Drug-related pneumonitis, interstitial lung disease (ILD), and pulmonary toxicity are direct adverse events of some systemic anticancer therapies.1 The causative agents in oncology include chemotherapy, targeted therapy, cellular therapy, and immunotherapy.1 The mechanism of pulmonary toxicity has been associated with oxidative stress, direct cytotoxic effects, and immune-mediated injuries.2 The oncolytic agents that are frequently associated with drug-related pneumonitis are bleomycin, epidermal growth factor receptor inhibitors, mTOR (mammalian target of rapamycin) inhibitors, and immunotherapy; however, other anticancer therapies can also carry a risk for drug-related pneumonitis.1-6
For example, docetaxel and paclitaxel, which are microtubule inhibitors that are prescribed for the treatment of various cancer types, can cause drug-related pneumonitis at an incidence of <1% to 4.6%, which does not include patients with preexisting lung disease.4-6 Antimetabolites, such as gemcitabine, can cause pneumonitis at an incidence of up to 0.2%.7 Immunotherapies, particularly PD-1 inhibitors, have an incidence of pneumonitis from approximately 1% to 2.7% and require prompt diagnosis and treatment.8 Of the more recent approved anticancer agents, cyclin-dependent kinase (CDK) 4/6 inhibitors can also carry a risk for pneumonitis, particularly abemaciclib, at a frequency of 0.2% to 3%.9-13 Antibody drug conjugates, such as fam-trastuzumab deruxtecan and ado-trastuzumab emtansine, have some risk for pneumonitis.14,15 The results of a pooled analysis of patients who received fam-trastuzumab deruxtecan showed an incidence of 15.4% for pneumonitis.14 Treatment with ado-trastuzumab emtansine is associated with a significantly lower incidence of pneumonitis than fam-trastuzumab deruxtecan, at approximately 1% to 2%.15
The onset of pneumonitis for anticancer therapies can also vary depending on the agent. Case reports of paclitaxel-induced pneumonitis in patients with early breast cancer showed a median time to onset of 15 days16; in another study, the onset of gemcitabine-induced pneumonitis in a patient with pancreatic cancer was 2 months.17 Furthermore, patients who are receiving immunotherapy can have pneumonitis approximately 2.6 months after starting therapy; for abemaciclib, the most frequently reported cases of pneumonitis were within 5 months of starting therapy.18,19
ILD and pulmonary toxicity are usually nonspecific to lung pathology, and can present as cough, dyspnea, or wheezing with varying degrees of onset.1,20,21 Histopathologic confirmation of ILD/pulmonary toxicity with a bronchoalveolar lavage and radiologic findings can assist with diagnosis, because this adverse event is considered a diagnosis of exclusion.20 The radiographic findings for drug-related pneumonitis include nonspecific interstitial pneumonia pattern or ground glass opacities.20,21 Additional findings can also include hypersensitivity pneumonia, pulmonary fibrosis, and pleural effusions, which are frequently associated with taxane therapy.20,21 Tissue confirmation will show the infiltration of lymphocytes and plasma cells or the evidence of inflammatory tissue.20,21
COVID-19 pneumonia differs from drug-related pneumonitis in clinical presentation, onset, and diagnosis. COVID-19 pneumonia often manifests similar to the common cold, with fever, dyspnea, cough, myalgia, and fatigue.20 Because COVID-19 pneumonia can lead to severe interstitial pneumonia, radiographic findings can mimic drug-related pneumonitis in alveolar damage and ground glass opacities, but a computed tomography (CT) scan within the first 48 hours of symptom onset can appear normal.20 In addition, COVID-19 can also lead to acute respiratory distress syndrome, pulmonary thrombosis, and multiorgan failure at a rapid pace.20 Furthermore, bronchoscopy is not recommended in patients with COVID-19 pneumonia because it can increase the risk for viral spread and may not be a valuable tool in diagnosis.20
In the era of COVID-19, the risk for using anticancer therapy agents that can cause pneumonitis in patients who had COVID-19 pneumonia or who received the COVID-19 vaccination series is unclear. Data related to vaccine-related pneumonitis are rather sparse; however, in a case report of a man with COVID-19 pneumonia, the pathology of vaccine-related pneumonitis was similar to that of a hypersensitivity reaction, but there is a lack of information on how to risk stratify patients who had COVID-19 pneumonia or who received a COVID-19 vaccination and are receiving anticancer agents that are known to cause pneumonitis.22 Information about the management and rechallenging of patients after they had drug-induced pneumonitis is limited as well. Although COVID-19 infection and vaccination may predispose patients to pneumonitis, further investigation is needed.
At our academic cancer center, MD Anderson Cancer Center at Cooper University Hospital, which is a tertiary care center in the Northeast Corridor, we have observed a number of patients with drug-related pneumonitis after recovering from COVID-19 or after receiving the COVID-19 vaccine series. The purpose of this study is to highlight the incidence of drug-related pneumonitis in patients at our institution who had COVID-19 or who received the COVID-19 vaccine, and to provide information regarding the duration of onset, diagnosis, and management strategies of drug-related pneumonitis. The primary study outcomes were the time from the COVID-19 primer to having symptoms of drug-related pneumonitis, and the time from the administration of anticancer therapy to having symptoms of drug-related pneumonitis.
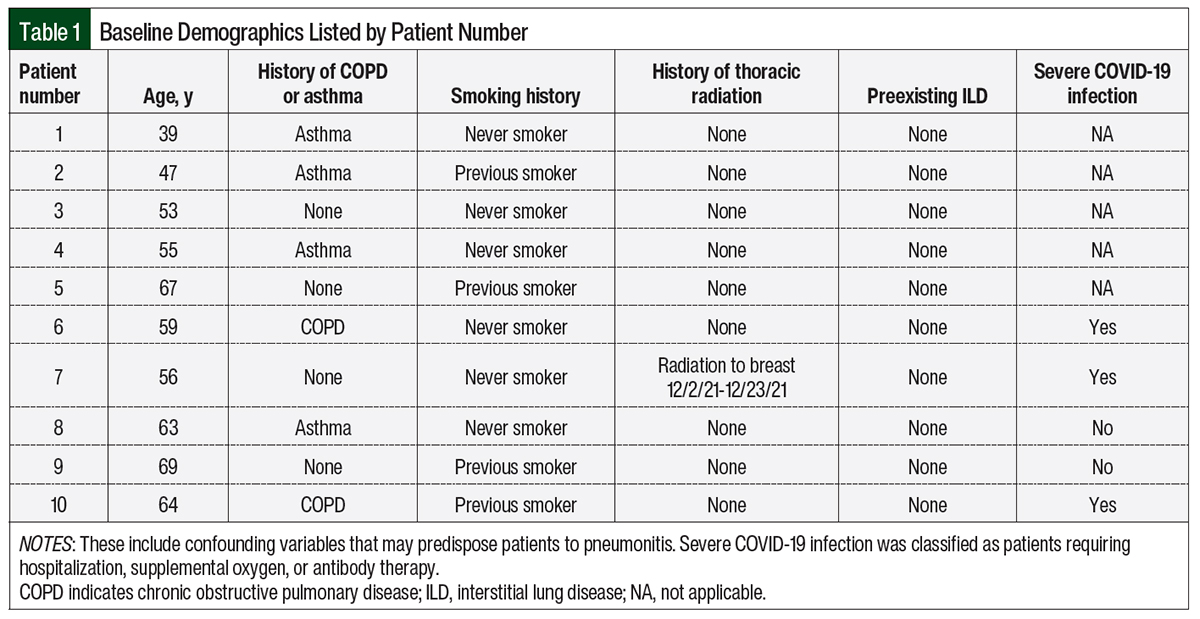
Methods
We performed a single-center, retrospective chart review of 10 patients who received anticancer treatment and had drug-induced pneumonitis between July 1, 2021, and May 31, 2022, at MD Anderson Cancer Center at Cooper University Hospital. Our data points included the patients’ cancer diagnosis, drug regimen, date of COVID-19 infection or vaccination, date of the last drug administration, duration of the anticancer treatment, date of symptom onset or CT chest imaging, evaluation by a pulmonologist, and treatment for pneumonitis. We also collected the baseline demographics with confounding factors that predispose patients to pneumonitis, such as chronic obstructive pulmonary disease (COPD), smoking history, preexisting ILD, previous thoracic radiation, and the severity of each COVID-19 infection (Table 1). There were no statistical methods used in this study.
Results
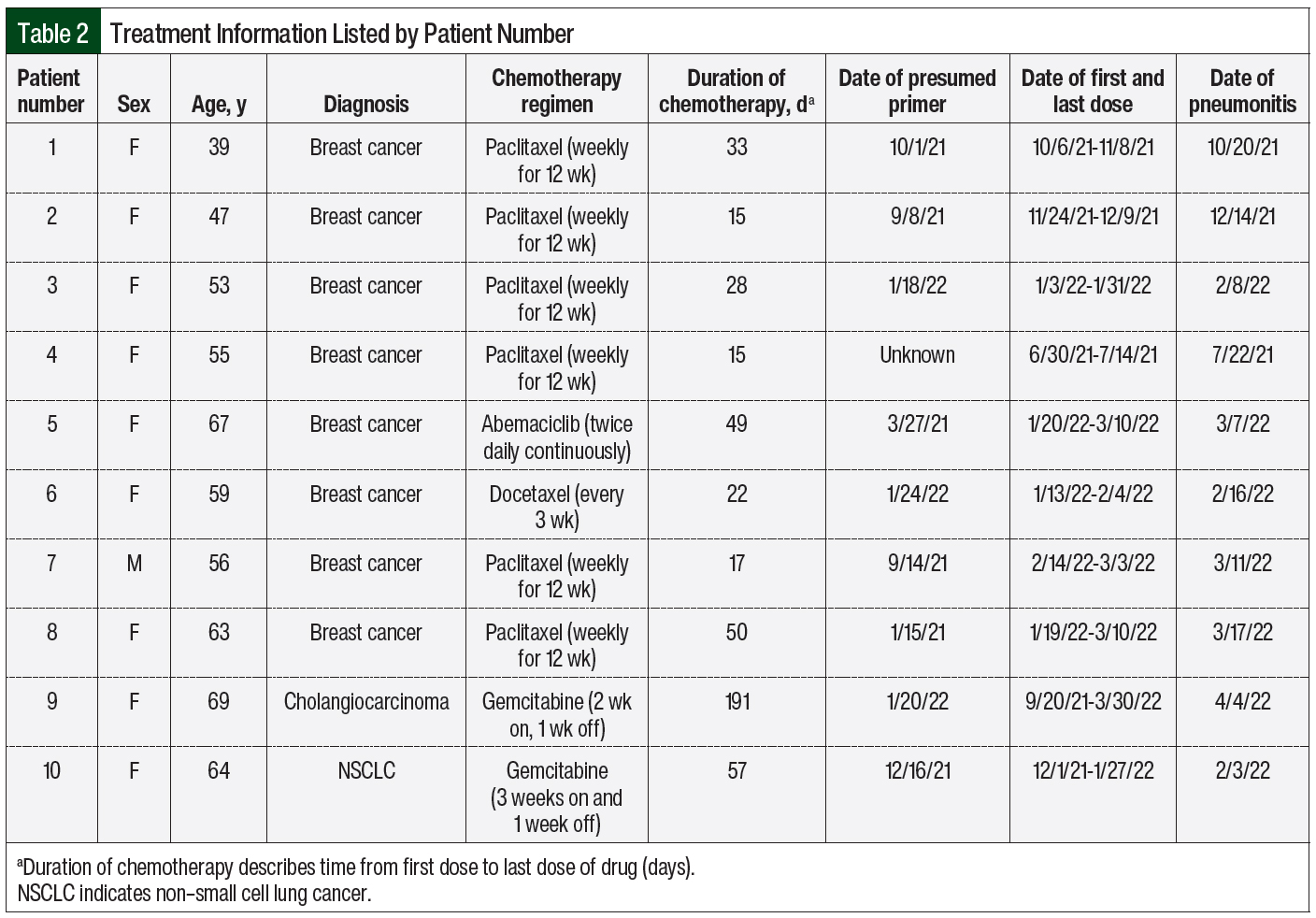
Of the 10 patients in the study, 8 had a diagnosis of breast cancer, 1 had cholangiocarcinoma, and 1 had non–small cell lung cancer. Pneumonitis was defined by either CT findings of ground glass opacities or nodular opacities that were thought to represent inflammation, or a diagnosis of pneumonitis after an assessment by a pulmonologist. All cases of drug-induced pneumonitis were preceded by having COVID-19 or receiving a COVID-19 vaccination (Table 2).
Of the 10 patients, 7 received a taxane, 2 received gemcitabine, and 1 received a CDK 4/6 inhibitor (abemaciclib) as the suspected causative agent. In all, 2 of the 10 patients who received chemotherapy also received immunotherapy before having pneumonitis. It is nearly impossible to distinguish whether the chemotherapy or the immunotherapy resulted in their symptoms, because pneumonitis secondary to immunotherapy can occur at any time point. For the purpose of this study, we are presuming that chemotherapy could have been the causative agent.
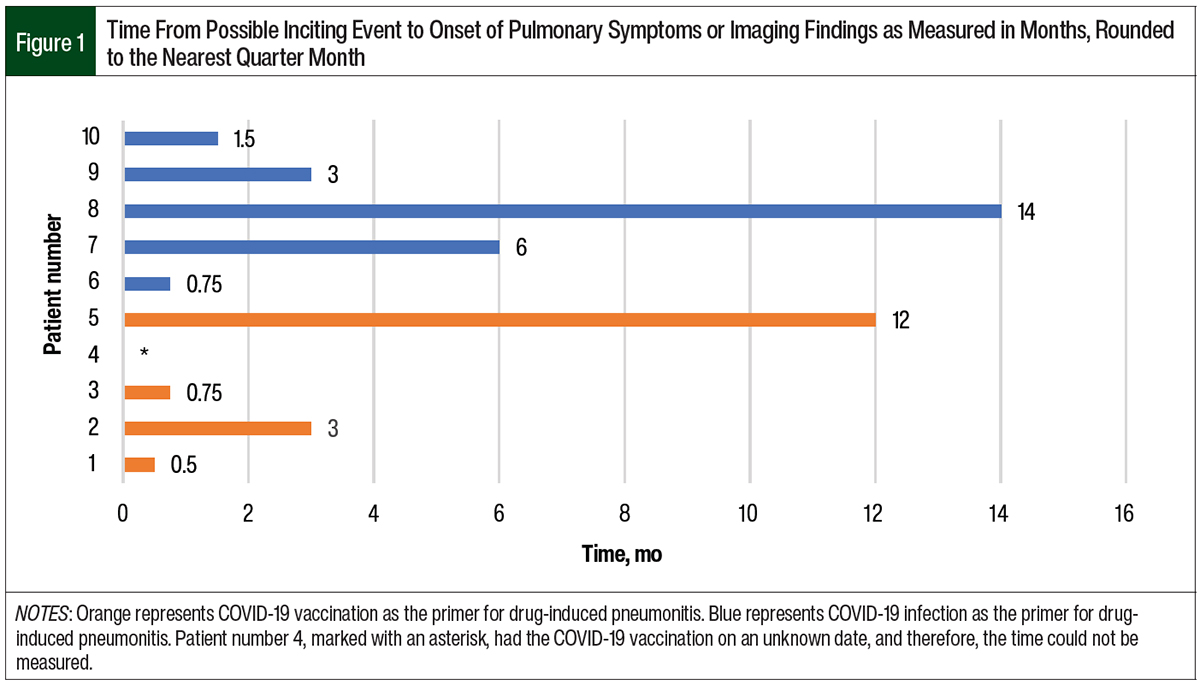
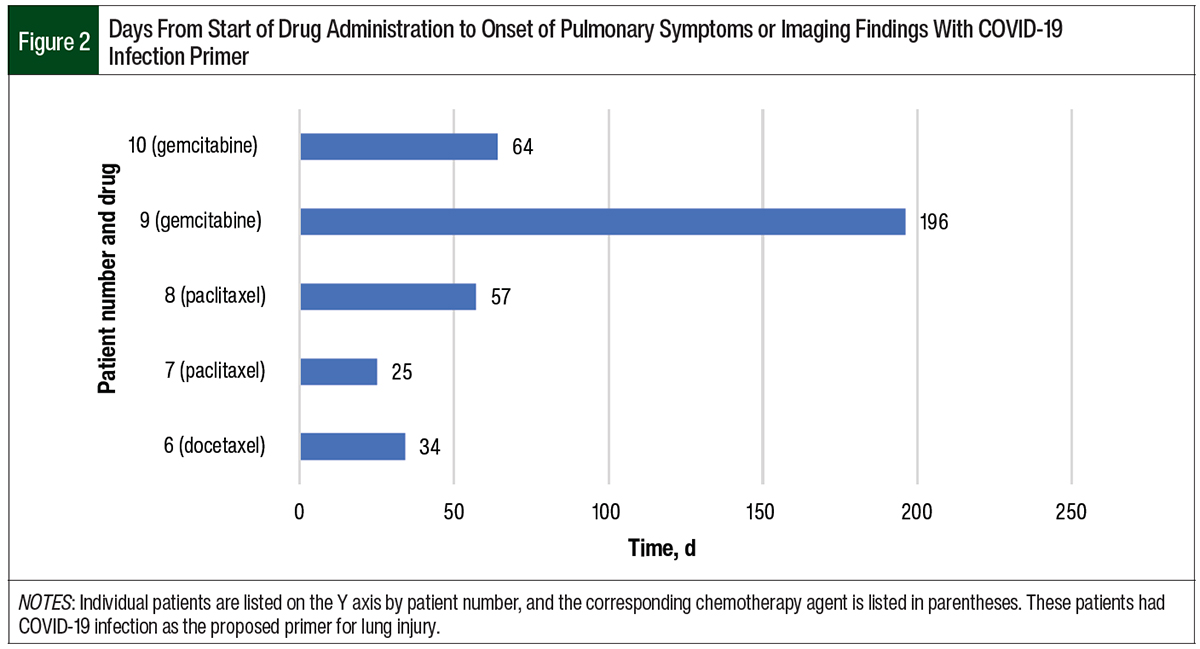
The data were separated by presumed primer, COVID-19 infection and vaccination. Of the 10 patients, 5 had COVID-19 before drug-induced pneumonitis. The time from a positive COVID-19 test to pulmonary symptoms or imaging findings ranged from 0.75 months to 14 months (Figure 1). The time from the start of chemotherapy to the onset of pneumonitis in the patients who previously had COVID-19 ranged from 25 days to 196 days (Figure 2). In this group, the median time to the onset of pneumonitis for the patients who received paclitaxel was 41 days. The median time to the onset of pneumonitis for those who received gemcitabine was 130 days. Of note, 2 of these 5 patients who had COVID-19 also received the COVID-19 vaccine; however, we suspect that having COVID-19 was the primer in these patients.
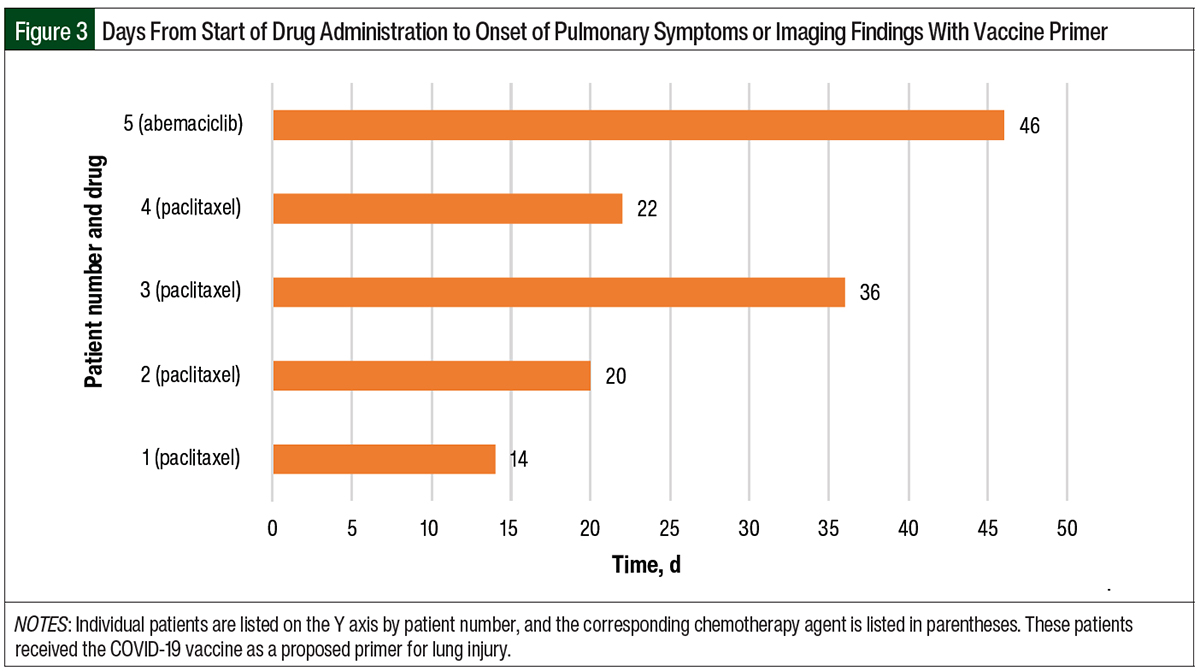
The remaining 5 patients had only the COVID-19 vaccination as a presumed primer for pneumonitis. One of these 5 patients did not have documentation for the COVID-19 vaccine date and therefore was not included in our descriptive data set. The timeline from COVID-19 vaccination to pulmonary symptoms or imaging findings ranged from 0.5 months to 12 months (Figure 1). The time from the start of chemotherapy to the onset of pneumonitis in this group ranged from 14 to 46 days (Figure 3). The median time to the onset of pneumonitis in the patients who received paclitaxel was 21 days.
When comparing the patients who had COVID-19 as the presumed primer with the patients who had a COVID-19 vaccine as the presumed primer, the average time from the start of receiving an anticancer drug to the onset of pneumonitis was 75 days in the COVID-19 group and 28 days in the COVID-19 vaccination group.
In regard to management, 9 of the 10 patients either received treatment in the emergency department or were hospitalized for their pulmonary symptoms. One patient was managed completely in the outpatient setting. A total of 8 of the 10 patients received a steroid taper and their symptoms improved. In all, 5 of the 10 patients required long-term supplemental oxygen via a nasal cannula. The presumed causative anticancer drug was discontinued in all of the patients.
Discussion
There is a paucity of data on the relationship between having COVID-19 or receiving the COVID-19 vaccination and drug-related pneumonitis, especially in patients with cancer. A study from Memorial Sloan Kettering Cancer Center showed that receiving anticancer therapy and having concurrent COVID-19 pneumonia was not associated with severe or critical COVID-19, but these data were not specific to adverse events that were related to drug-related pneumonitis.23 In addition, a case report of a 65-year-old man with head and neck cancer who had COVID-19 pneumonia that was resolving had worsening CT scan findings and immunotherapy-induced pneumonitis after the initiation of therapy, but there is a lack of information on how to quantify the risk for immunotherapy-related pneumonitis with a history of COVID-19 pneumonia.24 Furthermore, there are case reports of patients who had ILD after receiving the COVID-19 vaccination; however, there is limited information on how this information relates to the patients with cancer.25-27
There is a significantly higher incidence of drug-related pneumonitis in patients with cancer and preexisting ILD, which suggests that parenchymal injury at baseline can predispose patients to drug-related pneumonitis.28 If having COVID-19 or receiving the COVID-19 vaccine serves as a baseline parenchymal injury, it is plausible that the incidence of drug-related pneumonitis may be increased in this population. Among our patients, we noted that 4 were previous smokers and 6 had COPD or asthma, which could have conferred an additional risk for drug-related pneumonitis independent of having COVID-19 or receiving the COVID vaccination series.
We documented 10 patients over the course of 8 months who had drug-related pneumonitis at our institution. Although there may be confounding variables, we hypothesize that having COVID-19 or receiving the COVID-19 vaccination may predispose patients to oncologic drug-related pneumonitis; however, we were unable to collect the incidence of pneumonitis before the COVID-19 pandemic and therefore are not able to make a conclusive comparison at our institution.
Interestingly, we noted that drug-related pneumonitis was delayed in our study when compared with historical data, especially in the patients who had COVID-19 as the possible primer.16 For example, the median duration of the onset of pneumonitis in the patients who received paclitaxel was 21 days in those who received the COVID-19 vaccine and 41 days in those who had had COVID-19. A previous study suggests that the median duration of the onset of pneumonitis for paclitaxel is 15 days.16 Similarly, the historical data for gemcitabine show that the median duration of the onset of pneumonitis was approximately 60 days, whereas it was 130 days in our study patients who had COVID-19.17 In our study, the patients with COVID-19 had a longer average length of time from the start of an anticancer drug to pneumonitis compared with patients who received the COVID-19 vaccine.
In regard to management, drug-related pneumonitis can be resolved with high-dose steroids to suppress the acute inflammation.1,20,29 However, this strategy may not be useful in patients with pulmonary fibrosis or severe pneumonitis, which can be irreversible.21 Along with corticosteroids, the treatment strategy for drug-related pneumonitis is often to discontinue the causative agent.20,21 Two patients, one who received immunotherapy and paclitaxel and another who received abemaciclib, continued anticancer treatment for a short period of time after having symptoms of pneumonitis. Treatment with these drugs was stopped when the patients’ symptoms did not improve. In the absence of the real-world incidence of drug-related pneumonitis in patients with cancer who received the COVID-19 vaccine or had COVID-19 pneumonia, prompt evaluation by a pulmonologist and CT chest imaging will help establish a diagnosis. In our study, corticosteroids and oxygen therapy were widely employed and provided relief to the patients, but ultimately, the initiation of alternative anticancer therapies was delayed subsequent to their diagnosis of pneumonitis.
Limitations
Our study has several limitations, including its observational, retrospective nature and its small sample of patients diagnosed with drug-related pneumonitis who had COVID-19 or received a COVID-19 vaccination. There may be many confounding variables that contributed to this study, such as previous lung injury, smoking status, or a history of other pulmonary diseases. We cannot prove any causality between a COVID-19 primer and drug-induced pneumonitis, although we did observe a possible association with increased sensitivity to lung injury. We do not know the incidence of pneumonitis at our center before COVID-19, because this was not a part of our study.
An additional limitation is that there were 2 patients in our study who received immunotherapy or chemotherapy and had pneumonitis. For the purpose of this study, we presumed that chemotherapy was the implicated drug, but the use of immunotherapy is a major confounding variable because it can result in pneumonitis.
Conclusion
Now that the COVID-19 pandemic has become part of daily living, many patients with cancer have had COVID-19 or have received the COVID-19 vaccine before initiating anticancer therapy. This study provides examples of having COVID-19 or receiving the COVID-19 vaccine as a possible primer for drug-related pneumonitis. Our study results show a longer duration from the start of anticancer drug treatment to the onset of pneumonitis than in the historical data. This discrepancy was the most notable in the patients who had COVID-19 as the possible primer for drug-related pneumonitis.
It may be important for oncologists to have a low threshold for the early workup and treatment of new pulmonary symptoms in this ever-growing patient population. Future studies should examine the rate of pneumonitis in patients who had COVID-19 or who received the COVID-19 vaccine series.
Author Disclosure Statement
Dr Yenamandra, Dr Greidinger, and Dr Jenab-Wolcott have no conflicts of interest to report.
References
- Nishino M, Hatabu H, Hodi FS, Ramaiya NH. Drug-related pneumonitis in the era of precision cancer therapy. JCO Precis Oncol. 2017;1:PO.17.00026.
- Erasmus JJ, McAdams HP, Rossi SE. High-resolution CT of drug-induced lung disease. Radiol Clin North Am. 2002;40:61-72.
- O’Sullivan JM, Huddart RA, Norman AR, et al. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol. 2003;14:91-96.
- Tamiya A, Naito T, Miura S, et al. Interstitial lung disease associated with docetaxel in patients with advanced non-small cell lung cancer. Anticancer Res. 2012;32:1103-1106.
- Bielopolski D, Evron E, Moreh-Rahav O, et al. Paclitaxel-induced pneumonitis in patients with breast cancer: case series and review of the literature. J Chemother. 2017;29:113-117.
- Ostoros G, Pretz A, Fillinger J, et al. Fatal pulmonary fibrosis induced by paclitaxel: a case report and review of the literature. Int J Gynecol Cancer. 2006;16(suppl 1):391-393.
- Roychowdhury DF, Cassidy CA, Peterson P, et al. A report on serious pulmonary toxicity associated with gemcitabine-based therapy. Invest New Drugs. 2002;20:311-315.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Management of Immunotherapy-Related Toxicities. Version 3.2023. October 11, 2023. Accessed November 7, 2023. www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5.
- Johnston SRD, Harbeck N, Hegg R, et al; for the monarchE committee members and investigators. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2–, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38:3987-3998.
- Kisqali (ribociclib) tablets, for oral use [prescribing information]. Novartis; August 2023. Accessed November 7, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2023/209092s016lbl.pdf
- Wilgus ML, Rosenberg J. CDK4/6 inhibitor-induced pneumonitis: a case report and review of the literature. Proc UCLA Health. 2020;24. Accessed November 7, 2023. https://proceedings.med.ucla.edu/index.php/2020/09/30/cdk46-inhibitor-induced-pneumonitis-a-case-report-and-review-of-the-literature/
- Ahsan I, Malik F, Jafri S. Palbociclib related pnemotoxicity: a rare side effect. Am J Respir Crit Care Med. 2017;195:A5546.
- Powell CA, Modi S, Iwata H, et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 2022;7:100554.
- Kadcyla (ado-trastuzumab emtansine) for injection, for intravenous use [prescribing information]. Genentech, Inc; February 2022. Accessed November 7, 2023. www.gene.com/download/pdf/kadcyla_prescribing.pdf
- Ardolino L, Lau B, Wilson I, et al. Case report: paclitaxel-induced pneumonitis in early breast cancer: a single institution experience and review. Front Oncol. 2021;11:701424.
- Poole BB, Hamilton LA, Brockman MM, Byrd DC. Interstitial pneumonitis from treatment with gemcitabine. Hosp Pharm. 2014;49:847-850.
- Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22:6051-6060.
- Chen Y, Noma S, Taguchi Y, et al. Characteristics of interstitial lung disease in patients from post-marketing data on metastatic breast cancer patients who received abemaciclib in Japan. Breast Cancer. 2021;28:710-719.
- Dumoulin DW, Gietema HA, Paats MS, et al. Differentiation of COVID-19 pneumonitis and ICI induced pneumonitis. Front Oncol. 2020;10:577696.
- Schwaiblmair M, Behr W, Haeckel T, et al. Drug induced interstitial lung disease. Open Respir Med J. 2012;6:63-74.
- Matsuzaki S, Kamiya H, Inoshima I, et al. COVID-19 mRNA vaccine-induced pneumonitis. Intern Med. 2022;61:81-86.
- Jee J, Foote MB, Lumish M, et al. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol. 2020;38:3538-3546.
- Dipasquale A, Persico P, Lorenzi E, et al. COVID-19 lung injury as a primer for immune checkpoint inhibitors (ICIs)-related pneumonia in a patient affected by squamous head and neck carcinoma treated with PD-L1 blockade: a case report. J Immunother Cancer. 2021;9:e001870. Erratum in: J Immunother Cancer. 2021;9:e001870corr1.
- Yoshifuji A, Ishioka K, Masuzawa Y, et al. COVID-19 vaccine induced interstitial lung disease. J Infect Chemother. 2022;28:95-98.
- DeDent AM, Farrand E. Vaccine-induced interstitial lung disease: a rare reaction to COVID-19 vaccination. Thorax. 2022;77:9-10.
- Park JY, Kim JH, Lee IJ, et al. COVID-19 vaccine-related interstitial lung disease: a case study. Thorax. 2022;77:102-104.
- Cherri S, Noventa S, Fanelli M, et al. Drug-related pneumonitis in cancer treatment during the COVID-19 era. Cancers (Basel). 2021;13:1052.
- Kim R, Meyer KC. Therapies for interstitial lung disease: past, present and future. Ther Adv Respir Dis. 2008;2:319-338.