The oncology patient population is at an elevated risk for severe infections associated with increased mortality. The early recognition of fever is critical in patients with febrile neutropenia, because a fever may be the only sign of infection.1 According to the 2010 Infectious Diseases Society of America (IDSA) guidelines, febrile neutropenia is defined as an absolute neutrophil count (ANC) of ≤500 cells/mm3, with a single oral temperature of ≥101°F or a temperature of ≥100.4°F sustained over a 1-hour period.2
Treatment of febrile neutropenia is guided by patient classification to high or low risk for adverse outcomes. Patients are considered at high risk if the neutropenia is expected to be prolonged (ie, ≥7 days), or if comorbidities are present.3 High-risk patients may require hospitalization and empiric administration of intravenous (IV) antipseudomonal beta-lactam. The 2010 IDSA guidelines list cefepime, meropenem, imipenem-cilastatin, and piperacillin-tazobactam as the agents of choice for this patient population.2
International sepsis and febrile neutropenia guidelines recommend antibiotic administration within 1 hour of recognition, because of the influence time that antibiotic administration has on mortality.4,5 A cohort study of patients with febrile neutropenia showed that the 28-day mortality rate rose by 18% for every 1-hour delay in time to antibiotic administration.3 Lower mortality rates were seen with ≤30 minutes time to administration compared with a duration of 31 minutes to 60 minutes, which further supports the direct impact that the time to antibiotic administration has on mortality.3
The utilization of treatment protocols and computer-based standardized order sets may aid in achieving the shortest time to antibiotic administration possible for patients with febrile neutropenia and with other serious infections, as well. To help improve first-dose administration time, our institution also keeps antibiotics loaded in automated dispensing cabinets that are located on each patient’s unit and are available immediately after the orders are placed by a provider and are reviewed and verified by a pharmacist.
In September 2017, Hurricane Maria contributed to severe IV fluid shortages that affected health systems nationwide. Patient care was negatively affected, because IV preparations and maintenance fluid administration were subject to change daily, based on supply constraints.6 Puerto Rico is responsible for 44% of the United States’ IV fluid bag production.6 Typically,3 IV fluid manufacturers supply the average monthly demand of 40 million bags in the United States. In response to an extensive shortage, hospitals were required to develop alternative solutions for IV fluid preparation and administration.
Small-volume bag availability became scarce, causing hospitals to rely on expensive premixed drugs when available, to utilize larger-volume bags, or to administer drugs IV push.6 Concentration changes and other alternatives to standard preparation were also required to use the inventory appropriately.7,8 This had an extensive impact on electronic resource utilization (eg, editing of computer-based order sets, compounding technology) as inventory had to be continually reviewed for appropriate prescribing.7,8 As part of the institution’s continuing quality improvement assessments, 2 retrospective audits were compared from specific time points to assess antibiotic drug delivery times before and after the IV fluid shortage.
Methods
Avera McKennan Hospital and University Health Center is a 545-bed tertiary care referral institution that conducts periodic reviews for successful antibiotic time to administration in adult patients with febrile neutropenia. Antibiotic administration is defined as the time to initiation of the antibiotic documented in the medication administration record. In November 2017, a quality improvement audit was completed at the institution to examine the success rate of antibiotic administration in patients with febrile neutropenia between June 1, 2017, and October 31, 2017. Success was defined as the administration of an IV antipseudomonal beta-lactam within 60 minutes of a documented temperature of 100.5°F in patients with an ANC of <500 cells/mm3.
Patients were excluded from the audit if the first dose of the drug was administered outside of the institution. Failures, defined as antibiotics administered more than 1 hour after the antibiotic administration criteria are being met, are screened, and appropriate actions are taken to fix the processes that need improvement. After the completion of the November 2017 audit, the institution was greatly affected by the IV fluid shortages from Hurricane Maria.
A sequential quality improvement audit was completed in May 2018 to determine the impact that fluid shortages had on the time to antibiotic administration in the febrile neutropenia protocols between December 1, 2017, and March 31, 2018. As a result of a slight change in the institutional protocol, the definition of success included administration of an IV antipseudomonal beta-lactam within 60 minutes of a documented temperature of ≥100°F (which was 0.5°F different from the previous audit criteria) in patients with an ANC of <500 cells/mm3. Patients with a temperature of 100°F to 100.4°F also had to have a heart rate of >100 bpm or a systolic blood pressure of <100 mm Hg to meet the criteria to start antibiotic therapy.
The primary objective of this review was to compare the rates of successful antibiotic administration during the 2 audits. A Fisher’s exact test was used to determine statistical significance for the small sample size.
Results
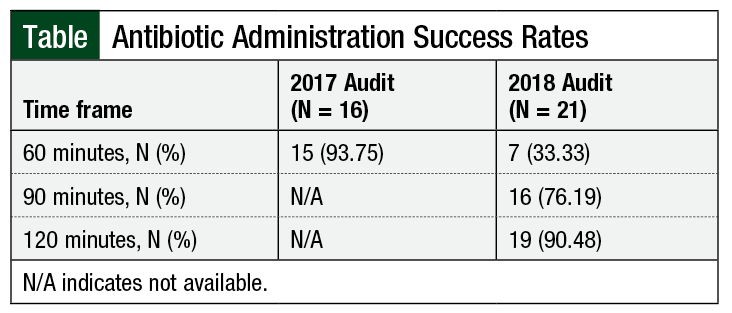
The first audit (from November 2017) included 16 patients who met the institution’s criteria for febrile neutropenia. A total of 15 patients successfully received treatment with an appropriate antibiotic within the 60-minute time frame, yielding a 93.75% success rate.
Of the 21 patients reviewed in the second audit (in May 2018), 7 patients successfully received treatment with an appropriate antibiotic within 60 minutes of a documented fever. Successful antibiotic administration at 60, 90, or 120 minutes is shown in the Table. In the 2017 audit, 93.75% (15 of 16) of patients received antibiotics within the 60-minute time target for the treatment of febrile neutropenia compared with the 2018 audit, in which only 33% of the patients received antibiotics within 1 hour of meeting the criteria for treatment. Overall, 19 of 21 (90.5%) patients received antibiotic treatment for febrile neutropenia starting within 2 hours of meeting the criteria. The average time to antibiotic administration in the second audit was 110.33 minutes. After excluding 2 outliers (ie, antibiotic administration >180 minutes), that average time to antibiotic administration was 75.63 minutes after the documented fever. In both cases, the time to antibiotic treatment was more than the 60-minute time frame targeted for treatment of febrile neutropenia.
We conducted statistical analysis to compare the 60-minute antibiotic administration success rates of the 2 audits, which included 15 of the 16 (93.75%) patients in the 2017 audit and 7 of the 21 (33.33%) patients in the 2018 audit. The Fisher’s exact test yielded a P value of .0002, deeming the 60.42% difference between the 2 audits statistically significant.
Discussion
The increased time to antibiotic administration and increased failure rate in the second audit is most likely the result of continual changes to drug ordering, drug compounding, and IV fluid availability. Delays in drug compounding because of fluctuating IV fluid availability decreased the utility of the protocols and order sets that were previously in place to promote the early administration of antibiotics. Dispensing pharmacists had to alter orders to fit the available IV fluid inventory, which caused small, but significant, delays in the delivery and administration of IV drugs.
Should future shortages occur, careful evaluation of the fluid shortage will be completed on an institutional basis for carrier fluids. Batch compounding, when available, will be used to optimize the storage and supply of medications on nursing units to replicate more closely situations when shortages are not occurring. Furthermore, the American Society of Health-System Pharmacists also provided strategies to conserve and control inventory that institutions may refer to for additional approaches to drug shortages.8
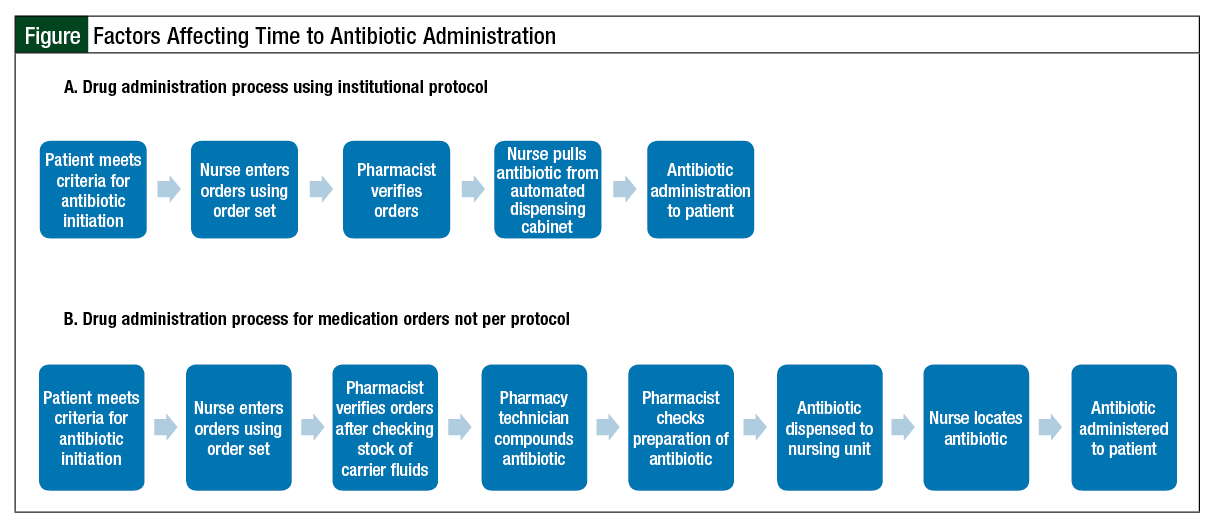
The Figure depicts 2 examples of processes for antibiotic dispensing in our institution. The first row (A) depicts the process for drug administration using an order set. Institutional order sets prompt the nurses to identify correctly and order drugs that have been previously compounded and stored in the automated dispensing cabinets on the unit. Compounding antibiotics ahead of time allows the drugs to be stored in close proximity to the patient, with quick accessibility by the nurse, ensuring an efficient and rapid administration of antibiotics to patients.
The second row (B) in the Figure depicts the process for drug administration during fluid shortages. The nurses continue to use order sets to order antibiotics; however, these orders no longer reflect drugs that can be prepared ahead of time. Pharmacists must check the inventory of carrier fluids and correct orders before those drugs can be compounded. This process is slower than when drugs are prepared ahead of time and readily available in dispensing cabinets on the nursing stations, because it involves on-demand compounding, as well as delivery to the nurses’ station where the patient is located.
Limitations
We identified 3 important limitations that may prevent this analysis from being generalizable to patients with febrile neutropenia at other sites: the 2 audits were retrospective, limiting the available data to what was documented in the specific medical record. there was also a slight difference in the inclusion criteria between the 2 audits as a result of a change in institutional protocol, and site-specific mortality rates were not reviewed in the audits, but these would be useful in determining the impact of the increased time failure to antibiotic administration.
Finally, in our institution, fluid supplies were changing daily, leading to unpredictable changes in how drugs were being dispensed from the hospital pharmacy. As a result of this daily variability, order sets could not be optimized to the full extent that electronic health records are proposed to offer.
Conclusion
Our analysis showed a strong statistical difference in achieving successful and timely antibiotic administration before and during national drug shortages. National IV fluid shortages led to significant delay in time to antibiotic administration, a known risk for mortality in patients with febrile neutropenia. Institutions should remain aware that significant fluid shortages requiring changes to routine dispensing practices can affect some of the most vulnerable patient populations, such as those with febrile neutropenia.
Acknowledgment
We thank Nicole Stenzel, PharmD, for helping with the quality improvement audits.
Author Disclosure Statement
Dr Beck has received Advisory Board honorarium from Heron Therapeutics; Dr Anderson, Dr McNamara, and Ms Elgersma have no conflicts of interest to report.
References
- Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;730-751.
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guidelines for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93.
- Rosa RG, Goldani LZ. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother. 2014;58:3799-3803.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637.
- Rolston KVI. Challenges in the treatment of infections caused by gram-positive and gram-negative bacteria in patients with cancer and neutropenia. Clin Infect Dis. 2005;40(suppl 4):S246-S252.
- Patiño AM, Marsh RH, Nilles EJ, et al. Facing the shortage of IV fluids: a hospital-based oral rehydration strategy. N Engl J Med. 2018;378:1475-1477.
- Hick JL, Hanfling D, Courtney B, Lurie N. Rationing salt water: disaster planning and daily care delivery. N Engl J Med. 2014;370:1573-1576.
- American Society of Health-System Pharmacists. Small-volume parenteral solutions shortages: suggestions for management and conservation. October 2017. www.ashp.org/-/media/assets/drug-shortages/docs/drug-shortages-svp--shortages-suggestions-for-management-conservation.ashx. Accessed May 2018.