Diffuse large B-cell lymphoma (DLBCL), a form of cancer that starts in the lymphatic system, is the most common type of non-Hodgkin lymphoma (NHL) in adults.1,2 In the United States, approximately 72,000 new cases of NHL are diagnosed annually; more than 20,000 people were estimated to die from the disease in 2017.3
B-cells—the lymphocytes (ie, white blood cells) that secrete antibodies in response to foreign pathogens—are implicated in several subtypes of NHL, including DLBCL, follicular lymphoma, Burkitt lymphoma, and mantle-cell lymphoma.2
For patients with DLBCL that does not respond to chemotherapy or subsequent regimens and disease that relapses within a year after having autologous stem-cell transplantation, the prognosis is particularly poor.4,5
Yescarta Second Gene Therapy Approved by the FDA
On October 18, 2017, Yescarta (axicabtagene ciloleucel; Kite Pharma) was approved by the US Food and Drug Administration (FDA) for the treatment of adults with certain types of relapsed or refractory large B-cell lymphoma after receiving 2 or more lines of systemic therapy; these types include DLBCL not otherwise specified, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.6 Axicabtagene ciloleucel, a gene-based immunotherapy, is the first chimeric antigen receptor (CAR) T-cell therapy to receive FDA approval for large B-cell lymphoma and the second gene therapy to be approved by the FDA.6,7 Axicabtagene ciloleucel is not indicated for the treatment of patients with primary central nervous system lymphoma.6,8
“Today marks another milestone in the development of a whole new scientific paradigm for the treatment of serious diseases. In just several decades, gene therapy has gone from being a promising concept to a practical solution to deadly and largely untreatable forms of cancer,”6 said FDA Commissioner Scott Gottlieb, MD.
The FDA granted axicabtagene ciloleucel a priority review, orphan drug status, and a breakthrough therapy designation for this indication.6
Mechanism of Action
Axicabtagene ciloleucel is a CD19-directed, genetically modified, autologous T-cell immunotherapy, which is a customized treatment that uses the patient’s own immune system to help fight lymphoma.6,8 This therapy binds to CD19-expressing cancer cells and normal B-cells. Axicabtagene ciloleucel targets the CD19-expressing cancer cells and their downstream signaling cascades that lead to T-cell activation, proliferation, and the release of inflammatory cytokines, which results in apoptosis of CD19-expressing cells.8
The patient’s peripheral blood cells are obtained via a standard leukapheresis procedure. After the cells are modified, expanded, and formulated into a suspension, they are infused back into the patient. Once reinfused into the patient, the anti-CD19 CAR T-cells recognize and eliminate CD19-expressing target cells.8
Dosing and Administration
A leuko-depleting filter should not be used with axicabtagene ciloleucel. Before axicabtagene ciloleucel is infused, a lympho-depleting regimen of cyclophosphamide and fludarabine should be administered. The patient’s identity should be verified before infusion. The patient should be premedicated with acetaminophen and an H1 antihistamine.8
The dose of axicabtagene ciloleucel is based on the number of CAR-positive viable T-cells. The recommended target dose is 2 × 106 CAR-positive viable T-cells per kg of body weight, with a maximum of 2 × 108 CAR-positive viable T-cells. Axicabtagene ciloleucel should only be administered in a certified healthcare facility. Before infusing axicabtagene ciloleucel, confirm that tocilizumab is available.8
Axicabtagene ciloleucel is available as a cell suspension for infusion; it is constituted as a suspension of 2 × 106 CAR-positive viable T-cells per kg of body weight, with a maximum of 2 × 108 CAR-positive viable T-cells in approximately 68 mL.8
ZUMA-1 Clinical Trial
The FDA approval of axicabtagene ciloleucel was based on safety and efficacy results from the ZUMA-1 study, a single-arm, open-label, phase 2 clinical trial of adults with relapsed or refractory aggressive B-cell NHL.4,8 After receiving lympho-depleting chemotherapy, 101 patients (median age, 58 years) received axicabtagene ciloleucel as a single intravenous infusion at a target dose of 2 × 106 CAR-positive viable T-cells/kg (maximum permitted dose of 2 × 108 cells). All patients were hospitalized for the infusion and remained hospitalized for at least 7 days thereafter.8
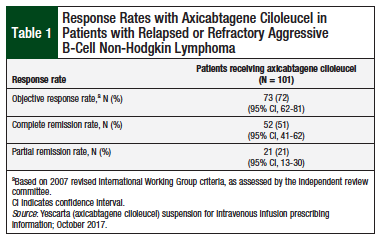
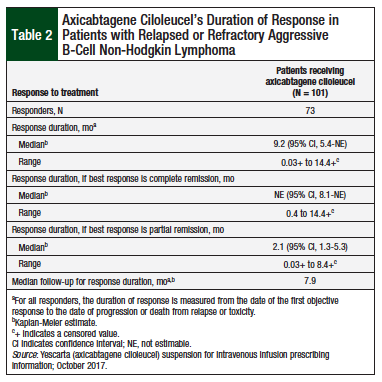
In ZUMA-1, 72% of the patients demonstrated an objective response to treatment with axicabtagene ciloleucel, and 51% of patients achieved a complete remission (Table 1).8 The median time to treatment response was 0.9 months (range, 0.8-6.2 months). At a median follow-up of 7.9 months, the median duration of response for patients in complete remission had not been reached (Table 2).8
Adverse Reactions
The most common (incidence ≥20%), any grade, nonlaboratory adverse reactions were cytokine release syndrome (CRS; 94%), fever (86%), hypotension (57%), encephalopathy (57%), tachycardia (57%), fatigue (46%), headache (45%), decreased appetite (44%), chills (40%), diarrhea (38%), febrile neutropenia (36%), infections-pathogen unspecified (26%), nausea (34%), hypoxia (32%), tremor (31%), cough (30%), vomiting (26%), dizziness (21%), constipation (23%), and cardiac arrhythmias (23%).8
Axicabtagene ciloleucel has no contraindications.8
Warnings and Precautions
Axicabtagene ciloleucel was approved with a boxed warning about the potential for CRS, including fatal or life-threatening reactions. Patients must be advised of the potential serious side effects associated with axicabtagene ciloleucel and the importance of immediately returning to the treatment site if side effects occur.6 Axicabtagene ciloleucel should not be administered to patients with active infection or inflammatory disorders. Severe or life-threatening CRS should be treated with tocilizumab or tocilizumab and corticosteroids.8
The boxed warning also cautions about neurologic toxicities, including fatal or life-threatening reactions that have occurred after treatment with axicabtagene ciloleucel, such as toxicities that can occur concurrently with or after the resolution of CRS. Patients should be monitored for neurologic toxicities after axicabtagene ciloleucel treatment and receive supportive care and/or corticosteroids, as necessary. Axicabtagene ciloleucel is only available through a restricted Risk Evaluation and Mitigation Strategy (REMS) program (Yescarta REMS).8
Patients should be monitored for hypersensitivity reactions during infusion, as well as for signs and symptoms of infection, and be treated appropriately.8
After infusion with axicabtagene ciloleucel, patients may exhibit grade 3 or higher cytopenias for several weeks. Patients should be monitored via complete blood counts.8
Patients should also be monitored for hypogammaglobulinemia and provided with replacement therapy.8
Any secondary malignancy that occurs after treatment with axicabtagene ciloleucel should be reported to the manufacturer.8
Patients should be advised to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks after receiving axicabtagene ciloleucel.8
Use in Specific Populations
Axicabtagene ciloleucel is not recommended for pregnant women; posttreatment pregnancy should be discussed with the treating physician. The pregnancy status of women of reproductive potential should be verified, or tested, if appropriate. The data are insufficient to determine the effect of axicabtagene ciloleucel on fertility or to provide a clear recommendation on the duration of posttreatment contraception.8
The data are also insufficient to determine whether patients aged ≥65 years respond to axicabtagene ciloleucel different from, or have different safety outcomes than, younger patients.8
Conclusion
With its FDA approval, axicabtagene ciloleucel became only the second gene therapy to be approved in the United States and the first CAR T-cell/gene therapy to be approved for the treatment of adults with several types of relapsed or refractory large B-cell lymphoma. In the pivotal clinical trial YUMA-1, 72% of patients who received axicabtagene ciloleucel achieved an objective response, of whom 51% achieved a complete remission. Axicabtagene ciloleucel, a CD19-directed CAR T-cell immunotherapy, is a new treatment option that may improve outcomes for patients with certain types of large B-cell lymphoma.
References
- American Cancer Society. Types of non-Hodgkin lymphoma. www.cancer.org/cancer/non-hodgkin-lymphoma/about/types-of-non-hodgkin-lymphoma.html. Accessed December 7, 2017.
- Mayo Clinic. Non-Hodgkin’s lymphoma. www.mayoclinic.org/diseases-conditions/non-hodgkins-lymphoma/symptoms-causes/syc-20375680. Accessed December 5, 2017.
- National Cancer Institute. SEER cancer stat facts: non-Hodgkin lymphoma. https://seer.cancer.gov/statfacts/html/nhl.html. Accessed December 6, 2017.
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531-2544.
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800-1808.
- US Food and Drug Administration. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma: Yescarta is the second gene therapy product approved in the U.S. October 18, 2017. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm581216.htm. Accessed December 4, 2017.
- Gilead. Kite’s Yescarta (axicabtagene ciloleucel) becomes first CAR T therapy approved by the FDA for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy. October 18, 2017. www.gilead.com/news/press-releases/2017/10/kites-yescarta-axicabtagene-ciloleucel-becomes-first-car-t-therapy-approved-by-the-fda-for-the-treatment-of-adult-patients-with-relapsed-or-refractory-large-bcell-lymphoma-after-two-or-more-lines-of-systemic-therapy. Accessed December 12, 2017.
- Yescarta (axicabtagene ciloleucel) suspension for intravenous infusion [prescribing information]. Santa Monica, CA: Kite Pharma; October 2017.