to increase our sample size and potentially research this strategy in patients with other malignancies.
Author Disclosure Statement
Dr Mahmoudjafari is a consultant to and on the Advisory Board of Omeros, Medexus, Coherus, Fresenius, Genentech, and AstraZeneca, and is on the Speakers Bureau of Omeros; Dr Gao is a Biostatistician at Bristol Myers Squibb, but was a graduate student at University of Kansas Medical Center when this work was done; Dr Weise, Dr Cascone, Dr Tran, Dr Martin, and Dr Khan have no conflicts of interest to report.
References
- Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13:e552-e561.
- Liutkauskiene S, Grizas S, Jureniene K, et al. Retrospective analysis of the impact of anthracycline dose reduction and chemotherapy delays on the outcomes of early breast cancer molecular subtypes. BMC Cancer. 2018;18:453.
- Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015;33:3199-3212.
- Neupogen (filgrastim) injection, for subcutaneous or intravenous use [prescribing information]. Amgen; April 2023. www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/neupogen/neupogen_pi_hcp_english.pdf. Accessed August 13, 2023.
- Neulasta (pegfilgrastim) injection, for subcutaneous use [prescribing information]. Amgen; February 2021. www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Neulasta/neulasta_pi_hcp_english.pdf. Accessed August 14, 2023.
- US Food and Drug Administration. FDA approves first biosimilar to Neulasta to help reduce the risk of infection during cancer treatment. June 4, 2018. www.fda.gov/news-events/press-announcements/fda-approves-first-biosimilar-neulasta-help-reduce-risk-infection-during-cancer-treatment#:~:text=The%20U.S.%20Food%20and%20Drug,number%20of%20infection%2Dfighting%20white.Accessed August 28, 2023.
- Meropol NJ, Miller LL, Korn EL, et al. Severe myelosuppression resulting from concurrent administration of granulocyte colony-stimulating factor and cytotoxic chemotherapy. J Natl Cancer Inst. 1992;84:1201-1203.
- Townley C, Porter C, McMullen N. Comparing Grade 4 neutropenia associated with pegfilgrastim administered via the Onpro device versus manual injection with a prefilled syringe. J Hematol Oncol Pharm. 2018;8:119-125.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Hematopoietic Growth Factors. Version 2.2023. March 6, 2023. www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed September 21, 2023.
- Burris HA, Belani CP, Kaufman PA, et al. Pegfilgrastim on the same day versus next day of chemotherapy in patients with breast cancer, non–small-cell lung cancer, ovarian cancer, and non-Hodgkin’s lymphoma: results of four multicenter, double-blind, randomized phase II studies. J Oncol Pract. 2010;6:133-140.
- Weycker D, Bensink M, Lonshteyn A, et al. Risk of chemotherapy-induced febrile neutropenia by day of pegfilgrastim prophylaxis in US clinical practice from 2010 to 2015. Curr Med Res Opin. 2017;33:2107-2113.
- Weycker D, Hanau A, Lonshteyn A, et al. Risk of chemotherapy-induced febrile neutropenia with same-day versus next-day pegfilgrastim prophylaxis among patients aged ≥65 years: a retrospective evaluation using Medicare claims. Curr Med Res Opin. 2018;34:1705-1711.
- Gerberich AJ, Attilio MR, Svoboda A. Revisiting same day administration of pegfilgrastim in the age of biosimilars: a review of literature. J Oncol Pharm Pract. 2020;26(3):1970-1976.